Abstract
BACKGROUND AND PURPOSE: Permanent balloon occlusion (PBO) of the carotid artery has been previously shown to be an effective means to treat carotid blowout syndrome (CBS). However, despite the effectiveness of this endovascular technique, concern remains regarding its potential for producing delayed cerebral ischemic complications in 15% to 20% of patients. This significant limitation of carotid PBO led our group to evaluate an alternative management strategy, consisting of endovascular reconstruction of the carotid artery (ERCA) in patients thought to be at particularly high risk for carotid occlusion (ie, provocative balloon test occlusion, angiographic documented incomplete circle of Willis, or contralateral carotid artery occlusion).
METHODS: We reviewed all cases of CBS referred to our service, in which ERCA was chosen as a management strategy for patients thought to be at high risk for PBO, based on previously defined criteria.
RESULTS: Sixteen carotid blowout events occurred in 12 patients with CBS who were deemed to be at high risk for cerebral ischemic complications, which were managed with ERCA by using a variety of stent devices and techniques. Adjunctive embolization of carotid pseudoaneurysms was performed in five of these patients by using platinum coils or acrylic glue. Hemostasis was achieved in all cases, although one patient with traumatic CBS and three patients with aggressive head and neck cancer-related CBS, required retreatment with ERCA. Recurrent CBS rates were similar to those reported in other studies using PBO. Overall, no treatment-related strokes or deaths occurred.
CONCLUSION: CBS managed with ERCA can be performed safely and with efficacy of outcomes at least equivalent to those previously reported in association with conventional carotid PBO, therefore representing an excellent alternative endovascular technique for patients who are at increased risk of stroke after PBO.
Carotid blowout is a term that has commonly been applied to cases of rupture of the extracranial carotid artery or its branches. Usually, carotid blowout results from trauma or as a complication of head and neck cancer (HNC) therapy, with resulting extravasation, pseudoaneurysm, or AVF. The clinical spectrum of signs and symptoms related to carotid artery rupture has been described as the carotid blowout syndrome (CBS), which can manifest as either an episode of acute transoral or transcervical hemorrhage or as a potential threat of bleeding in situations in which a portion of the carotid artery is injured or exposed (eg, secondary to trauma or wound dehiscence) (1). CBS historically has been associated with approximately 60% neurologic morbidity and 40% mortality on average (2). Such poor outcomes have been substantially improved with the advent of various endovascular surgical techniques, including permanent balloon occlusion (PBO) of an affected internal carotid artery (ICA) or common carotid artery (3, 4). However, as many as 15% to 20% of patients whose CBS is managed with PBO may develop immediate or delayed cerebral ischemia (5) as a consequence of one or more factors, such as an incomplete circle of Willis, thromboembolism arising from an acutely occluded carotid artery, and delayed collateral failure (6).
An alternative strategy for the management of CBS is to reconstruct the damaged artery through the various mechanical and biologic effects of endovascular stents (7–9). With the increasing use of stent-assisted angioplasty for the treatment of extracranial carotid occlusive disease, it is becoming more widely acknowledged that such endovascular reconstructive techniques can be performed with a relatively low rate of perioperative cerebral ischemic complications (10, 11). These ischemic complications, on average, seem to be lower than those for therapeutic occlusion of the carotid artery using either open or endovascular surgical techniques.
Consequently, our group evaluated stent-assisted endovascular reconstruction of the carotid artery (ERCA) as a treatment for CBS in patients at high risk for stroke from PBO of the carotid artery, with the expectation that such a shift in therapeutic strategy could serve the dual purpose of diminishing hemorrhagic complications and reducing the risk of perioperative stroke. We present our preliminary, evolving, clinical experience with this reconstructive therapeutic strategy.
Methods
We retrospectively reviewed all cases referred to the Neurointerventional Radiology Service for endovascular management of CBS through a neurointerventional database maintained by the senior author (J.C.C.). Those cases managed by ERCA were identified and analyzed in detail through medical record abstraction and a review of the imaging studies. Patients were treated by stent-assisted ERCA if at least one of the following case selection criteria were fulfilled: 1) presence of an incomplete circle of Willis, 2) occluded contralateral carotid artery, 3) anatomic deterrents from safely performing balloon test occlusion (eg, proximal atherosclerotic stenoses), 4) unstable clinical scenario precluding balloon test occlusion, and 5) failure to tolerate balloon test occlusion either on clinical or cerebral blood flow criteria (relative reduction of cerebral blood flow of ≥20% shown by single photon emission tomography). Balloon test occlusion was performed in a standardized fashion as described by Dare et al (6).
