Abstract
Background:
Bioaerosol plays an important role in human life with potentially infectious, allergic and toxic effects. Active and passive methods can be used to assess microbial air contamination, but so far there is not a unanimous consensus regarding the indications about methods to be used and how to interpret the results. The passive method has been standardized by the Index of Microbial Air contamination (IMA). Classes of contamination and maximum acceptable levels of IMA have been proposed, related to different infection or contamination risks. The aim of this study was to provide information about the use of the passive sampling method, with reference to the IMA standard.
Methods:
We searched PubMed and Scopus for articles published until January 2020 reporting the citation of the article by Pasquarella et al. “The index of microbial air contamination. J Hosp Infect 2000”. Only studies in English language where the IMA standard was applied were considered. Studies regarding healthcare settings were excluded.
Results:
27 studies were analyzed; 12 were performed in Europe, 8 in Asia, 5 in Africa, 2 in America. Cultural heritage sites, educational buildings and food industries were the most common indoor monitored environments; in 8 studies outdoor air was monitored.
Conclusions:
This review has provided a picture of the application of standard IMA in different geographic areas and different environments at risk of airborne infection/contamination. The analysis of the results obtained, together with a wider collection of data, will provide a useful contribution towards the definition of reference limits for the various types of environments to implement targeted preventive measures.
Keywords: air sampling, bioaerosol, IMA, indoor, outdoor, passive method.
Introduction
Bioaerosol plays an important role in human life with potentially infectious, allergic and toxic effects (1-5). Measuring microbial air quality is a fundamental step for risk management (6-8): it allows to confirm the presence of biological agents, identify critical situations and validate the preventive measures adopted; air sampling is also a useful tool for scientific research, quality assurance and educational purposes. So far, there is not a unanimous consensus regarding the indications for air sampling, what method should be used, and how to interpret the results in order to implement targeted preventive and control measures. Methods used for microbial air sampling can be classified in two categories: passive and active (6, 9). The active method allows the measurement of the concentration of culturable microorganisms in the air and is based on the use of some devices which collect a known volume of air, blown on to a nutrient media; the results are expressed as colony forming unit per cubic metre (CFU/m3). Several types of devices are available, such as air impactors, impingers, centrifugal machines or filtration systems, which differ for biological and physical efficiency therefore providing different results, difficult to compare. The passive method measures the rate at which microorganisms settle on surfaces; it is based on sedimentation and relies on the use of settle plates being exposed to air for a defined period of time; results are expressed as CFU/plate/time. The passive method has been standardized by the Index of Microbial Air contamination (IMA) which corresponds to the number of CFU counted on a Petri dish (9 cm in diameter) left open to the air according to the 1/1/1 scheme (for 1 hour, 1 meter above the floor and about 1 meter away from walls and major obstacles) (10). The IMA can be expressed also as CFU/m2 or dm2 or cm2/time. Five classes of IMA have been defined, representing a different increasing level of contamination: 0-5 very good; 6-25 good; 26-50 fair; 51-75 poor; >76 very poor. Maximum acceptable values of IMA have been proposed, related to different infection or contamination risks; these are 5, 25 and 50, in places at very high, high and medium risk, respectively (10). It is up to whoever is in charge to state the level of infection risk and adopt the corresponding maximum acceptable IMA level.
The aim of this study was to provide information about the use and diffusion of the passive sampling method for assessing the microbial air quality, with reference to the IMA standard (10). This paper deals with the results regarding non-healthcare settings.
Methods
We searched PubMed and Scopus for articles published until January 2020 reporting the citation of the article by Pasquarella et al. “The index of microbial air contamination”. J Hosp Infect 2000. Only studies in English language where the IMA standard was applied were considered. Studies performed in healthcare settings were excluded and will be object of a specific paper. Only studies using nutrient media for total bacteria and/or fungi count were included. When the exposure of settle plates was longer or shorter than one hour, values measured in the sampling time considered were proportioned to one hour. The studies were analysed with reference to the Countries, settings, monitored environments and results obtained.
Results
Figure 1 shows the flow diagram of the review process. The reference “The index of microbial air contamination” was reported in n. 187 articles, 151 from Scopus and 36 from PubMed. After the screening by title, 29 duplicates were identified and removed. After the exclusion of the reviews (n. 29) and the studies performed in healthcare settings (n. 66), n. 63 articles studies performed in non-healthcare setting were considered for the review. Articles in which the citation of “The Index of microbial air contamination” was not referred to the air sampling method used, articles written in other than English language (11-16) and articles dealing with studies where specific microorganisms were searched (17), were excluded. A total of 27 studies were included in the review; in 25 studies quantitative or quantitative and qualitative air microbial contamination was evaluated (18-42); in 2 studies qualitative contamination only was evaluated (43,44).
Figure 1.
