Abstract
Background and aim of the work:
Colorectal mucosal precancerous lesions, metabolic syndrome (MetS) and psychiatric disorders may share a common low-grade local and systemic inflammation. Aim is to report on preliminary data concerning a research adopting a psycho-neuro-endocrine-immune (PNEI) approach to study outpatients undergoing colonoscopy.
Methods:
A sample of patients undergoing colonoscopy was cross-sectionally investigated. Data on colorectal adenomas, MetS, early atherosclerosis, anxious-depressive symptoms, personality traits, and inflammatory markers were statistically analyzed.
Results:
Sixty-two patients were recruited (female 50%, mean age: 60.8±9.4 years). The prevalence of adenomas and MetS was respectively of 45.2% and 41.9%. Anxiety and depressive symptoms were detected in 16 (32.7%) and 9 (18.4%) subjects, respectively. The presence of adenomas positively correlated with male sex (p=0.01), age (p<0.01), IL-6 (p=0.03), hsCRP (p=0.04), and MetS (p=0.03); it was also associated with hsCRP concentration (aOR=3.81, p=0.03).
Conclusions:
Proinflammatory atherogenic status, psychological traits, increased mucosal inflammation, and metabolic parameters may share a common a pathogenic mechanism, worth studying.
Keywords: colorectal adenoma, metabolic syndrome, psycho-neuro-immuno-endocrinological approach, anxiety, depression
Introduction
A major cause of disability and mortality in Western countries is represented by colorectal cancer (CRC). CRC develops from benign precursors that transform into cancer in a stepwise manner; several molecular abnormalities accompany determine colorectal tumorigenesis (1).
The risk of CRC is increased in patients affected by Metabolic Syndrome (MetS), a cluster of cardiovascular risk factors affecting about a quarter of adult population worldly, clinically defined as a combination of visceral obesity, increased blood pressure, insulin resistance and dyslipidaemia (2). MetS components seem to share a common underlying pathophysiology that may also contribute to the progression of atherosclerosis (3). MetS frequency is higher in Western countries and among people aged 40-80, and its prognostic implications are considerable: people with MetS have a doubled risk of dying of cardiovascular diseases and a tripled risk to suffer from heart attack or stroke, as well as all-cause mortality (4).
Anxiety and depressive disorders are common among patients diagnosed with cancer, as well as among patients affected by MetS (5,6). Such disorders are related to extreme distress, low self-perceived health-related quality of life and significant economic costs. Moreover, according to recent studies, personality traits could be considered psychological risk factors for CRC, in addition to environmental and social risk factors (7). Psychological traits may also contribute to MetS, suggesting a mediation by reciprocal risk factors, both behavioural such as eating habits, physical inactivity and smoking, and biological such as inflammation and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (8).
Elevated levels of Myeloperoxidase (MPO), a member of the superfamily of heme-peroxidases, are associated with inflammation and increased oxidative stress. Evidence suggest that MPO could be associated with increased risk of cardiovascular diseases, and might be a marker of colorectal cancer risk in normal colorectal mucosa (9).
In the light of the above, the psycho-neuro-endocrine-immune (PNEI) perspective could represent a useful and feasible model to understand the complex bio-psycho-social comorbidities between CRC, MetS, anxious-depressive symptoms, personality traits and inflammation. Therefore, aim of the present study was to describe a sample of outpatients undergoing colonoscopy, by adopting a PNEI perspective, to assess whether such an integrated approach could help understand the complex cross-talk between different risk factors and states of health and disease, driving the multi-assessment of patients in the clinical practice. In particular, we aimed to assess the coexistence of multidistrict low-grade inflammation with metabolic and psychological conditions.
Materials and Methods
Study design and sample
Cross-sectional study. Data were collected from 1 January 2015 to 30 June 2016. Patients were enrolled from a population referred to colonoscopy because of either a history or new evidence suspicious for colorectal neoplastic disease (e.g. positive fecal occult blood), or non-specific abdominal symptoms.
Statement of ethics
The study was approved by the competent Ethic Committee and the Local Health Agency of Modena. Every patient enrolled in the study signed a detailed written informed consent. The study was carried out according to the Declaration of Helsinki, to the Good Clinical Practice principles for medical research and to the current regulations relating to the protection and processing of personal and sensitive data (European Regulation n. 679/2016).
