Abstract
Background and aim:
The low measles vaccination coverage contributes to the re-emerging of measles in Italy. This study aimed to estimate the measles burden, expressed in Disability Adjusted Life Years (DALYs), in Umbria, for the period 2013-2018.
Methods:
Data on measles cases in Umbria were obtained from the MoRoNet. While data related to the resident population, were obtained from the website of the National Institute of Statistics. The estimated DALYs was calculated using the Burden of Communicable Diseases in Europe toolkit. The results are expressed in DALYs per year, per case and per 100,000 subjects, for acute illness and for sequelae.
Results:
The estimated incidence in mean for the entire period was 52.50 cases per year. Resulting in an average loss of 3.10 DALYs per year.
Conclusions:
The data obtained from this analysis provide important information on the impact of measles in the Umbria region, and offer useful data to the Health Authorities that can be used to reduce measles incidence in the region.
Keywords: measles, burden of disease, Italy, vaccination, disability-adjusted life years
Background
Measles is an acute (RNA) viral, vaccine-preventable disease, transmitted by droplets, and still responsible of recurrent epidemics (1). Measles is an important cause of death and disability among children worldwide , responsible of 100 million of acute infections and 6 millions of deaths per year all over the word. Immunisation against measles started in the 1960s and dramatically changed the epidemiology of the disease, preventing 99% of cases in many industrialized countries. Considering the several efforts adopted, measles appears eliminated in 43 out of 53 WHO European Region member states (2), but not in Italy where the last epidemic outbreak started at the end of 2016 and it is still ongoing (2019), counting for more than 9,000 cases. In Italy, the first measles vaccine (mono-component, single-dose) was introduced in late 1970s, and later replaced in 1980s with the trivalent vaccine (measles-mumps-rubella, MMR) (3). However, despite the high vaccine effectiveness (95%), which is able to induce a life-long immunity (4), measles vaccine coverage in Italy remained very low (approximately 40%) for several decades, until the early 1990s (5). However, the rate highly improved after the reinforcement of mandatory vaccination law, which increased by 6% points the coverage (6).
Considering the re-emerging of measles, and its related health outcomes, it is important for Health Authorities and policy makers to have the best possible evidence in order to identify the most cost-effective interventions able to promote and guarantee the health of citizens. In order to do so, it is necessary to identify mixed measures, able to measure the loss of health in terms of years, thus allowing to make quantitative comparisons between the various phenomena, and that are also representative of the complex phenomena related to human health. In other words, it is necessary to estimate the burden of infectious diseases, so that an effective public health planning can be carried out (7). Regarding measles, even though the infection is mainly acute and mostly evolves into a resolution, it is also associated with both short and long-term complications, impacting on health and quality of life. However, the burden of disease of measles is not entirely known yet, and no studies aimed to estimate the measles burden during an epidemic have been conducted so far. Furthermore, there are no studies available to evaluate the burden at a national level in Italy, and above all there are no regional assessments. This information is largely needed in order to support public health policies (8). In this perspective, this work aimed to: i) describe the epidemiology of measles in Umbria for the period 2013-June 2019; ii) assess the burden of measles, expressed in Disability Adjusted Life Years (DALYs), in Umbria, in different age groups, for the period 2013-2018.
Methods
Data source
Data on measles cases in Umbria were obtained from the MoRoNet (Measles and Rubella Network) notification system for the period 2013-June 2019 (last update July 2019). While data related to the resident population, were obtained from the website of the National Institute of Statistics (9).
Estimation of the burden
DALYs is a composite measure that considers the years of life lost to disabilities (YLD), and the years of life lost due to premature death (YLL). The YLD is calculated considering the impact of the disease on the quality of life, while the YLL is calculated considering the years of life lost due to premature death, according to the life expectancy. The information needed to build the mathematical model are related to disease progression, rate of sequelae and underreporting. However, since a mathematical model is based on some assumptions, and in order to express the uncertainty in the outputs, given the random nature of its inputs, the Monte Carlo simulation was recommended (10).
In order to assess the burden, only data referring to the 2013-2018 were considered. The analysis was divided in three sub-analysis: the pre-epidemic period (2013-2016), then the epidemic year (2017), and lastly the entire 2013-2018 period. The analyses for the 2013-2016 and 2013-2018 were carried out considering both the cumulative incidence recorded in the two periods and the average incidence per year. The estimated DALYs was calculated using the Burden of Communicable Diseases in Europe (BCoDE) toolkit, a software developed by the European Centre for Disease Prevention and Control (ECDC) (11).