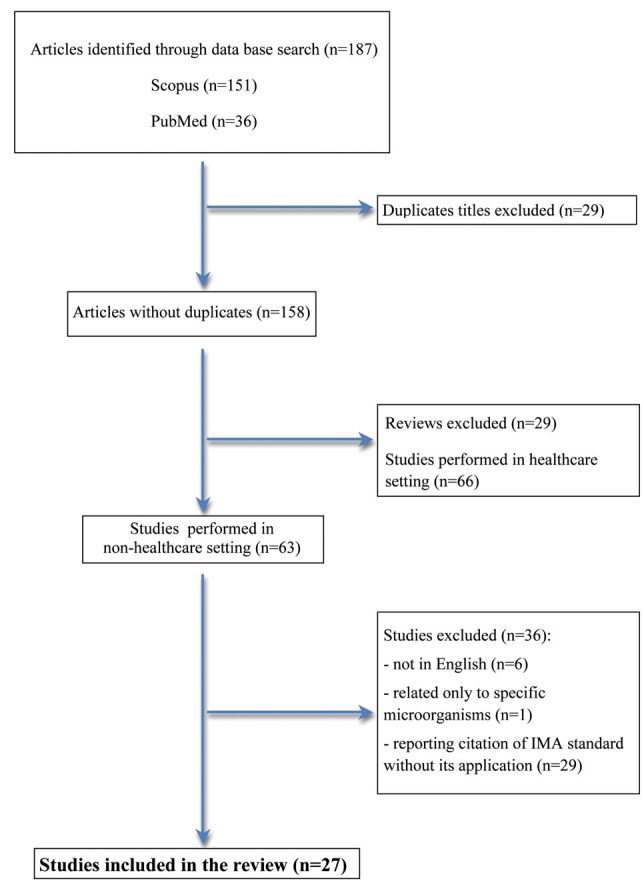
Flow diagram of study selection for review.
Table 1 and Table 2 list the 25 studies yielding quantitative data, with reference, in particular, to the study setting, sampling period, sampling time and environments monitored, reporting the IMA values obtained for bacteria, fungi or total count. Ten studies were performed in Europe, including eight in Italy (18-22,24,26,31), one in Romania (39) and one in Norway (34); eight studies were performed in Asia, two in Malaysia (26,35) and one for each of the following countries: Iran (32), Israel (36), Japan (30), Thailand (33), Turkey (28), Vietnam (37); five studies were conducted in Africa, two in Ethiopia (38,41) and one for each of the following countries, Egypt (25), Nigeria (23), South Africa (29); two studies were conducted in America, one in the USA (40) and one in Cuba (42). Twenty-one studies (18-33, 38-42) evaluated indoor air contamination, mainly in cultural heritage sites, educational buildings and food processing plants; five of these studies assessed also the outdoor microbial contamination (25,31-34); three studies evaluated only outdoor air quality (35-37). In five studies (38-42), listed in Table 2, the air was sampled using the IMA standard scheme, but the results obtained (CFU/plate/time) were transformed in CFU/m3 by using the Omelyansky’s formula: N = 5a x 104 (bt)-1 where N = CFU/m3, a = number of colonies per Petri dish, b = dish square centimeter, t = exposure time (min.) (45), based on the estimation that on the area of 100 cm2, in 5 minutes are deposited as many microbes as there are in 10 m3 of air. Table 2 reports both the original values in CFU/m3 and the IMA values obtained after the conversion based on the Omelyansky’s formula.
Table 1.
Characteristics of studies using the Index of Microbial Air contamination (IMA) standard
| Country Publication year (Ref) | Study setting | Sampling period | N. of monitored environments | Sampling time/ condition | Bacteria (IMA) | Fungi (IMA) | Microbial Total Count (IMA) | ||
| Mean (SD)/mean range/median | Range | Mean (SD)/mean range/median | Range | Mean/mean range/median | |||||
| Indoor environments | |||||||||
| Cultural heritage | |||||||||
| Italy 2011 (18) | Museum | January | 7 rooms | before opening time | 2.86* | 1* - 7* | 0.45* | 0* - 2* | |
| during opening time | 11.51* | 3* - 21* | 0.89* | 0* - 3* | |||||
| Italy 2012 (19) | Library | April | 8 rooms | 0* - 31* | 0* - 3* | ||||
| Italy 2014 (20) | Museum | May | 3 rooms | during opening time | 1 - 48○ | ||||
| October | 0 - 10○ | ||||||||
| Italy 2015 (21) | Library | July | 1 room | 8 | 3 - 35 | 1 | 0 - 3 | ||
| December | 8 | 4 - 15 | 2 | 0 - 3 | |||||
| Italy 2015 (22) | Library | Spring | 1 room | 0 - 28* | |||||
| Nigeria 2018 (23) | Library | Rainy season | 7 (2) - 30 (3) | 22 (3) - 73 (4) | |||||
| Dry season | 5 (3) - 22 (4) | 13 (2) - 27 (5) | |||||||
| Italy 2019 (24) | Library | February | 1 room | 3.33 | 0 - 7 | 0.5 | 0 - 2 | ||
| May | 3.33 | 1 - 6 | 3.67 | 0 - 9 | |||||
| September | 3.33 | 0 - 5 | 4.33 | 1 - 9 | |||||
| December | 1.33 | 0 - 3 | 0.67 | 0 - 2 | |||||
| Educational Buildings | |||||||||