Data collection
For each patient, demographic (age, gender) and anthropometric characteristics (height, waist and hip circumference, in centimeters; body weight, in kilograms) were collected. BMI (body weight in kilograms/height in metres2) and waist-to-hip ratio were then calculated manually. Systolic and diastolic arterial blood pressure (mmHg) were measured by means of a manual sphygmomanometer at the right arm.
A detailed medical history was obtained for each patient, including on-going medications (psychotropic medication: antidepressant and/or anxiolytic drug therapy; cholesterol-lowering medications: statins). Dichotomous data (yes/no) on alcohol consumption, smoking habit and sedentary lifestyle were also collected. Finally, the Cumulative Illness Rating Scale (CIRS) was calculated for each patient to assess multimorbidity.
Results were then collected of the following assessments and laboratory analyses:
1) Immunofluorescence by confocal microscopy on colonic biopsies
Three samples of normal colorectal mucosa (NM) were collected during colonoscopy (right colon, descending colon, and sigmoid colon-rectum).
The samples of NM collected, were fixed in 3% formalin and embedded in paraffin for immunofluorescence analysis. The immunofluorescence experiments were performed as previously described (10). To each sample was assigned a code number and the score, referred to as Immuno Fluorescence Intensity Score (IFIS), was determined by an observer who was blind to tissue groups during the analysis (10). Data were expressed as a mean of the IFIS in the three segments of the intestine collected for each patient as previously described (11).
Blood samples were drawn before performing colonoscopy, immediately processed for serum extraction by centrifugation and stored frozen at -80 °C until test execution. The following biochemical analyses were performed: high sensitive (hs) CRP (mg/L); glycaemia (mg/dL), total cholesterol (mg/dL), low-density lipoprotein (LDL) cholesterol (mg/dL), high-density lipoprotein (HDL) cholesterol (mg/dL), very low-density lipoprotein (VLDL) cholesterol (mg/dL), triglycerides (mg/dl). Serological analyses were performed on a platform validated for clinical purposes (Roche Diagnostics S.p.A., Cobas® System, Monza, Italy). Serological and immunohistochemistry tests were performed in blind for anthropometric data and other clinical records.
2) Enzyme Linked ImmunoSorbent Assays (ELISA)
A highly sensitive quantitative sandwich enzyme immunoassay technique, Quantikine® HS ELISA KIT (R& D systems, Minneapolis, USA), was used to measure circulating serum levels of IL-6 in accordance with the manufacture’s instruction starting from 200 μL of diluted sera or standard solutions. After adding Stop Solution to each well and the optical density was measured by using a Thermo Scientific Multiskan® FC microplate reader, at 490 and 690 nm.
IL-1 levels were measured by pre-coated ELISA using Boster Human IL-1 Picokine TM ELISA KIT according to the manufacturer’s instruction staring from 100 µL of standard solutions, samples (dil. 1:3) or control. The reaction was stopped by adding 100 µL of Stop Solution and O.D. absorbance read by using the microplate reader, at 450 nm. IL-1 was decantable in 18 out of 62 patients.
TNF-and IFN-levels were also measured in the serum of patients by using comedically available highly sensitive Picokine ELISA KITs, but the cytokine levels were under the limit of detection of these KITs.
3) Ultrasound vascular evaluation
To measure early atherosclerosis, an ultrasound technique of intima-media thickening measurement of the common carotid artery wall (qIMT, carotid intima-media thickness) was used. Enrolled patients underwent ultrasound examination on an Esaote MyLab25 platform (Esaote Medical Systems, Italy) using a high-resolution 7–13 MHz linear-array transducer (LA523) by an experienced angiographer. The system employed dedicated software RF-tracking technology to obtain RF Quality IMT (RF-QIMT). The sonographer was blinded to the patients’ results of the other tests performed during this study. Image acquisition included the evaluation of the right and left common carotid arteries (LS-qIMT, left side carotid intima-media thickness; RS-qIMT, right side carotid intima-media thickness), 1 cm proximal to the carotid bulb. All measurements were taken in the supine position with patient at rest and comfortable. The thickness of the intima-media layer was expressed in micrometer with a minimum resolution of 12 micrometers, and the result accepted when the standard deviation of the multiple automated acquisitions was within 20 micrometers.