As previously defined, the clinical severity of CBS for each patient was classified into three groups (1, 2). Group I, “threatened” carotid blowout, consisted of patients with either a visibly exposed carotid artery segment (likely to eventually rupture if not covered with healthy, vascularized tissue) or evidence on diagnostic angiograms of neoplastic invasion of the carotid artery or nonhemorrhagic pseudoaneurysm. Group II patients presented with a sentinel hemorrhage that typically resolves spontaneously or with surgical packing. Group III, classic carotid blowout, was comprised of patients with profuse, poorly controlled hemorrhage. Any patients who experienced subsequent episodes of recurrent CBS (rCBS) were also identified (1).
Written, informed consent was obtained from all patients. Using a percutaneous, transfemoral arterial route, the common, internal, and external carotid and intracranial vascular anatomy was evaluated with high resolution biplane, digital subtraction angiography. If a likely causative lesion was identified by digital subtraction angiography in groups II and III CBS, it was primarily targeted for stent-assisted ERCA. For group I CBS, either the likely causative lesion or the exposed arterial segment was targeted for ERCA. Stent-assisted ERCA was performed with the patient under conscious sedation by using IV administered fentanyl and midazolam. Blood pressure, EKG, arterial oxygen saturation, and respiratory rate were continuously monitored.
Using biplane, fluoroscopic road map guidance, one or more stents were deployed across the involved portions of the extracranial carotid artery after placement of an appropriate 0.014- or 0.018-in exchange length (330 cm) guidewire. The choice of a particular stent was determined by its availability and by both the diameter and length of the target lesion. The following stents were used: Palmaz (Cordis Corp., Miami, FL), Precise (Cordis Corp.), SMART (Cordis Corp.), AVE (Arterial Vascular Engineering, Inc., Santa Rosa, CA), NIR (Scimed Life Systems, Inc., Maple Grove, MN), Wallstent (Boston Scientific, Minneapolis, MN), and Wallgraft (Boston Scientific). Platinum coils (GDC) (Target Therapeutics, Fremont, CA) or n-butyl cyanoacrylate (Histoacryl; Braun, Melsungen, Germany) were used with stent placement to manage five patients with extracranial carotid pseudoaneurysms. At the conclusion of the surgery, control cerebral and carotid angiograms were obtained for all patients. Prophylactic heparin was not administered to any patient at high risk for re-hemorrhage (groups II and III) during or after ERCA. An antiplatelet regimen consisting of clopidogrel (Plavix; Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, New York, NY) (75 mg administered orally every day) and aspirin (325 mg administered orally every day) was begun immediately in group I patients and in the recipients of the Wallgraft stent. ERCA and rCBS outcomes were reviewed for ≤2 years.
Results
Summaries of the technical and clinical results are provided in the Table, and images from selected illustrative cases are shown in Figures 1 and 2. Sixteen episodes of CBS occurred in 12 patients who fulfilled one or more of the above defined case selection criteria. These included seven patients each with an incomplete circle of Willis revealed by conventional angiography, two patients each with a contralateral carotid occlusion, and three patients for whom balloon test occlusion failed. Ten (83%) patients had underlying HNC, whereas two (17%) had posttraumatic CBS. Acute hemorrhagic arrest was initially achieved in all group II and III patients. However, four patients each experienced one episode of rCBS that necessitated additional endovascular intervention. Fortunately, in all cases of rCBS, re-treatment resulted in no recurrent episodes of bleeding (ie, durable hemostasis) during the follow-up period. A total of 26 stents were used, 22 (85%) of which were uncovered and the remaining four of which were covered. A 96% technical success rate was achieved in placing the stents; the sole technical failure occurred when an autologous vein-covered Palmaz stent failed to deploy in the carotid artery. In six (37.5%) of the 16 procedures, pseudoaneurysm embolization was also performed by using adjuvant acrylic glue or platinum coils.