| Italy 2009 (26) | University | April - June | 14 rooms Buildings A, B, C Research laboratories | in the morning (m.) in the afternoon (a.) | A m. 1.1 - 41.8 a. 0.1 - 6 | ||||
| B m. 0.5 - 3.8 a. 0.5 - 4.3 | |||||||||
| C m. 1.1 - 20.3 a. 0.7- 29 | |||||||||
| October - December | A m. 0 - 11 a. 0 - 45 | ||||||||
| B m. 2.1 - 5.7 a. 0.5 - 4.2 | |||||||||
| C m. 1.3 - 17 a.0.5 - 10.3 | |||||||||
| Malaysia 2017 (27) | University | 5 environments | in the morning (m.) in the afternoon (a.) | ||||||
| University Service Center | m. 12.08* a. 5.09* |
||||||||
| Top Management Office | m. 5.72* a. 3.81* |
||||||||
| Tissue Culture Laboratory | m. 6.99* a. 17.8* |
||||||||
| Cafè | m. 22.25* a. 15.26* |
||||||||
| Library | m. 27.98* a. 27.98* |
||||||||
| Other environments | |||||||||
| Turkey 2011 (28) | Morgue Department | Spring - Summer | 1 autopsy room | before autopsy | Spring 9.1 (5.7) | Spring 2.7 (1.7) |
|||
| Summer 27.4 (22.1) |
Summer 16.7 (26.3) |
||||||||
| during autopsy | Spring 51.1 (17.1) |
Spring 117.8 (271.6) |
|||||||
| Summer 60.9 (65.7) |
Summer 99.3 (175.6) |
||||||||
| after autopsy | Spring 21.6 (49.3) |
Spring 13 (28.4) |
|||||||
| Summer 19.7 (21.6) |
Summer 9.2 (10.5) |
||||||||
| South Africa 2017 (29) | Fruit handling environments | Over three years period |
11 | 27 - 900• 105• |
|||||
| Japan 2019 (30) | Animal housing system | November - January | 1 | 0.3• | 9.3• | ||||
| Indoor and outdoor environments | |||||||||
| Norway 2009 (34) | Dry-cured meat production facility | February, August, December | 16 rooms indoor outdoor | operational | 15 | ||||
| Iran 2014 (32) | School dormitory | One year period | 1 | indoor 10 - 112 |
indoor 11 - 36 |
||||
| and retirement home | 1 | outdoor 15 - 96 | outdoor 8 - 40 | ||||||
| Thailand 2016 (33) | Fitness centre A (indoor) | 2.09 (1.50) | 0.97 (1.69) | ||||||
| Fitness centre B (indoor) | 3 | 8.44 (5.74) | 5.07 (2.34) | ||||||
| Fitness centre C (outdoor) | 7.52 (3.73) | 5.59 (3.57) | |||||||
| Italy 2017 (31) | Buffalo farms | at rest | 10 - 76 | ||||||
| 3 Feeding rooms (outdoor) | operational | 39 - 76 | |||||||
| at rest | 6 - 76 | ||||||||
| 3 Milking rooms (indoor) | operational | 12 - 24 | |||||||
| Egypt 2020 (25) | Museum | Two years period | 6 Buildings | working hours | 1 - 256** | ||||
| Outdoor environments | |||||||||
| Malaysia 2015 (35) | Residential areas: Case study: | 2 | |||||||
| built on dumping site | 48• | 36• | |||||||
| Control: at 20 km from | 27• | 36• | |||||||
| case study | |||||||||
| Israel 2016 (36) | Areas closeness to domestic GW-treatment systems (RVFCWs) | June - February | 3 | early in the morning | |||||
| 0.3 m away from GW-t systems | 0.22 - 616.7** | ||||||||
| 1 m away from GW-t systems | 0 - 15.2** | ||||||||
| Vietnam 2019 (37) | Ho Chi Minh City | Three years period | 4 | four times a day | |||||
| road area | 80.3** | 4.37** | |||||||
| zoo area | 33.3** | 52.48** | |||||||
| residential area | 42.26** | 3.02** | |||||||
| rural area | 99.27** | 14.17** | |||||||
| Legenda: *calculated from CFU/dm2/h; **calculated from CFU/m2/h; • IMA calculated for 1 h; ○only fungal count on Saburand Dextrose Agar medium was considered | |||||||||
Table 2.