In our analysis a standard life expectancy was considered (maximum age 85 years). The mathematical model used for the current analysis was developed by the ECDC through a literature review and expert consultation. Regarding the multiplication factor (MF) used to correct for the underreporting, this should ideally be sex, age group, and country specific (depending on the type of notification systems) (11). However, in literature, these data are not often reported. A previous study conducted in Germany, aimed to estimate the measles burden, used a single MF of 2.5 for each age group and sexes (12). It was identified by literature search and estimating a low rate of underreporting, considering that measles notification systems, as well as diagnosis, reached a high-quality level in Europe.
For each sub-analysis the DALYs are reported per year, per case and per 100,000 subjects (each of which is specified on the total), for acute illness and for sequelae. Finally, the DALYs per year and per case are presented in both aggregated and disaggregated forms (YLL and YLD). Values are expressed as medians with 95% uncertainty interval (95% IU) quantified by performing a Monte Carlo simulation (10,000 iterations).
Statistical analysis
Descriptive analysis of measles cases was reported either as a percentage or as an average with standard deviation (SD). The cumulative incidence was calculated considering all notified cases in the 2013-2018 period referring to the average of the resident population in the same period. Resident population was stratified by sex and age.
Ethical approval
This study has been conducted using data routinely collected within the Italian Ministry of Health mandate; no ethical approval was needed.
Results
126 cases were reported in Umbria in the period January 2013-June 2019, with a cumulative incidence of 14 measles cases per 100,000 residents, 58% of which occurred in females. The mean age was 29±16.3 years, and 15.3% cases were reported in children aged ≤5 years. Laboratory confirmation was performed in 75.6% of cases, 93.9% of which were positive for measles (PCR or IgM). The vaccination status was known for 97% of the cases, 79%of which were not vaccinated, while the remaining had received only one dose. One case was recorded in a pregnant woman, and 16% occurred among health care workers (HCW). The most observed complications were diarrhoea (18%), stomatitis (11.7%), pneumonia (9.4%), keratoconjunctivitis (7.8%), hepatitis (6.3%) and thrombocytopenia (5.5%). Hospitalization (or at least emergency room access) occurred in 57% of the cases, and the mean length of stay was 4 days (range 1-12 days).
Burden of measles
Considering the pre-epidemic period 2013-2016, a cumulative incidence of 23 notified measles cases was observed, 47.8% of which in females. After correction for underreporting, the estimated number of new cases was 57.5. Modelling the long-term sequelae, the expected rates were 0.02 cases of permanent disability due to encephalitis, 0.004 cases of post-infectious encephalitis, 0.04 deaths and 0.002 cases of subacute sclerosing panencephalitis (SSPE). Considering the pre-epidemic period 2013-2016 as a mean, 5.75 cases occurred on average per year. After correction for underreporting, the estimated number of new cases was 14.38 per year. Modelling the long-term sequelae, the expected rates are 0 cases of permanent disability due to encephalitis, 0.001 cases of post-infectious encephalitis, 0 deaths and 0.001 cases of SSPE. Considering the epidemic year (2017), 91 new cases occurred, 64.8% of which in females. After correction for underreporting, the estimated number of new cases was 227.50. Modelling the long-term sequelae, the expected rates were 0.06 cases of permanent disability due to encephalitis, 0.15 cases of post-infectious encephalitis, 0.004 deaths and 0.004 cases of SSPE. Considering the period 2013-2018, a cumulative incidence of 131 notified cases was observed, 59.5% of which in females. After correction for underreporting, the estimated number of new cases was 315. Modelling the long-term sequelae, the expected rates were 0.08 cases permanent disability due to encephalitis, 0.21 cases of post-infectious encephalitis, and 0.003 cases of SSPE. Lastly, considering the pre-epidemic period 2013-2018 as a mean, 21 cases occurred on average per year. After correction for underreporting, the estimated number of new cases was 52.5 per year. Modelling the long-term sequelae, the expected rates were 0.01 cases of permanent disability due to encephalitis, 0.03 cases of post-infectious encephalitis, 0.001 deaths and 0.001 cases of SSPE. The estimated DALYs are reported in Table 1. The DALYs – in both aggregated and disaggregated forms – per year by sex and age groups are depicted in Figures from 1 to 3.
Table 1.