To measure endothelial function, we used flow-mediated dilation technique executed on the brachial artery in the non-dominant arm. Brachial flow-mediated dilation (brachial FMD) was performed according to the literature (12). Brachial artery diameter measurements were performed on a segment of about 2 cm in length. These were performed at baseline, 60 seconds after hyperemia, and 3 minutes after hyperemia. The hyperemia (endothelium-dependent dilation) is induced by the inflation of an adult cuff of a common sphygmomanometer for blood pressure measurement. The cuff is positioned in the proximal portion of the arm to stop the flow of the artery and it is kept for 5 minutes. Brachial artery diameter was expressed in millimeters with two decimals. The flow mediated dilation (FMD) is defined as the maximum percentage increase in the artery diameter during hyperemia following the release of the pressure in the cuff, and the subsequent reperfusion of the artery.
4) Psychometric assessment
Patients were asked to fill in the following 4 self-administered psychometric instruments:
The Hospital Anxiety and Depression Scale (HADS), Italian version: rating scale made of 7 questions for anxiety and 7 for depression, developed to assess the presence and severity of depressive and/or anxious symptomatology in the week before administration. Although originally designed to be used with hospital populations, it has been found to be a valid and reliable instrument among medical and/or psychiatric outpatients and for screening procedures; clinically-significant symptoms of anxiety and/or depression are suggested by a score of 8 or more on each subscale;
The Temperament and Character Inventory (TCI), Italian version: 240-items test for personality traits to assess the intensity of and the relationships between the 4 basic dimensions of temperament (Novelty Seeking (NS), Harm Avoidance (HA), Reward Dependence (RD) and Persistence (P)) and the 3 dimensions of character (Self-Directedness (SD), Cooperativeness (C) and Self-Transcendence (ST)). It is based on a psychobiological model that attempts to describe and explain the causes of individual differences in personality traits;
The INTERMED (INTERdisciplinary MEDicine) Self-Assessment (IMSA): it is a recently developed tool, validated in the Italian language, consisting in 27 multiple-choice questions and aiming at measuring bio-psycho-social complexity impacting on organization of care;
The 36-item Short Form Survey (SF-36), Italian version: assess patients’ perception of own general health status, consisting in 8 scaled scores, each exploring one of the following domains: vitality, physical functioning, bodily pain, general health perceptions, mental health, physical role functioning, emotional role functioning, social role functioning.
Statistics
All data were recorded anonymously in a Microsoft Excel sheet and further analyzed by means of STATA 13.1 (College Station, TX, USA). Descriptive statistics were performed by means of means, frequencies, standard deviations and ranges. Correlation analyses between variables were performed by means of Pearson’s coefficient (r). To reduce the risk of type I error, q-value throughout Holm’s correction were calculated. Level of statistical significance was set at alpha = 0.05. Finally, simple and multiple binary logistic regressions models were run. The outcome was represented by a binary variable, equal to 1 when at least one adenoma was detected at the colonoscopy, 0 otherwise. Explanatory variables were all those collected from anamnesis and physical examination, colonoscopy, immunofluorescence by confocal microscopy on colonic biopsies, ELISA analysis, ultrasound vascular evaluation, and psychometric assessment. A stepwise multiple regression analysis was run, including all those variables that had reached a P < .25 level of statistical significance (the latter set to reduce type II error). Differently, in the multivariate regression analysis the usual level of significance (P < .05) was set.
Results
Descriptive analysis
Sixty-two patients were recruited (31 women, 50%, and 31 men, 50%) with a mean age of 60.8±9.4 years. Table 1 displays the absolute (on the left) and gender-related (on the right) prevalence in the sample of risk factors and/or diseases, as dichotomous variables. Twenty-two subjects (35.5%) were current smokers, 42 (67.7%) declared to generally drink alcoholic beverages and 25 (40.3%) admitted having a sedentary lifestyle. Only 3 patients (4.8%) currently used antidepressants and 4 (6.5%) anxiolytic medications; 15 patients (24.2%) currently used statins.
Table 1.
General features of the sample (dichotomous variables).