Characteristics of studies using the Index of Microbial Air contamination (IMA) standard with values expressed as CFU/m3 calculated by Omeliansky’s formula
| Country Publication Year (Ref) | Study setting | Sampling period | N. of monitored environments | Sampling time/condition | Bacteria | Fungi | Microbial Total Count | |||||
| Range | Mean | Range | Mean | |||||||||
| Educational Buildings | ||||||||||||
| CFU/m3 | IMA* | CFU/m3 | IMA* | CFU/m3 | IMA* | CFU/m3 | IMA* | |||||
| Ethiopia 2015 (38) | at 6 a.m. | 747 - 9960 | 57 - 760 | 531 - 6568 | 41 - 501 | |||||||
| University | April, May | 30 dormitory rooms | at 7 p.m. | 511 - 4010 | 39 - 306 | 730 - 6403 | 56 - 489 | |||||
| Romania 2016 (39) | University (U) | March, April, May | 5 rooms | between 12 a.m. and 5 p.m. | ||||||||
| High school (Hs) | ||||||||||||
| Primary school (Ps) | ||||||||||||
| U - A3 | 497.3 | 37.94• | ||||||||||
| Romania 2016 (39) | U - A5 | 414 | 31.59• | |||||||||
| U - microbiol lab | 175.3 | 13.38• | ||||||||||
| Hs - classroom | 829 | 63.25• | ||||||||||
| Ps - classroom | 149.7 | 11.42• | ||||||||||
| March 122 -862 | March 9.31 - 65.67• | |||||||||||
| All monitored environments | April 145 - 830 | April 11.06 - 63.33• | ||||||||||
| May 176 - 795 | May 13.43 - 60.66• | |||||||||||
| USA 2019 (40) | High school | October - February | 8 rooms | 135 | 10 | |||||||
| Primary school | 293 | 22 | ||||||||||
| Ethiopia 2019 (41) | Primary school | March - April | 51 classrooms | at 6:30 a.m. and at 5:00 p.m. | 613.29 | 47 | 136.5 - 2164.5 | 10 - 164 | ||||
| Food industry | ||||||||||||
| Cuba 2019 (42) | 2 Food production plants: | March - November | between 1:00 p.m. and 2:00 p.m. | |||||||||
| Artisanal chocolate plant | 5 rooms | 0 - 1507 | 0 - 115 | |||||||||
| Product for special regime plant | 2 rooms | 39 - 1638 | 3 - 125 | |||||||||
| *IMA values calculated from CFU/m3, •IMA calculated for 1 h multiplying for 3 (20 min) or 4 (15 min) | ||||||||||||
As for cultural heritage sites, six studies were performed in Italy (18-22,24) and two in Africa (Nigeria and Egypt) (23,25). Considering the Italian studies, bacterial air contamination values ranged from 0 to 35 IMA without visitors (21); fungal contamination increased during opening time up to 48 IMA (20). Higher values were found in a museum library in Nigeria, where the heaviest microbial contamination means both for fungi (73 IMA) and bacteria (30 IMA) were found during the rainy season compared with the dry season (23). Fungal contamination values found in an Egyptian museum, where six rooms were monitored, ranged from 1 to 256 IMA, with median values from 8 to 30 IMA (25); in this study also outdoor environment was monitored, and indoor /outdoor ratio confirmed that outdoor environment was the main source of indoor fungal pollution. Microbial air contamination in educational buildings was evaluated in 6 studies. Di Giulio et al. (26) performed a study in 14 University research laboratories located in three different buildings over a period of six months, in the morning and in the afternoon; the IMA values showed a seasonal fluctuation of total microbial contamination, which were always within the threshold values of 50 and 25 IMA defined respectively for the common laboratory rooms and for the bacteriology laboratory with a controlled microbial charge. An IMA value below 25 was also found in the University Tissue culture Laboratory in Malaysia by Chong et al (27); in this study the lowest bacterial mean contamination values were found in the Top Management Office, from 5.72 IMA in the morning to 3.81 IMA in the afternoon, while the highest mean IMA value (27.98) was found in the library. Other four studies, carried out in educational buildings, 2 in Ethiopia (38,41), 1 in Romania (39) and 1 in the USA (40), converted the IMA value to CFU/m3 by using the Omelyansky’s formula. Going back to CFU/plate/time values (IMA) we found in a University microbiology laboratory in Romania (39) a mean charge converted value of 13.38; about classrooms, mean fungal contamination values ranged from 11.42 to 63.25. In a University dormitory in Ethiopia (38) very high values up to 760 IMA for bacteria and 501 IMA for fungi were reached. In Cuba, Anaya et al. (42) monitored the fungal contamination for a period of nine months in two food production plants, one for artisanal chocolate and one for products for special regime plant; IMA values, calculated from the CFU/m3 obtained by Omelyansky’s formula, ranged from 0 to 125 IMA. In the study by Scholtz (29) fungal contamination was assessed along the pear export chain from South Africa to the UK over a three year period, obtaining a median range from 52 to 1725 IMA, with a median IMA value of 201. The assessment of indoor airborne fungal contamination was also performed in a dry-cured meat production facility, and outdoor in a study by Asefa et al. in Norway (34); overall, in the production rooms, the mean value of 15 IMA was observed with the heaviest contamination in the brining, smoking, and sorting processes rooms, showing the last one the highest IMA value (about 90 IMA, graphic data); the outdoor fungal contamination was about 25 IMA (graphic data). In Italy, Vella et al. (31) carried