Overview of the measles burden, Umbria 2013-2018. Disability-adjusted life years (DALYs), Years Lived with Disability (YLD), Years Life Lost due to premature death (YLL).
| 2013-2016 cumulative | 2013-2016 average per year | 2017 | 2013-2018 cumulative | 2013-2018 average per year | |
| DALYs per year, total | 3.63 (3.25-4.00) | 0.91 (0.81-1.00) | 13.17 (12.09-14.24) | 18.58 (17.01-20.13) | 3.10 (2.84-3.36) |
| YLD | 0.91 (0.71-1.13) | 0.23 (0.18-0.28) | 3.34 (2.74-3.97) | 4.69 (3.92-5.49) | 0.78 (0.65-0.93) |
| YLL | 2.72 (2.41-3.03) | 0.68 (0.61-0.75) | 9.84 (8.88-10.78) | 13.88 (12.54-15.17) | 2.32 (2.10-2.53) |
| DALYs per year, acute disease | 2.74 (2.43-2.94) | 0.69 (0.61-0.76) | 10.08 (9.15-11.02) | 14.19 (12.85-15.46) | 2.37 (2.15-2.58) |
| YLD | 0.12 (0.11-0.13) | 0.03 (0.03-0.03) | 0.48 (0.46-0.51) | 0.67 (0.63-0.71) | 0.11 (0.11-0.12) |
| YLL | 2.62 (2.32-2.94) | 0.66 (0.58-0.73) | 9.60 (8.65-10.54) | 13.52 (12.17-14.80) | 2.26 (2.03-2.47) |
| DALYs per year, sequelae | 0.88 (0.69-1.11) | 0.22 (0.17-0.28) | 3.09 (2.51-3.72) | 4.39 (3.63-5.17) | 0.73 (0.60-0.87) |
| YLD | 0.78 (0.58-1.01) | 0.20 (0.15-0.25) | 2.85 (2.27-3.47) | 4.02 (3.27-4.82) | 0.67 (0.54-0.81) |
| YLL | 0.10 (0.09-0.11) | 0.02 (0.02-0.03) | 0.24 (0.22-0.26) | 0.37 (0.33-0.40) | 0.06 (0.06-0.07) |
| DALYs per case | 0.06 (0.06-0.07) | 0.06 (0.06-0.07) | 0.06 (0.05-0.06) | 0.06 (0.05-0.06) | 0.06 (0.05-0.06) |
| DALYs per case, acute disease | 0.05 (0.04-0.05) | 0.05 (0.04-0.05) | 0.04 (0.04-0.05) | 0.05 (0.04-0.05) | 0.05 (0.04-0.05) |
| DALYs per case, sequelae | 0.02 (0.01-0.02) | 0.02 (0.01-0.02) | 0.01 (0.01-0.02) | 0.01 (0.01-0.02) | 0.01 (0.01-0.02) |
| DALYs/100.000 | 0.41 (0.36-0.45) | 0.10 (0.09-0.11) | 1.48 (1.36-1.60) | 2.09 (1.91-2.26) | 0.35 (0.32-0.38) |
| DALYs/100.000 acute disease | 0.10 (0.08-0.12) | 0.08 (0.07-0.09) | 1.13 (1.03-1.24) | 1.59 (1.44-1.74) | 0.27 (0.24-0.23) |
| DALYs/100.000 sequelae | 0.31 (0.27-0.34) | 0.02 (0.02-0.03) | 0.35 (0.28-0.42) | 0.49 (0.41-0.58) | 0.08 (0.07-0.10) |
Figure 1.
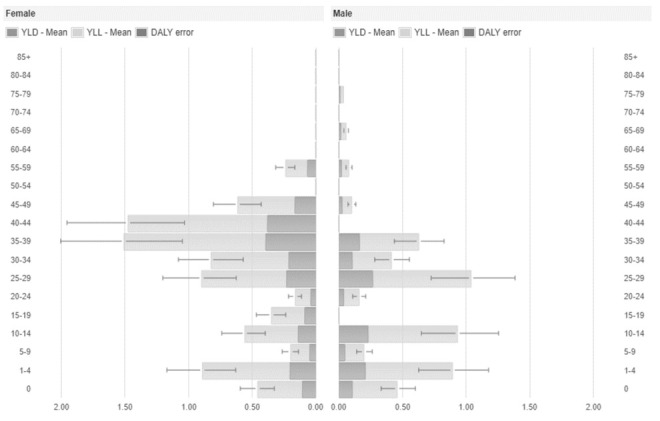
Disability-Adjusted Life Years (DALYs), Years Lived with Disability (YLD) and Years of Life Lost (YLL) per year and by sex and age group of measles cases, Umbria 2017.