| Dichotomous Variable | Number | % | Missing | Variable | M/F (%) |
| Sex M/F | 31/31 | 50/50 | 0 | Presence of adenomas | 19/9(67.9/32.1) |
| Smoke Y/N | 22/40 | 35.5/64.5 | 0 | MetS negative | 16/20 (44.4/55.6) |
| Alcohol Y/N | 42/20 | 67.7/32.3 | 0 | ||
| Sedentary Y/N | 25/37 | 40.3/59.7 | 0 | ||
| Adenoma Y/N | 28/34 | 45.2/54.8 | 0 | Absence of adenomas | 13/22(39.4/71.0) |
| MetS Y/N | 26/36 | 41.9/58.1 | 0 | MetS positive | 15/11(57.7/42.3) |
| Antidepressants Y/N | 3/59 | 4.8/95.2 | 0 | ||
| Anxiolytics Y/N | 4/58 | 6.5/93.5 | 0 | ||
| Statins Y/N | 15/47 | 24.2/75.8 | 0 | ||
| HADS-A pos/neg | 16/33 | 32.7/67.3 | 13 | ||
| HADS-D pos/neg | 9/40 | 18.4/81.6 | 13 | ||
| HADS-AD pos/neg | 4/45 | 8.2/91.8 | 13 | ||
| IMSA pos/neg | 0/28 | 0/100 | 34 |
List of abbreviations: HADS, Hospital Anxiety and Depression Scale; A, anxiety; D, depression; IMSA, InterMed Self-Assessment
Twenty-eight participants were affected by at least one adenoma (45.2% of the total sample); in this group, 19 (67.9%) were men. MetS was diagnosed in 26 participants (41.9% of the sample, according to the ATP-III-R criteria). Clinically significant symptoms of anxiety were reported by 16 patients (32.7%); 9 individuals reported the presence of depressive symptoms (18.4%), and 4 presented combined anxiety-depressive symptoms (8.2%).
Figure 1 displays the prevalence and comorbidity of adenomas, MetS and symptoms of anxiety and/or depression, among the 49 participants for whom all variables were collected and available. The intersections between the closed curves show the areas of comorbidity between different diseases. In particular, 1 patient was simultaneously affected by all these conditions (adenoma, MetS, symptoms of anxiety and depression); 3 patients by both anxiety and adenoma; 1 patient by adenoma and comorbid anxious-depressive symptomatology; 2 patients by adenoma, MetS and symptoms of anxiety; and 1 patient by adenoma, MetS and depression.
Figure 1.
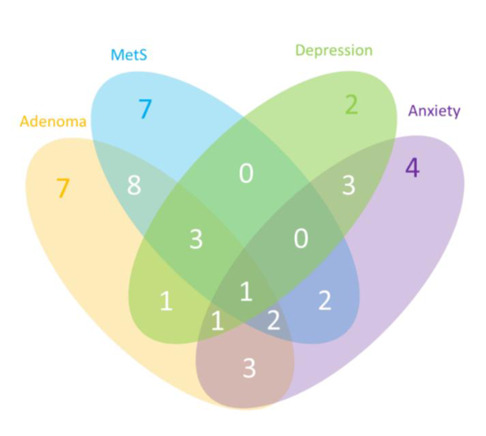
Venn Diagram. Prevalence of adenoma, Metabolic Syndrome, symptoms of anxiety and symptoms of depression, and their comorbidity, among 49 patients of the sample. Abbreviation: MetS=Metabolic Syndrome.
Demographic, anthropometric, and clinical characteristics of the patients, as continuous variables, are displayed in Table 2.
Table 2.
General features of the sample (continuous variables).
| Continuous Variable | Size | Missing | Mean | Mean | Mean | Min |
| Age in years | 62 | 0 | 60.8 | 9.4 | 82 | 44 |
| BMI Kg(m-2) | 62 | 0 | 27.3 | 4.6 | 38.1 | 18.8 |
| w/h ratio | 62 | 0 | 0.9 | 0.1 | 1.2 | 0.8 |
| SBP mmHg | 62 | 0 | 146.0 | 18.2 | 180 | 110 |
| DBP mmHg | 62 | 0 | 83.2 | 10.3 | 105 | 60 |
| Glyc mg/dl | 62 | 0 | 94.1 | 18.3 | 194 | 64 |
| Chol tot mg/dl | 62 | 0 | 196.2 | 19.2 | 241 | 156 |
| HDL mg/dl | 61 | 1 | 49.9 | 10.8 | 77 | 32 |