Endovascular repair of carotid blowout syndrome: summary of clinical data
| Age (yr)/Sex | Clinical History | Event (No.) | Pathologic Lesions | CBS Group | Treatment | Outcome/Follow-up | Complication |
|---|---|---|---|---|---|---|---|
| 34/M | Trauma (Fig 1) | 1 | Petrous ICA Psa | I | GDCs placed in Psa | rCBS/10 mo | |
| 2 | Petrous ICA Psa | I | 4 × 8 mm AVE stent; GDCs placed through stent in Psa | DH/2 yr | |||
| 36/F | Trauma | 1 | ICA Psa | I | 4 × 9 NIR and 6 × 20 mm SMART stents | DH/1.4 yr | |
| 70/M | SCCa, ND, XRT | 1 | Direct tumor involvement of ICA/CCA | I | 8 × 40 Wallstent and 10 × 40 mm SMART stent | DH/3 mo* | |
| 74/M | Thyroid CA, ND, XRT | 1 | Direct tumor involvement of CCA | I | 10 × 30 and 10 × 40 mm SMART stents | DH/2.5 mo; deceased 1.5 yr* | |
| 72/M | SCCa, ND, XRT | 1 | CCA luminal ulcer, mild stenosis, and irregularity | II | Two 8 × 40 mm SMART stents | DH/1.5 mo* | |
| 75/M | SCCa, XRT, ND | 1 | Psa (2) proximal ICA | II | 4 × 18 and 4 × 12 mm AVE stents | rCBS/3 days | |
| 2 | Increase in size of ICA Psa | II | Attempted vein-covered Palmaz stent; 6 × 20 and 7 × 20 mm Wallstents and GDCs | DH/deceased 4 days as result of PE* | Palmaz stent placed in R EIA | ||
| 54/M | SCCa, ND, XRT, rCBS | 1 | ICA Psa | II | Gortex covered Palmaz stent (P104) | DH/3 mo | |
| 56/M | SCCa, TLa, ND, XRT, Pc | 1 | CCA/ICA luminal irregularity and small Psa; ECA superior thyroidal irregularity and Psa | III | GDCs in ECA Psa; Two 8 × 40 mm Wallstents ICA/CCA | DH/1.6 year | |
| 26/M | SCCa, XRT, ND, rCBS | 1 | ICA luminal irregularity and stenosis; 2 Psa of ECA trunk | III | 4 × 16/4 × 9 mm NIR ON Ranger stents in ICA; GDCs in ECA trunk | DH/deceased 1.5 mo* | |
| 66/F | SCCa, XRT, ND | 1 | ICA Psa | III | 8 × 20 and 8 × 60 mm Wallstents | rCBS/2 days | |
| 2 | ICA Psa | III | DPAE under flow arrest | DH/1 yr* | TIA | ||
| 46/M | SCCa, ND, Pc | 1 | ICA Psa | II | Three overlap Precise stents | rCBS/1 day | |
| 2 | ICA Psa | II | 7 × 30 mm Wallgraft | DH/1 mo* | |||
| 41/F | SCCa, TLa (Fig 2) | ECA origin Psa | II | 8 × 30 mm Wallgraft | DH/1 yr |
Note.—CBS indicates carotid blowout syndrome; M, male; F, female; SCCa, squamous cell carcinoma; ND, surgical neck dissection; XRT, radiation therapy; CA, cancer; rCBS, recurrent carotid blowout syndrome; TLa, total laryngectomy; Pc, pharyngocutaneous fistula; ICA, internal carotid artery; Psa, pseudoaneurysm; CCA, common carotid artery; ECA, external carotid artery; DH, durable hemostasis; PE, pulmonary embolus; R EIA, right external iliac artery; TIA, transient ischemia.
Advanced head and neck cancer.
Fig 1.
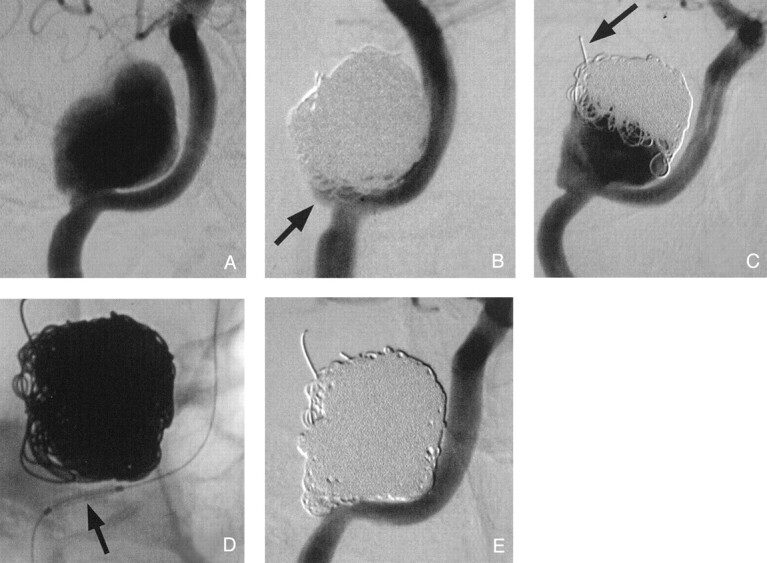
Endovascular treatment of traumatic ICA pseudoaneurysm.