out a study in three buffalo farms, including indoor and outdoor air microbial evaluation, at rest and in operational conditions: mean IMA values for fungal contamination ranged from 6 to >76 IMA in indoor milking rooms and from 10 to >76 IMA in the outdoor areas (feeding rooms). In the study by Sonmez et al. the presence of bacteria and fungi was determined in an autopsy room, in summer and spring seasons, before, during and after autopsy. The microbial air contamination was significantly higher at the time of the autopsy than that found in pre and post- autopsy sessions, reaching the highest values of 117.8 IMA for fungi in spring and 60.9 IMA for bacteria in summer; maximum acceptable IMA values were considered 75 for bacteria and 19 for fungi. In Japan, Tasaki et al. (30) monitored, for a period of thirteen months, a cargo van rabbit housing system obtaining a mean IMA value of 0.30 and 9.30, for bacteria and fungi, respectively. Other two studies, one in Iran (32) and one in Thailand (33), monitored the indoor and outdoor microbial contamination. In the first one a school dormitory and a retirement home were monitored, and bacterial IMA values for the two environments ranged from 10 to 112, while fungal contamination from 11 to 36; outdoor bacterial and fungal IMA values ranged from 15 to 96 and from 8 to 40, respectively. The second one dealt with three fitness centers, two indoor and one outdoor, locating settle plates at 1.5 m from the floor considering this height representing the human breathing zone; indoor mean IMA values ranged from 2.09 to 8.44 for bacteria and from 0.97 to 5.07 for fungi, while in the outdoor center bacterial and fungal mean IMA values were 7.52 and 5.59 respectively. Studies dealing with only outdoor microbial air sampling were carried out in Malaysia (35) and in Israel (36), both regarding waste treatments areas, and in Vietnam (37) where air was sampled in Ho Chi Minh city. In the study by Ithnin et al. (35), air sampling was performed around a former area dumping site, the case location, and 20 kilometers away, the control location; the mean bacterial air contamination values were 48 and 27 IMA, respectively, while mean fungal contamination was the same at both sites (36 IMA). Benami et al. sampled bioaerosols emitted from domestic grey water (GW) treatment systems; low amount of bacteria, with mean values ranging from 0 to 15.2 IMA were found to aerosolized up to 1 m away from the GW treatment system, while at the 0.3 m distance the mean values reached value of 616.7 IMA. In Ho Chi Minh city, airborne bacteria and fungi in the atmosphere were assessed from 2014 to 2016, covering two wet and dry seasons, at four sites of the city (zoo, road, rural and urban areas). The highest bacterial contamination was found at rural area while the lowest at zoo (33.3 IMA), where the heaviest fungal contamination was found (52.48 IMA).
Table 3 shows bacteria and fungi isolated in the different monitored environments by using settle plates according to IMA standard; in two studies (43,44) only qualitative evaluation was performed. Studies in which both active and passive air sampling were performed, but microorganisms isolated were reported without distinguishing which method allowed their isolation were not considered (21,24,25,32). Among bacteria, Bacillus spp., Staphylococcus spp., Micrococcus spp., Pseudomonas spp. and Enterococcus spp., were the most frequently isolated genera, while Penicillium spp., Aspergillus spp., Cladosporium spp. and Fusarium spp. were the predominant fungi.
Table 3.
Fungi and bacteria isolated in the different environments
| Fungi | Bacteria | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Environments Country Publication year (Ref) | Acremonium spp. | Alternaria spp. | Ascochyta spp. | Aspergillus spp. | Aureobasidium pullulans | Beauveria spp. | Bipolaris spp. | Botryotinia spp. | Botrytis cinerea | Candida spp. | Chaetomium spp. | Chrysonilia sitophila | Chrysosporium spp. | Cladosporium spp. | Cochliobolus sp. | Curvularia spp. | Eurotium spp. | Fusarium spp. | Geotrichum spp. | Maya benzeri mantar | Microsporum spp. | Monilia sitophila | Mucor spp. | Neurospora spp. | Paecilomyces spp. | Penicillium spp. | Phaeospheria spp. | Pithomyces spp. | Pseudopestalotiopsis spp. | Rhizopus spp. | Rhodotorula spp. | Scedosporium apiospermum | Scopulariopsis spp. | Sporotrichum spp. | Stemphylium spp. | Syncephalastrum spp. | Thielaviopsis spp. | Trichoderma spp. | Trichophyton spp. | Trichotecium spp. | Ustilago spp. | Verticillium spp. | Wallemia spp. | Yeasts | Sterile mycelia | Acinetobacter spp. | Actynomyces spp. | Aerococcus spp. | Aeromonas spp. | Alcaligenes faecalis | Bacillus spp. | Corynebacterium spp. | Cochliobolus indoltheticum | Curtobacterium spp. | Escherichia coli | Enterobacteriaceae | Enterococcus spp. | Klebsiella spp. | Microbacterium spp. | Micrococcus spp. | Mycobacterium smegmatis | Myroides spp. | Neisseria meningitidis | Pseudomonas spp. | Rathayibacterium caricis | Staphylococcus spp. | Streptococcus spp. | Vagococcus spp. | Heterotrophic | |