| LDL mg/dl | 61 | 1 | 119.9 | 16.7 | 154 | 84 |
| VLDL mg/dl | 62 | 0 | 26.6 | 9.1 | 44 | 11 |
| TRGL mg/dl | 62 | 0 | 132.7 | 45.3 | 220 | 57 |
| RS-qIMT | 57 | 5 | 817.8 | 286.4 | 1500 | 434 |
| LS-qIMT | 57 | 5 | 841.2 | 305.0 | 2000 | 503 |
| FMD basal-1 min % | 56 | 6 | 9.6 | 5.6 | 21.1 | 0.0 |
| FMD basal-3 min % | 56 | 6 | 8.6 | 5.9 | 20 | -7.3 |
| FMD 1 min -3 min % | 56 | 6 | -1.3 | 6.2 | 15.5 | -22.5 |
| HADS-A | 49 | 13 | 5.7 | 3.9 | 15 | 0 |
| HADS-D | 49 | 13 | 4.4 | 3.1 | 11 | 0 |
| TCI NS | 43 | 19 | 22.0 | 3.8 | 29 | 15 |
| TCI HA | 43 | 19 | 18.4 | 2.9 | 24 | 10 |
| TCI RD | 43 | 19 | 11.1 | 2.7 | 18 | 6 |
| TCI P | 43 | 19 | 5.4 | 1.6 | 8 | 3 |
| TCI SD | 43 | 19 | 26.4 | 3.9 | 34 | 18 |
| TCI C | 43 | 19 | 21.9 | 4.6 | 31 | 12 |
| TCI ST | 43 | 19 | 20.4 | 3.5 | 28 | 12 |
| SF36 PCS | 49 | 13 | 47.5 | 7.9 | 58.8 | 26.1 |
| SF36 MCS | 49 | 13 | 51.3 | 8.8 | 66.1 | 22.8 |
| SF36 CS | 49 | 13 | 2.8 | 0.9 | 1 | 4 |
| IMSA | 28 | 34 | 7.8 | 4.2 | 3 | 18 |
| hs-CRP (mg/L) | 62 | 0 | 0.6 | 0.4 | 2.1 | 0.2 |
| IL-6 (pg/ml) | 49 | 13 | 0.8 | 0.8 | 5.0 | 0.2 |
| IL-1 (pg/ml) | 18 | 44 | 6.0 | 9.3 | 41.7 | 0.8 |
| MPO mean | 62 | 0 | 60.9 | 8.9 | 80.7 | 37.3 |
| CIRS | 62 | 0 | 0.2 | 0.1 | 0.5 | 0 |
As to cardiometabolic parameters, the mean BMI of the sample was 27.3 kg/m2 (SD=4.6 kg/m2); mean Systolic Blood Pressure was 146.0 mmHg (SD=18.2 mmHg), mean Diastolic Blood Pressure was 82.2 mmHg (SD=10.3 mmHg). The mean level of blood glycaemia was 94.1 mg/dl (SD=18.3 mmHg), mean total cholesterol was 196.2 mg/dl (SD=19.2 mg/dl). RS q-IMT was 817.8 micrometers (SD=286.4 micrometers), while LS q-IMT was 841.2 micrometers (SD=305.0 micrometers).
As to results of psychometric tests, data are less complete (13 missing data for HADS and SF36; 19 for TCI; 34 for IMSA). Mean scores at the HADS were 5.7±3.9 for anxiety and 4.4±3.1 for depression. TCI and SF36 scores both demonstrate a complete range of different psychological profiles and conditions (different dimensions at TCI), as indicated by the mean values as well as by standard deviations and ranges.
Finally, as to parameters of inflammation, the hs-CRP mean value was 0.6 mg/dL, indicating a generally low level of inflammation. While TNF-and INF-levels were undetectable in the serum of patients, IL-1 mean level (available for 18 subjects) was 6.0 pg/mL in 18 out of 62 patients and IL-6 mean level was 0.8 pg/mL (available for 49 subjects). MPO mean value throughout colorectal mucosa was 60.9 (SD=8.9, available for 62 subjects).
Correlation analysis
The presence of adenomas was associated with male sex (r=0.32; p=0.01), age (r=0.34, p<0.01), IL-6 (r=0.31; p=0.03), hsCRP (r=0.27; p=0.04) and diagnosis of MetS (r=0.28; p=0.03).
MetS was associated with age (r=0.32; p<0.001), IL-6 (r=0.37; p<0.01), hsCRP (r=0.54; p=0.00), presence of adenomas (r=0.27; p=0.03) and CIRS (r=0.56; p=0.00).