Figure 3.
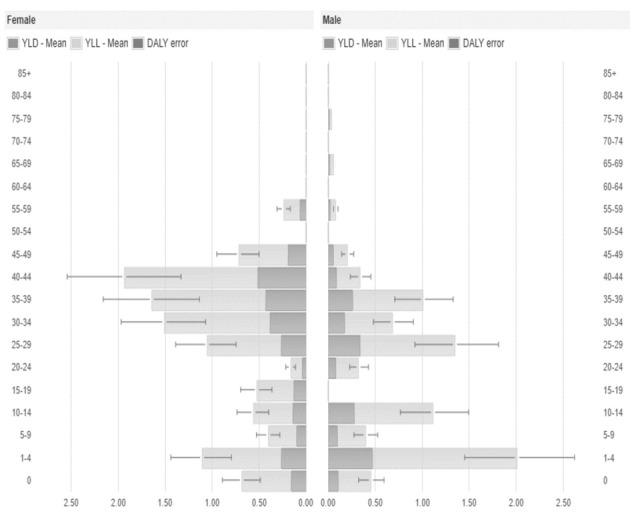
Disability-Adjusted Life Years (DALYs), Years Lived with Disability (YLD) and Years of Life Lost (YLL) per year and by sex and age group of cumulative measles cases, Umbria 2013-2018.
Figure 2.
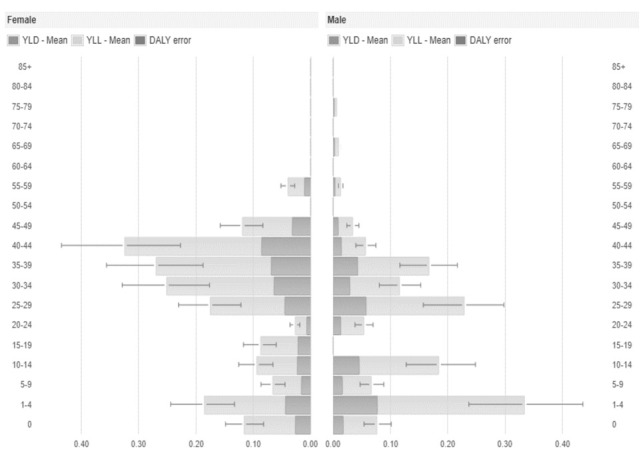
Average Disability-Adjusted Life Years (DALYs), Years Lived with Disability (YLD) and Years of Life Lost (YLL) per year and by sex and age group of measles cases, Umbria 2013-2018.
Conclusion
The most recent epidemic outbreak faced by Umbria, as well as by Italy, started at the end of 2016 and mainly occurred during 2017. The mean age of the cases notified during the whole period 2013-2018 was 29±16.3 years, with 58% recorded among children ≤5 years old. These results highlight the need for new vaccination strategies as the catch up policy, or offering the anti-MPR(V) vaccination during all possible occasions, and in particular in women of child-bearing age (13).