A, Initial angiogram reveals a large pseudoaneurysm at the carotid canal.
B, After endovascular repair with GDCs, a small aneurysmal neck remnant remains (arrow).
C, GDC packing and aneurysmal growth are noted on the 10-month follow-up angiogram; note coil herniation into middle cranial fossa (arrow). The aneurysmal remnant could not be fully packed with GDCs without the use of endovascular stent reconstruction.
D, As a result, a stent was placed at the aneurysmal orifice (arrow) and was deployed.
E, Additional GDCs could then be detached to complete the repair.
Fig 2.
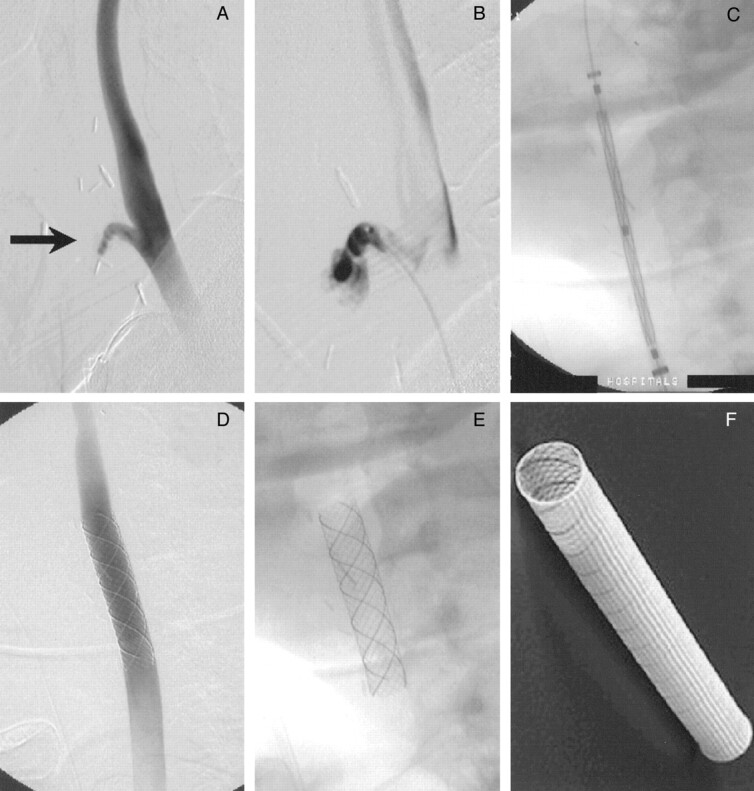
Endovascular treatment of HNC-related CBS by use of the self-expanding, covered Wallgraft stent.
A, Initial angiogram reveals a stump (arrow) at the proximal external carotid artery.
B, Microcatheter injection of the stump during endovascular exploration confirms a pseudoaneurysm as the source of hemorrhage.
C, An 8 × 30 mm Wallgraft stent is positioned within the common carotid artery and ICA junction, bridging the external carotid artery origin.
D, After deploying the stent, angiography shows exclusion of the pseudoaneurysm and normal caliber of the parent, stented artery.
E, Digital subtraction angiogram mask is shown for detail.
F, Photograph of the Wallgraft stent is shown for detail.
One patient developed postprocedural transient ischemia, but no permanent neurologic complications occurred in this series. No deaths were attributable to ERCA, although one death occurred within the 30-day perioperative period. Specifically, one patient with advanced HNC (group III) died as a result of complications of disease progression (pulmonary embolus) on the fourth postoperative day.
Discussion
CBS is an uncommon but feared complication of HNC occurring in 4.3% of patients with HNC (12). Certain risk factors have been identified for the development of CBS in patients with HNC, including thrombosis of the vasa vasorum secondary to wound infection, direct exposure and desiccation, stripping of the carotid sheath, exposure to saliva, adjacent tissue necrosis, and pharyngeal fistula formation (12, 13). Also, previous irradiation adds a 7.6-fold increased risk of developing CBS in patients with HNC (12). The mean age of patients with HNC who present with CBS is 60 years (12), which is similar to the mean age of 58 years for this current cohort of patients with HNC.