Both HADS-A (r=-0.65; p=0.00) and HADS-D (r=-0.45, p<0.01) scores were associated with SF36 MCS; HADS-D was also associated with SF36 PCS (r=-0.39, p<0.01). An ongoing antidepressant therapy was associated with diastolic blood pressure (r=0.26, p=0.04), glycemia (r=0.38, p<0.01) and RS-qIMT (r=0.37, p<0.01). Various inter-correlations related to temperament dimensions as measured by the TCI were also found. Novelty Seeking was associated with Harm Avoidance (r= 0.40, p<0.01) and Self-Transcendence (r=0.34, p=0.03); Self-Directedness was associated with Cooperativeness (r= 0.63, p<0.01) and Self-Transcendence (r=0.55, p<0.01); Cooperativeness was associated with Self-Transcendence (r=0.62, p<0.01). Novelty Seeking was associated with right qIMT (r=-0.31, p=0.04); Harm Avoidance was associated with right qIMT (r=-0.56, p<0.01); Reward Dependence was associated with weight (r=-0.51, p<0.05), height (r=-0.55, p<0.01), BMI (r=-0.35, p=0.02), waist circumference (r=-0.36, p=0.02) and presence of medical comorbidities (CIRS score) (r=0.38, p=0.01); Persistence was associated with weight (r=0.35, p=0.03) and height (r=-0.34, p=0.03); Cooperativeness was associated with statin therapy (r=-0.31, p=0.03). Novelty Seeking was associated with SF36 MCS (r=-0.43, p<0.05), Harm Avoidance was associated with SF36 PCS (r=0.30, p<0.05), and finally Cooperativeness was associated with SF36 MCS (r=-0.31, p<0.05).
In addition to the observed association with the presence of adenomas and the diagnosis of MetS, hsCRP resulted directly related to cholesterol values (total cholesterol: r= 0.42; p<0.01; LDL cholesterol: r=0.32; p= 0.01; VLDL cholesterol: r=0.26; p= 0.04), triglycerides (r= 0.27; p= 0.04), BMI (r=0.38, p<0.01), weight (r=0.39; p<0.01), waist (r=0.46, p<0.01) and hip circumferences (r=0.33; p<0.01) and to waist-to-hip ratio (r=0.39, p<0.01). Moreover, PCR was related to both diastolic (r=0.29, p=0.02) and systolic blood pressure (r=0.29, p=0.02), sedentary lifestyle (r=0.26, p=0.04) and CIRS (r=0.47, p<0.01).
A few correlations were detectable also with respect to results at the ultrasound vascular examination (IMT): RS-qIMT and LS-qIMT resulted to directly covariate (r=0.33, p<0.01), though in few cases different correlations have been found between a variable and RS-qIMT or LS-qIMT. Moreover, both right and left side qIMT resulted directly related to cholesterol values and particularly to LDL cholesterol (RS-qIMT: r=0.40, p<0.01; LC-IMT: r=0.37, p<0.01). qIMT resulted to be directly related to BMI, weight, glycaemic values, and MetS. Higher values of qIMT were significantly associated with the presence of one or more adenomas in the colonic tract (RS-qIMT: r=0.30, p=0.03; LS-qIMT: r=0.33, p=0.01). Mean MPO and LS-IMT were inversely related (r=-0.28; p=0.03).
After Holm’s correction, the following correlations remained significant: sex-BMI (q-value: 0.04); age-TCIHA (q-value: 0.05); age-smoke (q-value: 0.003); MetS-WHR (q-value: 0.05); MetS-SBP (q-value: 0.03); MetS-glycemia (q-value: 0.02); HADSD-SF36MCS (q-value: 0.04); TCIHA-RSqIMT (q-value: 0.003); TCISD-TCIST (q-value: 0.007); SF36PCS-sedentary life style (q-value: 0.007); BMI-SBP (q-value: 0.01); BMI-DBP (q-value: 0.04); BMI-CIRS (q-value: 0.009); WHR-DBP (q-value: 0.03); WHR-hsCRP (q-value: 0.05); FMD1min-FMD1-3min (q-value: 0.01); FMD3min-FMD1-3min (q-value: 0.006); CIRS-hsCRP (q-value: 0.003).
The full results of the correlation analysis are available from the corresponding author on request.
Binary logistic regression analysis
Table 3 shows the variables that had reached a P < .25 level of statistical significance at the simple regression analysis. The stepwise regression analysis carried out on the list of variables reported in Table 3 pointed out that only increased cholesterol levels were associated with increased presence of colorectal adenomas (aOR=1.07, p<0.01, 95%CI= 1.02; 1.10). Notably, a 10% significance level was reported by the association between IMTSX and outcome (aOR= 1.00, p= 0.09, 95%CI 1.00-1.04).
Table 3.