In this perspective, and considering the restricted resources for health (14), a crucial role is played by counselling and communication (15), especially for measles vaccination, to which a large population distrust is associated, mainly due to the alleged and false association with autism (16). In Italy, the reasons for missed measles vaccination are routinely collected, and show a decreasing trend during the last three years (17). This achievement is mainly due to the approval of the new Immunisation Plan 2017-2019 (13), the reinforcement of a mandatory vaccination law (18), and the implementation of the immunization information system (IIS), a useful instrument to counter vaccine hesitancy and to identify subjects under or unimmunized (19). The Umbria region has an advanced IIS, considering that all the Local Health Units use the same shared software, and data are individual-based (20). However, until now, electronically stored data are mainly related to infants’ vaccinations, while older data that had been recorded on paper in the past decades have not been digitalized yet. Considering our results, future efforts in electronically recording immunization data of older cohorts is needed (21). Moreover, a higher percentage of cases notified in HCWs has been recorded in Umbria compared to national data (16% vs 5% in Italy) despite measles vaccination is one of the highly recommended vaccinations for HCWs. Measles vaccination among HCWs is extremely important for several reasons. Firstly, to avoid potentially causing nosocomial outbreaks and secondly because HCWs represent a positive model. Indeed, even though health-related information are often searched on the Internet (22-27), HCWs are still the most trusted and most consulted source of information (28). Nevertheless, despite these aspects and the high efficacy and safety of vaccinations, vaccination coverages are still below the threshold (29). HCWs’ immunization, as well as for the general population, is another crucial strategy to reach elimination goal (30). The hospitalization rate was slightly lower in Umbria compared to the national data, but in both cases approximately 60% of the affected people were treated at the hospital. On one hand, these data confirm that measles can have severe manifestations requiring hospitalization. On the other hand, this high rate of hospitalization might be since the surveillance system is much more precise in the identification of hospitalized patients, compared to people treated in primary care. As a matter of fact, under-reporting is an intrinsic issue of surveillance systems, even in industrialized countries. In particular, under-reporting highly affects primary care mainly due to underdiagnosis. Considering this, we applied a MF to our measles burden estimation. Using both the MF and the notified cases of measles, we observed an estimated incidence of 52.50 cases per year. The estimated cases resulted in an average loss of 3.10 DALYs per year, whose major component is the YLL (75%). This is mainly due to the intrinsic characteristics of measles, resulting in a low rate of complicated infections but with a high fatality rate.
The DALY for 100,000 estimated confirmed results shown in a previous European study (period 2009-2013) (31). However, in our study, the DALYs per 100,000 considering only 2017, is twice higher compare the entire period. However, it should be kept in mind that the burden of measles could be completely avoided, thanks to the effective and safe vaccine. According to previous study estimated the measles burden in Germany, referring to the period 2005-2007, the most affected age group in term of burden was the 0-19 years old subjects, without gender differences (12). This was not confirmed in our study, in which the most affected age group was the 20-44 years old subjects, and mainly females. While the 0-19 age group ranked second, without gender differences. These differences might be explained considering the historical low measles vaccination coverage obtained in Italy. Indeed, measles vaccination coverage was around 40% from 1976 (when the measles vaccination was first introduced in Italy) to the end of the 1980s. Moreover, the second dose was introduced in the vaccination schedule in the year 2013 (5). Considering these aspects, it is extremely important to know both coverage and burden data to effectively plan interventions aimed to eliminate measles. In fact, identifying the most affected age group is mandatory to reduce the measles burden during potential future epidemic outbreaks, as recommended by the WHO (32).
This study has some limitations. Firstly, the measles incidence was calculated using the total population and not the susceptible population, potentially contributing to an underestimation of the burden. Secondly, the selected MF, although previously used in similar analysis, it must be noted that they were European studies, and therefore it may not be perfectly applicable to the Umbrian situation. However, it was relatively low, and this may have led to an underestimation of the burden. Indeed, surveillance systems are affected by a certain rate of under-reporting, which cannot be ignored. Lastly, we did not modify any parameters set in the software. These parameters are based on simplified generalizations of the disease evolution that, in the real world, might highly be heterogeneous. Moreover, they had been set based on the European context that, even though it could be representative of the Umbria region as well, it could also be partially different.
Despite the mentioned limitations, the study has important strengths. This is the first study presenting epidemiology and measles burden in Umbria, in both epidemic and non-epidemic periods. Another strength is the pathogen-based approach used to estimate the burden. This method is more precise compared to the disease-based approach, allowing to include all possible clinical manifestations of the disease, instead of considering only one (33). Furthermore, the pathogenic-based approach ensures greater comparability of results between various infectious diseases, as well as between different populations (12). Moreover, in this study data form the national surveillance system has been used, representing the most reliable and trustful source. Lastly, in order to estimate the burden we used the BCoDE toolkit, an intuitive software, listed by the European Food Safety Authority (EFSA) among the risk ranking tools (34).
The data obtained from this analysis provide important information on the impact of measles in Umbria, and offer useful data to the Health Authorities that can be used to reduce measles incidence in the region, thus contributing to the achievement of the elimination goal.