| Cultural heritage | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Italy 2015 (21) | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Italy 2015 (22) | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nigeria 2018 (23) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Educational buildings | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Italy 2010 (26)* | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poland 2013 (43)** | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Italy 2014 (20)* | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Romania 2016 (39) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| USA 2019 (40) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ethiopia 2019 (41) | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Food industry | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Norway 2009 (34) | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| South Africa 2017 (29) | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Portugal 2017 (44) | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cuba 2019 (42) | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Autopsy room | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Turkey 2011 (28) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||||||||||||||||||||
| Outdoor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Israel 2016 (36) | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vietnam 2019 (37) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||||||||||||||||||||||||||||
*Genera most frequently found; **Other isolated microorganisms: Acanthurus blochii, Artrographis Kalrae, Arxula adeninivarans, Bipolaris spicifera,
Bjerkandera adusta, Blastomyces dermatididis, Cladophiarophora boppi, Corynespora cassiicola, Cystfilobasidium informominiatum,
Debaryomyces hansenii, Debaryomyces polimorphus, Debariomyces occidentalis, Debariomyces vanrijiae, Emericella quadrilineata, Emmonsia crescens,
Epidermophyton floccosum, Gymnoascus dancaliensis, Hormographiella aspergillata, Hormographiella verticillata, Kluyveromyces lactis,
Kluyveromyces marxianus, Kluyveromyces thermotolerans, Kluyveromyces varrowii, Kluyveromyces wickerhamii, Lipomyces starkeyi, Madurella grisea,
Mrakia frigida, Nadsonnia commutata, Oosporidium margaritiferum, Phialophora bubakii, Phoma cruris-hominis, Pichia anomala, Pichia farinosa,
Pichia membranifaciens, Rhizomucor pusillus, Rhodosporidium dacryoideum, Saccharomyces cerevisiae, Saccharomycopsis capsularis,
Saccharomyces fructuum, Scytalidum lignicola, Yarrovia lipolytica
Conclusions
This review has provided a picture of the application of IMA standard in different geographic areas and in different environments at risk of airborne infection/contamination. The use of settle plates, whose sampling efficiency is not influenced by engineering factors, standardized with the IMA, yields comparable results wherever and whenever they were obtained, providing the basis for the definition of threshold limits towards an effective risk prevention. In some studies (26,27,28,29,31,33), the IMA threshold values initially proposed for the different environments (10) were considered, and proved to be useful for the interpretation of results. A wide range of microbial contamination has been observed, in the same settings of several studies; a larger collection of data, recording also variables which can affect the microbial air contamination, will provide a useful contribution towards the definition of limit values referred to specific environments. In particular, exposure times and incubation temperature for fungal search need to be defined, for a complete standardization of the air sampling.
A consideration should be made regarding the use of Omelyansky’s formula which was applied in order to convert the CFU/plate values (IMA) in CFU/m3. Both active and passive sampling can be used for a general evaluation of microbial air quality, but they have specific aims: while active sampling measures the concentrations of microorganisms, passive sampling measures the fall-out of the biological particles, as a mirror of the airborne risk for critical surfaces (e.g. object, material, food). In any case, considering the relationship provided by the EC GGMP Guidelines to Good Manufacturing Practice (46), it can be observed that the CFU/m3 results obtained with the Omelyansky’s formula are much higher, giving an overestimation of the risk. It could be suggested to keep the IMA value without converting in CFU/m3, and to use the EC GGMP active and passive methods relationship for a possible estimation of the CFU/m3. However, it is questionable to assume that a predefined correspondence between active and passive sampling exists, as some Authors do when using specific formulae to obtain the number of CFU/m3 from the number of CFU/settle plates.