Variables included in the stepwise logistic regression (observations: 37; pseudo-R squared: 0.32).
| Variable | Odds Ratio | P-value | 95%CI |
| Diagnosis of MetS | 0.13 | 0.16 | 0.01; 2.17 |
| Cholesterol levels | 1.06 | 0.05 | 9.99; 1.13 |
| IMT (left) | 1.00 | 0.10 | 0.99; 1.01 |
| CIRS-SI | 0.90 | 0.92 | 0.12; 6.92 |
| HADS-D | 0.97 | 0.87 | 0.67; 1.41 |
| SF36- MCS | 1.05 | 0.48 | 0.92; 1.20 |
| hsCRP | 6.96 | 0.15 | 0.50; 96.53 |
| IL6pgml1 | 1.30 | 0.88 | 0.05; 35.68 |
Discussion
The sample enrolled in the present study was consistent with the adult western population undergoing first-line diagnostic procedures: a quarter of the sample were currently following a statin prescription, suggesting that, despite an average level of cholesterol of almost 200 mg/dl, hypercholesterolemia was rather common, in line with Italian prevalence data (13). Psychological symptoms were generally low, as expected in a non-selected population, with mean scores at the HADS largely below the cut-off of clinical significance. MetS was suggested to be related to several of the other measures collected, and specifically: circulating levels of IL-6 and hsCRP, a well-known marker of systemic inflammation; carotid IMT, a relevant indicator of early atherosclerosis and powerful predictor of coronary artery disease and stroke (9); score at the CIRS, a significant proxy of bio-psycho-social complexity; and, finally, presence of at least one adenoma in the large bowel.
Adenoma is a well-known step of the adenoma-carcinoma sequence. Epidemiological studies have demonstrated that colorectal adenomas and CRC risk are higher in individuals with MetS or any of its components. A recent meta-analysis of cohort studies reported that MetS was associated with higher relative risks of CRC both in men and in women (14). The association of MetS and obesity with intestinal adenomas was already observed in larger populations (4), suggesting that MetS could be considered in risk stratification for surveillance intervals for colonoscopy. Moreover, an interesting result is the significant correlation of hsCRP and IL-6 with both the presence of colorectal adenomas and MetS, suggesting that even a low grade of inflammation may be a useful indicator of those common conditions.
MetS and psycho-social issues may be associated either directly, throughout shared biological pathways, or indirectly. In fact, personality traits widely affect behaviour, leading individuals to have various levels of attention of good health and positive lifestyle, as well as different attitudes toward screening procedures (15). Health behaviors may also be affected by socio-economic conditions and environmental factor (16).
The presence of anxiety and depressive symptoms was associated with a generally worse health status, consistently with previous research (17). Intra-correlations between TCI dimensions were only small, but in line with previous data mainly supporting Cloninger’s theory of independent dimensions (18). In contrast with previous scientific findings, we could not replicate the presence of significant associations between personality traits and anxiety-depressive symptomatology, as initially hypothesised, though this could be due to methodological problems or sample size dimensions. The negative association between Cooperativeness and ongoing therapy with statins, even if with moderate effect size, is consistent with other findings in literature. Indeed, this dimension of personality relates to facets of agreeableness/hostility, and it was hypothesised that people with higher levels of hostility are more likely to have more cardiovascular risk factors, such as dyslipidaemia, hypertension, obesity and even MetS (18). It could be argued that this association is mediated by both behavioural factors, such as diet and smoking, and biological factors, namely HPA dysregulation, that leads angry and hostile individuals to have chronically higher levels of cortisol, cardiovascular and neuroendocrine reactivity (8).
This study has several limitations that need to be acknowledged. First, the cross-sectional observational nature of the study does not allow to establish a causal or etiological association between the collected variables. Second, no control group was included. Third, the sample size was small, and further limited by the fact that not every patient completed the psychometric questionnaires. Such features limit the generalizability findings; nevertheless, the sample was enrolled from outpatients performing screening procedures, thus minimizing selection bias from highly selected populations. Fourth, the psychopathological assessment was performed exclusively by means of self-reported questionnaires, limiting extent, reliability and depth of collected data; anyway, the tools were all validated and commonly used for similar aims. Despite all limitations, the present study represents a first approach to implement at the clinical and research level a multi-disciplinary, PNEI approach adopted, increasingly accepted as a validated paradigm in the scientific community. Further studies, with larger sample sizes and adopting a prospective design are currently on our research agenda, to improve the limitations of this research and provide more evidence able to sustain this approach.