Acknowledges
The Authors would like to thanks M.D. Giulia Dallagiacoma, University of Pavia, for English revision.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Heymann David L. Control of comunicable diseases manual. 19th edition. Washington, DC: American Public Health Association; 2008. [Google Scholar]
- 2.World Health Organization. Measles and Rubella Surveillance Data. 2019 [Available from: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en. / [Google Scholar]
- 3.Filia A, Tavilla A, Bella A, Magurano F, Ansaldi F, Chironna M, et al. Measles in Italy, July 2009 to September 2010. Euro Surveill. 2011;16(29) [PubMed] [Google Scholar]
- 4.Pillsbury A, Quinn H. An assessment of measles vaccine effectiveness, Australia, 2006-2012. Western Pac Surveill Response J. 2015;6(3):43–50. doi: 10.5365/WPSAR.2015.6.2.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epicentro. Morbillo, aspetti epidemiologici. 2017 Available from: https://www.epicentro.iss.it/morbillo/epidemiologia-italia . [Google Scholar]
- 6.D’Ancona F, D’Amario C, Maraglino F, Rezza G, Iannazzo S. The law on compulsory vaccination in Italy: an update 2 years after the introduction. Euro Surveill. 2019;24(26) doi: 10.2807/1560-7917.ES.2019.24.26.1900371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakab Z. Why a burden of disease study? Euro Surveill. 2007;12(12):E1–2. doi: 10.2807/esm.12.12.00750-en. [DOI] [PubMed] [Google Scholar]
- 8.Gianfredi V, Balzarini F, Gola M, Mangano S, Carpagnano LF, Colucci ME, et al. Leadership in Public Health: Opportunities for Young Generations Within Scientific Associations and the Experience of the “Academy of Young Leaders”. Front Public Health. 2019;7:378. doi: 10.3389/fpubh.2019.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Istituto Nazionale di Statistica. Popolazione residente, Umbria. 2019 [Available from: http://dati.istat.it/Index.aspx?QueryId=18542 . [Google Scholar]
- 10.Kretzschmar M, Mangen MJ, Pinheiro P, Jahn B, Fevre EM, Longhi S, et al. New methodology for estimating the burden of infectious diseases in Europe. PLoS medicine. 2012;9(4):e1001205. doi: 10.1371/journal.pmed.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colzani E, Cassini A, Lewandowski D, Mangen MJ, Plass D, McDonald SA, et al. A Software Tool for Estimation of Burden of Infectious Diseases in Europe Using Incidence-Based Disability Adjusted Life Years. PloS one. 2017;12(1):e0170662. doi: 10.1371/journal.pone.0170662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plass D, Mangen MJ, Kraemer A, Pinheiro P, Gilsdorf A, Krause G, et al. The disease burden of hepatitis B, influenza, measles and salmonellosis in Germany: first results of the burden of communicable diseases in Europe study. Epidemiology and infection. 2014;142(10):2024–35. doi: 10.1017/S0950268813003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Immunization Plan 2017-2019 [Piano Nazionale Prevenzione Vaccinale 2017-2019], (2017) [Google Scholar]
- 14.Odone A, Landriscina T, Amerio A, Costa G. The impact of the current economic crisis on mental health in Italy: evidence from two representative national surveys. Eur J Public Health. 2018;28(3):490–5. doi: 10.1093/eurpub/ckx220. [DOI] [PubMed] [Google Scholar]
- 15.Gianfredi V, Grisci C, Nucci D, Parisi V, Moretti M. Communication in health. Recenti progressi in medicina. 2018;109(7):374–83. doi: 10.1701/2955.29706. [DOI] [PubMed] [Google Scholar]
- 16.Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32(29):3623–9. doi: 10.1016/j.vaccine.2014.04.085. [DOI] [PubMed] [Google Scholar]
- 17.Gianfredi V, D’Ancona F, Maraglino F, Cenci C, Iannazzo S. Polio and measles: reasons of missed vaccination in Italy, 2015-2017. Annali di igiene: medicina preventiva e di comunita. 2019;31(3):191–201. doi: 10.7416/ai.2019.2282. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health. Legge 31 luglio 2017, n. 119: «Disposizioni urgenti in materia di prevenzione vaccinale, di malattie infettive e di controversie relative alla somministrazione di farmaci.». Gazzetta Ufficiale Serie Generale. 2017:182. [Google Scholar]