In a context in which there are no generally accepted protocols for the evaluation of microbial contamination of air, the use of IMA standard, for the relevance of data providing the estimation of the airborne risk of contamination for critical surfaces and the cumulative measurements of microbial contamination, as well as for its characteristics of economy and simplicity of use, represents a valid tool in the identification of situations at risk and in the evaluation of effectiveness of prevention interventions.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosols health effects and exposure assessment: progress and perspectives. Ann Occup Hyg. 2003;47(3):187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- 2.Kim KH, Kabir E, Jahan SA. Airborne bioaerosols and their impact on human health. J Environ Sci. 2018;67:23–35. doi: 10.1016/j.jes.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai Km, Emberlin J, Colbeck J. Outdoor environments and human pathogens in the air. Environ Health. 2009;8(Suppl 1):S15. doi: 10.1186/1476-069X-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mora M, Mahnert A, Koskinen K, Pausan MR, Oberauner-Wappis L, et al. Microorganisms in Confined Habitats: Microbial Monitoring and Control of Intensive Care Units, Operating Rooms, Cleanrooms and the International Space Station. Front Microbiol. 2016;7:1573. doi: 10.3389/fmicb.2016.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandrioli P, Caneva G, Sabbioni C. Cultural heritage and aerobiology. Methods and measurement techniques for biodeterioration monitoring. Kluver, Dordrecht. 2003 [Google Scholar]
- 6.Pasquarella C, Albertini R, Dall’Aglio P, Saccani E, Sansebastiano G, Signorelli C. Air microbial sampling: the state of the art. Ig Sanita Pubbl. 2008;64(1):79–120. [PubMed] [Google Scholar]
- 7.Pitzurra M, Savino A, Pasquarella C. Microbiological environment monitoring (MEM) Ann Ig. 1997;9(6):439–454. [PubMed] [Google Scholar]
- 8.Istituto Nazionale Assicurazione Infortuni sul Lavoro. Il monitoraggio microbiologico negli ambienti di lavoro - Campionamento e analisi. 2010 [Google Scholar]
- 9.ISO 14698-1. Cleanrooms and associated controlled environments—biocontamination control. Part 1: general principles and methods. 2003 [Google Scholar]
- 10.Pasquarella C, Pitzurra O, Savino A. The Index of microbial air contamination. J Hosp Inf. 2000;46:241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 11.Guerrera E, Frusteri L, Giovinazzo R, Mariani M. Presenza del rischio nelle falegnamerie umbre. G Ital Med Lav Erg. 2006;28(4):466–471. [PubMed] [Google Scholar]
- 12.Garcia JCR. Evaluación aeromicrobiológica del depósito del Centro de Documentació del Museo Nacional de la Música de Cuba. Ge-Conservación. 2016;9:117–126. [Google Scholar]
- 13.Coelho AIM, Milagres RCRM, Martins JFL, Cordeiro de Azeredo RM, Campos Santana AMC. Contaminaçao microbiológica de ambientes e de superfícies em restaurantes comerciais. Ciéncia & Saude Coletiva. 2010;15 (Suppl. 1):1597–1606. doi: 10.1590/s1413-81232010000700071. [DOI] [PubMed] [Google Scholar]
- 14.Morais GR, da Silva MA, de Carvalho MC, Dos Santos JGS, von Dolinger EJO, de Brito DVD. Qualidade do ar interno em uma Instituiçao de ensino superior brasileira. Biosci J. 2010;26(2):305–310. [Google Scholar]
- 15.Schleibinger H, Laubmann D, Eis D, Samwer H, Mickelmann A, Ruden H. Discrimination between mouldy and non-mouldy homes with the detection of settling mould spores (OPD method). Results of a field study in greater Berlin, Germany. Umweltmedizin in Forschung und Praxis. 2004;9(5):289–297. [Google Scholar]
- 16.Bidaki MZ, Yazdanbakhsh A, Mohasel MA, Ghazi M. Comparing the effects of deep and surface aeration methods on density and type of airborne bacteria and fungi in municipal waste water treatment plant. J Mazandaran University of Medical Sciences. 2019;29(174):121–133. [Google Scholar]
- 17.Okraszewska-Lasica W, Bolton DJ, Sheridan JJ, McDowell DA. Airborne Salmonella and Listeria associated with Irish commercial beef, sheep and pig plants. Meat Science. 2014;97:255–261. doi: 10.1016/j.meatsci.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Pasquarella C, Sansebastiano GE, Saccani E, Ugolotti M, Mariotti F, Boccuni C, et al. Proposal for an integrated approach to microbial environmental monitoring in cultural heritage: experience at the Correggio exhibition in Parma. Aerobiologia. 2011;27:203–211. [Google Scholar]
- 19.Pasquarella C, Saccani E, Sansebastiano GE, Ugolotti M, Pasquariello G, Albertini R. Proposal for a biological environmental monitoring approach to be used in libraries and archives. Ann Agric Environ Med. 2012;19:209–212. [PubMed] [Google Scholar]
- 20.Lamonaca F, Pizzuti G, Arcuri N, Palemo AM, Morello R. Monitoring of environmental parameters and pollution by fungal spores in the National Gallery of Cosenza: a case study. Measurement. 2014;47:1001–1007. [Google Scholar]
- 21.Pasquarella C, Balocco C, Pasquariello G, Petrone G, Saccani E, Manotti P, et al. A multidisciplinary approach to the study of cultural heritage environments: experience at the Palatina Library in Parma. Sci Total Environ. 2015;536:557–567. doi: 10.1016/j.scitotenv.2015.07.105. [DOI] [PubMed] [Google Scholar]
- 22.Micheluz A, Manente S, Tigini V, Prigione V, Pinzari F, Ravagnan G, et al. The extreme environment of a library: Xerophilic fungi inhabiting indoor niches. Int Biodeterior Biodegrad. 2015;99:1–7. [Google Scholar]
- 23.Okpalanozie OE, Adebusoye SA, Troiano F, Catto’ C, Ilori MO, Cappitelli F. Assessment of indoor air environment of a Nigerian museum library and its biodeteriorated books using culture-dependent and independent techniques. Int Biodeterior Biodegrad. 2018;132:139–149. [Google Scholar]