The reported mortality rates associated with HNC-related CBS vary widely, ranging from 9% to 100% (12, 14), whereas an average 60% neurologic morbidity rate has been observed in cases of HNC-related CBS after open surgical ligation of the carotid artery (2, 3, 15). In a combined surgical and endovascular series reported by Citardi et al (3), in which 12 patients with CBS were managed by endovascular carotid occlusion, a 0% endovascular mortality rate and an 8% morbidity rate were observed (one patient had Horner’s syndrome after PBO). Although in the current study, one (8%) procedure-related transient ischemia occurred, no deaths or permanent morbidity attributable to ERCA occurred. These low complication rates are comparable with those reported in association with endovascular stent placement of carotid atherosclerotic stenosis (10).
Treatment of traumatic CBS with ERCA has been previously described (7–9, 16, 17). In the current study, two patients presented with CBS secondary to blunt trauma. As expected, these patients were relatively young (ages, 34 and 36 years) and each had a pseudoaneurysm of the ICA. Two stents were used in one patient to repair the pseudoaneurysm. The stents were overlapped with each other, resulting in diminished “porosity” between the stent struts. This, in turn, promoted sluggish flow and subsequent thrombosis within the pseudoaneurysm, which was confirmed by angiography 42 days later. The second patient’s pseudoaneurysm was initially managed with endovascular deposition of platinum coils (Fig 1). Because of a small residual neck, regrowth of the pseudoaneurysm was noted on follow-up angiograms. A single stent was deployed across the ICA rent, which provided a platform for additional and complete coil embolization. This allowed total exclusion of the pseudoaneurysm from the ICA circulation.
Endovascular occlusion of the carotid artery, performed by using detachable balloons for managing HNC-related CBS, has been described in depth since it was first reported in 1984 (18). However, both PBO and the requisite temporary balloon test occlusion add an additional 15% to 20% delayed and 0.4% immediate occurrence of permanent stroke, respectively (5, 6, 19, 20). Although ERCA maintains patency of the common carotid artery and ICA, thus minimizing both of these risks, thromboembolism due to stent placement or any adjuvant embolization technique (eg, coils, glue) will pose a hypothetical 1% to 6% risk of major stroke (11, 21). In fact, the upper limits of this risk may be artificially high, considering that this estimate presumes underlying atherosclerotic, stenotic disease. Our experience with ERCA in this series of 0% major stroke suggests that the risk of permanent neurologic morbidity is probably no more (and is likely less) than the reported risks associated with stent-assisted angioplasty of an atherosclerotic carotid artery.
rCBS after initial endovascular treatment occurred in four (33%) patients. One of the four had a traumatic ICA pseudoaneurysm, with treatment detailed above. The remaining three patients with rCBS had advanced HNC and presented with potentially life-threatening hemorrhage (groups II and III) that was initially managed with overlapping, noncovered stents. The case of one of the three patients has been previously reported (22). In short, this patient who had previously undergone HNC resection and irradiation presented with CBS due to ruptured pseudoaneurysm of the distal common carotid artery. Because of balloon test occlusion failure, overlapping Wallstents were placed across the pseudoaneurysm orifice. Hemorrhage was controlled until 18 hr later, when active bleeding recurred. Because no covered stent was available, the pseudoaneurysm was permanently embolized with the use of n-butyl cyanoacrylate glue injection.
The third patient with advanced HNC and rCBS due to ICA pseudoaneurysm was also initially treated with deployment of overlapping stents (AVE stents). Hemorrhage recurred 72 hr later. Using an endovascular approach, a P204 Palmaz stent (covered with autologous saphenous vein) was positioned within the previously placed stents. Unfortunately, the covered stent would only partially deploy, which prevented placement in the ICA as well as removal through the femoral sheath. Ultimately, deployment of the stent was satisfactorily deposited within the right external iliac artery. Next, two Wallstents were placed and overlapped within the previous overlapping ICA stents; GDC embolization of the pseudoaneurysm was performed to eliminate the sluggish but persistent filling of the pseudoaneurysm. No hemorrhage recurred before the patient died as a result of pulmonary embolism 4 days later.