- 19.Gianfredi V, Moretti M, Lopalco PL. Countering vaccine hesitancy through immunization information systems, a narrative review. Human vaccines & immunotherapeutics. 2019:1–19. doi: 10.1080/21645515.2019.1599675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Ancona F, Gianfredi V, Riccardo F, Iannazzo S. Immunisation Registries at regional level in Italy and the roadmap for a future Italian National Registry. Annali di igiene: medicina preventiva e di comunita. 2018;30(2):77–85. doi: 10.7416/ai.2018.2199. [DOI] [PubMed] [Google Scholar]
- 21.Derrough T, Olsson K, Gianfredi V, Simondon F, Heijbel H, Danielsson N, et al. Immunisation Information Systems - useful tools for monitoring vaccination programmes in EU/EEA countries, 2016. Euro Surveill. 2017;22(17) doi: 10.2807/1560-7917.ES.2017.22.17.30519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bragazzi NL, Barberis I, Rosselli R, Gianfredi V, Nucci D, Moretti M, et al. How often people google for vaccination: Qualitative and quantitative insights from a systematic search of the web-based activities using Google Trends. Human vaccines & immunotherapeutics. 2017;13(2):464–9. doi: 10.1080/21645515.2017.1264742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provenzano S, Santangelo OE, Giordano D, Alagna E, Piazza D, Genovese D, et al. Predicting disease outbreaks: evaluating measles infection with Wikipedia Trends. Recenti progressi in medicina. 2019;110(6):292–6. doi: 10.1701/3182.31610. [DOI] [PubMed] [Google Scholar]
- 24.Gianfredi V, Bragazzi NL, Mahamid M, Bisharat B, Mahroum N, Amital H, et al. Monitoring public interest toward pertussis outbreaks: an extensive Google Trends-based analysis. Public Health. 2018;165:9–15. doi: 10.1016/j.puhe.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Mahroum N, Bragazzi NL, Sharif K, Gianfredi V, Nucci D, Rosselli R, et al. Leveraging Google Trends, Twitter, and Wikipedia to Investigate the Impact of a Celebrity’s Death From Rheumatoid Arthritis. J Clin Rheumatol. 2018;24(4):188–92. doi: 10.1097/RHU.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 26.Gianfredi V, Bragazzi NL, Nucci D, Martini M, Rosselli R, Minelli L, et al. Harnessing Big Data for Communicable Tropical and Sub-Tropical Disorders: Implications From a Systematic Review of the Literature. Front Public Health. 2018;6:90. doi: 10.3389/fpubh.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bragazzi NL, Gianfredi V, Villarini M, Rosselli R, Nasr A, Hussein A, et al. Vaccines Meet Big Data: State-of-the-Art and Future Prospects. From the Classical 3Is (“Isolate-Inactivate-Inject”) Vaccinology 1.0 to Vaccinology 3.0, Vaccinomics, and Beyond: A Historical Overview. Front Public Health. 2018;6:62. doi: 10.3389/fpubh.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giambi C, Fabiani M, D’Ancona F, Ferrara L, Fiacchini D, Gallo T, et al. Parental vaccine hesitancy in Italy - Results from a national survey. Vaccine. 2018;36(6):779–87. doi: 10.1016/j.vaccine.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 29.Gianfredi V, Nucci D, Salvatori T, Orlacchio F, Villarini M, Moretti M, et al. “PErCEIVE in Umbria”: evaluation of anti-influenza vaccination’s perception among Umbrian pharmacists. Journal of preventive medicine and hygiene. 2018;59(1):E14–E9. doi: 10.15167/2421-4248/jpmh2018.59.1.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianfredi V, Dallagiacoma G, Provenzano S, Santangelo OE. Factors predicting health science students’ willingness to be vaccinated against seasonal flu during the next campaign. Ann Ist Super Sanita. 2019;55(3):209–16. doi: 10.4415/ANN_19_03_03. [DOI] [PubMed] [Google Scholar]
- 31.Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, McDonald SA, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23(16) doi: 10.2807/1560-7917.ES.2018.23.16.17-00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orenstein WA, Cairns L, Hinman A, Nkowane B, Olive JM, Reingold AL. Measles and Rubella Global Strategic Plan 2012-2020 midterm review report: Background and summary. Vaccine. 2018;36 Suppl 1:A35–A42. doi: 10.1016/j.vaccine.2017.10.065. [DOI] [PubMed] [Google Scholar]
- 33.Zou S. Applying DALYs to the burden of infectious diseases. Bulletin of the World Health Organization. 2001;79(3):267–9. [PMC free article] [PubMed] [Google Scholar]
- 34.EFSA Panel on Biological Hazards. Scientific opinion on the development of a risk ranking toolbox for the EFSA BIOHAZ Panel. EFSA Journal. 2015;13(1):3939. [Google Scholar]