- 24.Pasquarella C, Balocco C, Saccani E, Capobianco E, Viani I, Veronesi L. Biological and microclimatic monitoring for conservation of cultural heritage: a case study at the De Rossi room of the Palatina library in Parma. Aerobiologia. 2019 https://doi.org/10.1007/s10453-019-09610-1 . [Google Scholar]
- 25.Awad AHA, Saeed Y, Shakour AA, Abdellatif NM, Ibrahim YH, Elghanam M, Elwakeel F. Indoor air fungal pollution of a historical museum, Egypt: a case study. Aerobiologia. 2020 https://doi.org/10.1007/s10453-019-0909623-w . [Google Scholar]
- 26.Di Giulio M, Grande R, Di Campli E, Di Bartolomeo S, Cellini L. Indoor air quality in university environments Environ Monit Assess. 2010;170:509–517. doi: 10.1007/s10661-009-1252-7. [DOI] [PubMed] [Google Scholar]
- 27.Chong ETJ, Faizin KAK, Goh LPW, Lee P-C. Assessment of indoor airborne microorganisms in a densely populated Malaysian Public University. Malaysian J of Public Health Medicine. 2017;17(2):113–120. [Google Scholar]
- 28.Sonmez E, Ozdemir HM, Cem EM, Sonmez Y, Salacin S, Ismail OC, et al. Microbiological detection of bacteria and fungi in the autopsy room. Rom J Leg Med. 2011;19:33–44. [Google Scholar]
- 29.Scholtz I, Siyoum N. Korsten L. Penicillium air mycoflora in postharvest fruit handling environments associated with the pear export chain. Postharvest Biology and Technology. 2017;128:153–160. [Google Scholar]
- 30.Tasaki T, Kojima M, Suzuki Y, Tatematsu Y, Sasaki H. Creating and stable short-term housing environment for rabbits in a cargo van. J Am Ass Lab. Animal Science. 2019;58(4):456–461. doi: 10.30802/AALAS-JAALAS-19-000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vella FM, Laratta B. UV-based evaluation of ergosterol for monitoring the fungal exposure in Italian buffalo farms. FEMS Microbiol Lett. 2017;364(22):1–6. doi: 10.1093/femsle/fnx224. [DOI] [PubMed] [Google Scholar]
- 32.Faridi S, Hassanvand MS, Naddafi K, Yunesian M, Nabizadeh R, Sowlat M H, et al. Indoor/outdoor relationships of bioaerosol concentrations in a retirement home and a school dormitory. Environ Sci Pollut Res. 2014 doi: 10.1007/s11356-014-3944-y. [DOI] [PubMed] [Google Scholar]
- 33.Onchang R, Panyakapo M. The physical environments and microbiological contamination in three different fitness centres and the participants’ expectations: Measurement and analysis. Indoor Built Environ. 2016;25(1):213–228. [Google Scholar]
- 34.Asefa DT, Langsrud S, Gjerde RO, Kure CF, Sidhu MS, Nesbakken T, et al. The performance of SAS-super-180 air sampler and settle plates for assessing viable fungal particles in the air of dry-cured meat production facility. Food Control. 2009;20:997–1001. [Google Scholar]
- 35.Ithnin A, Shakirin M, Yusuf NM, Rahman SAA, Halim AA. Study on air quality and influences on human respiratory health among residents who occupy buildings at former landfill site. Nature Environment and Pollution Technology. 2015;14(2):385–390. [Google Scholar]
- 36.Benami M, Busgang A, Gillor O, Gross A. Quantification and risks associated with bacterial aerosols near domestic greywater-treatment systems. Sci Total Environ. 2016;562:344–352. doi: 10.1016/j.scitotenv.2016.03.200. [DOI] [PubMed] [Google Scholar]
- 37.Hai WD, Hoang SMT, Hung NTQ, Ky NM, Gwi-Nam B, Ki-Hong P, et al. Characteristics of airborne bacteria and fungi in the atmosphere in Ho Chi Minh City, Vietnam – a case study over three years. Int Biodet Biodegr. 2019:145. [Google Scholar]
- 38.Hayleeyesus SF, Ejeso A, Derseh FA. Quantitative assessment of bio-aerosols contamination in indoor air of University dormitory rooms. Int J Health Sci. 2015;9(3):249–256. [PMC free article] [PubMed] [Google Scholar]
- 39.Lipsa FD, Ulea E, Chiriac IP. Monitoring of fungal aerosols in some educational buildings from Iaşi Romania. Environ Engineering Management J. 2016;4:801–807. [Google Scholar]
- 40.Seong D, Norman RS, Hoque S. Influence of indoor conditions on microbial diversity and quantity in schools. E3S Web of Conferences. 111 01035 CLIMA 2019. [Google Scholar]
- 41.Andualem Z, Gizaw Z, Dagne H. Indoor culturable fungal load and associated factors among public primary school classrooms in Gondar City, Northwest Ethiopia, 2018: a cross-sectional study. Ethiop J Health Sci. 2019;29(5):623–630. doi: 10.4314/ejhs.v29i5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anaya M, Gámez-Espinosa E, Falco AS, Benítez E, Carballo G. Characterization of indoor air mycobiota of two locals in a food industry, Cuba. Air Qual Atmos Health. 2019 [Google Scholar]
- 43.Ejdys E, Dynowska M, Biedunkiewicz A, Sucharzewska E. An overview of the species of Fungi occurring in school rooms-a four-year study. Pol J Stud. 2013;6:1691–1700. [Google Scholar]
- 44.Meireles A, Fulgêncio R, Machado I, Mergulhão F, Melo L, Simões M. Characterization of the heterotrophic bacteria from a minimally processed vegetables plant. Food Science and Technology. 2017;85:293–300. [Google Scholar]
- 45.Omelyansky VL. Manual in Microbiology, USSR Academy of Sciences Moscow, Leningrad. 1940 [Google Scholar]
- 46.EC Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use Revision to Annex 1. Manufacture of Sterile Medicinal Products. Brussels European Commission (2008) 25 November. Available at http://ec.europa.eu/health/files/eudralex/vol-4/2008_11_25_gmp-an1_en.pdf . [Google Scholar]