The fourth patient with rCBS also initially presented with group II CBS due to an HNC-related ICA pseudoaneurysm. Despite placing three overlapping Precise stents across the lesion, hemorrhage recurred later that day. Repeat angiography showed persistent filling of the pseudoaneurysm. A 7-mm-diameter 30-mm-length Wallgraft was placed within the Precise stents without complication, resulting in prompt, permanent arrest of hemorrhage. The carotid artery remained patent at the 1-month follow-up, as revealed by angiography. The patient ultimately later succumbed to progressive HNC without further episodes of CBS.
These four cases of rCBS highlight the major continued shortcoming of endovascular management of CBS in completely preventing recurrent hemorrhages (1). In the current series, all cases of rCBS occurred in the same carotid vasculature that was originally targeted for ERCA, thus representing initial treatment failures as previously defined by Chaloupka et al (1). Of interest, however, is that these events of rCBS occurred only when either uncovered stent placement or adjuvant embolization was performed alone. When combined together, the efficacy of ERCA in treating rCBS seemed to be relatively better in preventing future hemorrhages. Although PBO of the affected carotid vasculature has been considered the gold standard for management of CBS, and may therefore also be considered the same for effectiveness in preventing rCBS, a careful review of the literature indicates that this complication has been frequently encountered with such a technique. In one series, six of seven patients (four with HNC) treated with PBO experienced seven episodes of rCBS, even though “nearly half” of these episodes occurred in the contralateral neck because of progressive HNC (1). Three of these patients underwent two procedures each, which increased the total number of PBO procedures to 10 (eg, ICA PBO complicated by rCBS, then treated with common carotid artery PBO). At best, approximately 35% of these PBO procedures failed to prevent ipsilateral rCBS. At worst, approximately 43% of these patients developed ipsilateral rCBS. Overall, in this previously reported series, rCBS developed in 26% of the patients with CBS who were referred for evaluation and treatment. The occurrence of rCBS and, therefore, the efficacy of treatment are comparable with those in the current series, in which 25% of ERCA procedures were complicated by rCBS and 33% of the patients treated with ERCA experienced rCBS as a related complication.
Advantages are afforded by stent reconstruction of the carotid artery that may ultimately enhance the efficacy of endovascular management of CBS. In particular, the scaffolding and secondary remodeling induced by a stent not only maintains patency but likely reinforces the mechanical integrity of the arterial wall, which in the case of CBS has been damaged by one or more processes, such as radiation, infection, trauma, or tumor encasement (2, 3). This ultimately should result in an artery that is more resistant to future spontaneous rupture. Another important potential benefit of stent-assisted ERCA is the favorable alteration in flow mechanics; disturbed blood flow and eddy formation are reduced, as is the risk of thromboembolic ischemic stroke (8).
Based on our experience in the current series and based on extensive cumulative experience with endovascular surgical revascularization of carotid occlusive disease in general (10), we think that a self-expanding stent is highly preferable to a balloon-mounted stent for the treatment of CBS because of a variety of technical and biomechanical factors. Self-expanding stents (eg, Wallgraft, Wallstent, Precise, SMART) easily accommodate varying diameters of the carotid tree, especially at the transition from the common carotid artery to the ICA. Such stents are more forgiving when determining the necessary diameter and have decreased porosity compared with balloon-mounted stents, which improves vessel support and promotes pseudoaneurysm thrombosis (Fig 2). Superior flexibility in conforming to tortuous segments of the carotid segment can be achieved with these stents.
Furthermore, a self-expanding stent is preferable to a balloon-mounted stent because of the potential for arterial injury resulting from the requisite high inflation pressures needed for stent deployment. This is especially relevant in the setting of CBS, because the target arterial segment has been weakened from a variety of processes that predispose to CBS (2, 3). These concerns have recently been confirmed by Kwok et al (23), who presented an example of HNC-related CBS in which a covered stent (Jostent) offered no protection to high pressure balloon stent expansion. Extravasation at both ends of the stent with persistent transoral hemorrhage required endovascular occlusion of the common carotid artery. Five other cases of CBS treated with covered stents have been reported (7, 24–27).
With the exception of one vein-covered balloon-expandable stent, all stents were placed in a technically successful manner. Marotta et al (28) reported the successful placement of a vein-covered Palmaz stent in treating traumatic CBS of the ICA. However, as a result of limited experience with autologous vein-covered stents in preventing both stent-related thromboembolism and rCBS, the potential benefits may not be known until the preparation and deployment of these unique stents can be easily duplicated with consistency and a lack of procedural complications.
We recently achieved excellent results with use of the currently available Wallgraft stent in two of our most recent patients (Table). In both cases, rapid deployment of this polytetrafluoroethylene-covered stent produced immediate exclusion of the targeted pseudoaneurysm while simultaneously providing reconstructive preservation of the carotid artery (Fig 2). However, it must be emphasized that the safe and effective use of the Wallgraft stent for CBS requires a variety of favorable anatomic and pathophysiological factors that were present in both of our patients. These factors include common femoral and iliac arterial anatomy that will permit placement of a large caliber (9 or 10 French) vascular sheath, simple curvature of the aortic arch, relatively straight carotid and brachiocephalic arteries, and lack of coexistent atherosclerotic or postradiation stenoses. Therefore, more widespread use of self-expanding, covered stents for the management of CBS will not be possible until significant technologic improvements are realized, such as the creation of lower profile delivery systems, increased overall flexibility, and enhanced deliverability. With the advent of these improvements, it is almost certain that constructive endovascular approaches, without adjuvant embolization, will become the treatment of choice for repairing both the common carotid artery and the ICA affected by CBS. We predict that the use of such devices will not only substantially diminish the immediate perioperative complications of conventional PBO but also decrease the rate of rCBS attributable to treatment failure that has remained a problem with uncovered stents.
Conclusion
Our preliminary experience with stent-assisted ERCA for the treatment of CBS in a high risk group of patients has been very favorable. We have established both the feasibility and safety of this alternative management approach. Considering the high risk nature of the patient population, it seems that such a treatment paradigm may be associated with a lower risk of perioperative stroke compared with conventional PBO of the affected carotid vascular tree. Although mostly uncovered stents were used in this series, rCBS, because of treatment failure, seemed to be no more frequent a problem when compared with previously published series using PBO.
Footnotes
This work was presented in part at the Joint Section Meeting, AANS/CNS/ASITN, Waikoloa, HI, 2001; the 39th Annual Meeting, ASNR, Boston, MA, 2001; and at the 40th Annual Meeting, ASNR, Vancouver, BC, 2002.
References
- 1.Chaloupka JC, Roth TC, Putman CM, et al. Recurrent carotid blowout syndrome: diagnostic and therapeutic challenges in a newly recognized subgroup of patients.AJNR Am J Neuroradiol 1999;20:1069–1077 [PMC free article] [PubMed] [Google Scholar]
- 2.Chaloupka JC, Putman CM, Citardi MJ, Ross DA, Sasaki CT. Endovascular therapy of the carotid blowout syndrome in head and neck surgical patients: diagnostic and managerial considerations.AJNR Am J Neuroradiol 1996;17:843–852 [PMC free article] [PubMed] [Google Scholar]
- 3.Citardi MJ, Chaloupka JC, Son YH, Ariyan S, Sasaki CT. Management of carotid artery rupture by monitored endovascular therapeutic occlusion (1988–1994).Laryngoscope 1995;105:1086–1092 [DOI] [PubMed] [Google Scholar]
- 4.Morrissey DD, Andersen PE, Nesbit GM, Barnwell SL, Everts EC, Cohen JI. Endovascular management of hemorrhage in patients with head and neck cancer.Arch Otolaryngol Head Neck Surg 1997;123:15–19 [DOI] [PubMed] [Google Scholar]
- 5.Yonas H. Defining candidates for cerebral revascularization. Presented at the Scientific Symposium I of the 5th Annual Joint Section Meeting of the AANS/ASITN, Dallas, February 3–6, 2002
- 6.Dare AO, Chaloupka JC, Putman CM, Fayad PB, Awad IA. Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayed hemodynamic ischemia after therapeutic carotid occlusion.Surg Neurol 1998;50:147–156 [DOI] [PubMed] [Google Scholar]
- 7.Simionato F, Righi C, Melissano G, Rolli A, Chiesa R, Scotti G. Stent-graft treatment of a common carotid artery pseudoaneurysm.J Endovasc Ther 2000;7:136–140 [DOI] [PubMed] [Google Scholar]
- 8.Coldwell DM, Novak Z, Ryu RK, et al. Treatment of posttraumatic internal carotid arterial pseudoaneurysms with endovascular stents.J Trauma 2000;48:470–472 [DOI] [PubMed] [Google Scholar]
- 9.Matsuura JH, Rosenthal D, Jerius H, Clark MD, Owens DS. Traumatic carotid artery dissection and pseudoaneurysm treated with endovascular coils and stent.J Endovasc Surg 1997;4:339–343 [DOI] [PubMed] [Google Scholar]
- 10.Chaloupka JC, Weigele JB, Mangla S, Lesley WS. Cerebrovascular angioplasty and stenting for the prevention of stroke.Curr Neurol Neurosci Rep 2001;1:39–53 [DOI] [PubMed] [Google Scholar]
- 11.Wholey MH, Wholey M, Mathias K, et al. Global experience in cervical carotid artery stent placement.Catheter Cardiovasc Interv 2000;50:160–167 [DOI] [PubMed] [Google Scholar]
- 12.Maran AG, Amin M, Wilson JA. Radical neck dissection: a 19-year experience.J Laryngol Otol 1989;103:760–764 [DOI] [PubMed] [Google Scholar]
- 13.Sanders EM, Davis KR, Whelan CS, Deckers PJ. Threatened carotid artery rupture: a complication of radical neck surgery.J Surg Oncol 1986;33:190–193 [DOI] [PubMed] [Google Scholar]
- 14.Porto DP, Adams GL, Foster C. Emergency management of carotid artery rupture.Am J Otolaryngol 1986;7:213–217 [DOI] [PubMed] [Google Scholar]
- 15.Coleman JJ III. Treatment of the ruptured or exposed carotid artery: a rational approach.South Med J 1985;78:262–267 [DOI] [PubMed] [Google Scholar]
- 16.Ditmars ML, Klein SR, Bongard FS. Diagnosis and management of zone III carotid injuries.Injury 1997;28:515–520 [DOI] [PubMed] [Google Scholar]
- 17.Higashida RT, Halbach VV, Tsai FY, et al. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases.AJR Am J Roentgenol 1989;153:577–582 [DOI] [PubMed] [Google Scholar]
- 18.Osguthorpe JD, Hungerford GD. Transarterial carotid occlusion: case report and review of the literature.Arch Otolaryngol 1984;110:694–696 [DOI] [PubMed] [Google Scholar]
- 19.Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases.AJNR Am J Neuroradiol 1995;16:749–754 [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman MC, Mickel RA, Kessler DJ, Mehringer CM, Hieshima GB, Calcaterra TC. Treatment of impending carotid rupture with detachable balloon embolization.Arch Otolaryngol Head Neck Surg 1987;113:1169–1175 [DOI] [PubMed] [Google Scholar]
- 21.Phatouros CC, Higashida RT, Malek AM, et al. Carotid artery stent placement for atherosclerotic disease: rationale, technique, and current status.Radiology 2000;217:26–41 [DOI] [PubMed] [Google Scholar]
- 22.Roth TC, Chaloupka JC, Putman CM, et al. Percutaneous direct-puncture acrylic embolization of a pseudoaneurysm after failed carotid stenting for the treatment of acute carotid blowout.AJNR Am J Neuroradiol 1998;19:912–916 [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok PC, Cheung JY, Tang KW, Wong WK. Re: Endovascular treatment of acute carotid blow-out syndrome.J Vasc Interv Radiol 2001;12:895–896 [DOI] [PubMed] [Google Scholar]
- 24.Macdonald S, Gan J, McKay AJ, Edwards RD. Endovascular treatment of acute carotid blow-out syndrome.J Vasc Interv Radiol 2000;11:1184–1188 [DOI] [PubMed] [Google Scholar]
- 25.Scavee V, De Wispelaere JF, Mormont E, Coulier B, Trigaux JP, Schoevaerdts JC. Pseudoaneurysm of the internal carotid artery: treatment with a covered stent.Cardiovasc Intervent Radiol 2001;24:283–285 [DOI] [PubMed] [Google Scholar]
- 26.Alexander MJ, Smith TP, Tucci DL. Treatment of an iatrogenic petrous carotid artery pseudoaneurysm with a Symbiot covered stent: technical case report.Neurosurgery 2002;50:658–66211841739 [Google Scholar]
- 27.Ruebben A, Merlo M, Verri A, et al. Exclusion of an internal carotid aneurysm by a covered stent.J Cardiovasc Surg (Torino) 1997;38:301–303 [PubMed] [Google Scholar]
- 28.Marotta TR, Buller C, Taylor D, Morris C, Zwimpfer T. Autologous vein-covered stent repair of a cervical internal carotid artery pseudoaneurysm: technical case report.Neurosurgery 1998;42:408–413 [DOI] [PubMed] [Google Scholar]