Conclusion
This study suggested the usefulness of implementing a clinical multidimensional model in a PNEI perspective; such approach provided hints of evidence concerning connections among a proinflammatory atherogenic status, some psychological traits, increased mucosal inflammation and metabolic parameters in a sample of outpatients screened for colonic adenomas. Also, the findings of this study provide a preliminary support that colonic adenomas might arise from complex and interconnected clinical and psychopathological substrates in humans.
Acknowledgement:
Authors wish to thank all participants to the study for their time and collaboration. Particularly, we thank Anita Calvreo, Silvia Tassi, Serena Saraceni, Giacomo Galli, Andrea Fabbrizi for their contribution to data collection.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Funding Sources:
The authors received a Research grant by a Local bank (Fondazione Cassa di Risparmio di Vignola). The work was also supported by a local no-profit organization (Associazione per la Ricerca sui Tumori Intestinali (ARTI)). The funding sources had no involvement in any phase of the study.
References
- 1.Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci Basel Switz. 2018 Apr 13;6(2) doi: 10.3390/medsci6020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005 Jan;149(1):33–45. doi: 10.1016/j.ahj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Hess PL, Al-Khalidi HR, Friedman DJ, Mulder H, Kucharska-Newton A, Rosamond WR, et al. The Metabolic Syndrome and Risk of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2017 Aug 23;6(8) doi: 10.1161/JAHA.117.006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butnoriene J, Bunevicius A, Saudargiene A, Nemeroff CB, Norkus A, Ciceniene V, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol. 2015;190:360–6. doi: 10.1016/j.ijcard.2015.04.122. [DOI] [PubMed] [Google Scholar]
- 5.Mattei G, Padula MS, Rioli G, Arginelli L, Bursi R, Bursi S, et al. Metabolic syndrome, anxiety and depression in a sample of italian primary care patients. J Nerv Ment Dis. 2018 May;206(5):316–24. doi: 10.1097/NMD.0000000000000807. [DOI] [PubMed] [Google Scholar]
- 6.Rioli G, Tassi S, Mattei G, Ferrari S, Galeazzi GM, Mancini S, et al. The Association Between Symptoms of Anxiety, Depression, and Cardiovascular Risk Factors: Results From an Italian Cross-Sectional Study. J Nerv Ment Dis. 2019 May;207(5):340–7. doi: 10.1097/NMD.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 7.Marchi M, Mattei G, Mancini S, Roncucci L, Galeazzi GM, Ferrari S. Personality traits and physical activity may be involved in colorectal carcinogenesis: preliminary data from a cross-sectional study on patients undergoing colonoscopy. Minerva Psichiatr. 2019;60(2):100–1. [Google Scholar]
- 8.Goldbacher EM, Matthews KA. Are psychological characteristics related to risk of the metabolic syndrome? A review of the literature. Ann Behav Med Publ Soc Behav Med. 2007 Dec;34(3):240–52. doi: 10.1007/BF02874549. [DOI] [PubMed] [Google Scholar]
- 9.Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J Gastroenterol. 2014 Aug 7;20(29):9716–31. doi: 10.3748/wjg.v20.i29.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvi F, Miller MD, Grilli A, Giorgi R, Towers AL, Morichi V, et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008 Oct;56(10):1926–31. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 11.Sena P, Mancini S, Benincasa M, Mariani F, Palumbo C, Roncucci L. Metformin Induces Apoptosis and Alters Cellular Responses to Oxidative Stress in Ht29 Colon Cancer Cells: Preliminary Findings. Int J Mol Sci. 2018 May 16;19(5) doi: 10.3390/ijms19051478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertens Dallas Tex 1979. 2010 May;55(5):1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giampaoli S, Palmieri L, Donfrancesco C, Lo Noce C, Pilotto L, Vanuzzo D, et al. Cardiovascular health in Italy. Ten-year surveillance of cardiovascular diseases and risk factors: Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey 1998-2012. Eur J Prev Cardiol. 2015 Sep;22(2 Suppl):9–37. doi: 10.1177/2047487315589011. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Monforte M, Sánchez E, Barrio F, Costa B, Flores-Mateo G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2017 Apr;56(3):925–47. doi: 10.1007/s00394-016-1305-y. [DOI] [PubMed] [Google Scholar]
- 15.Forghieri M, Longo C, Galeazzi GM, Rigatelli M, Seidenari S, Pellacani G. The different psychological profiles of subjects attending melanoma screening campaigns and those delaying diagnosis: an aid for designing preventive campaigns? Eur J Dermatol. 2010 Dec;20(6):802–7. doi: 10.1684/ejd.2010.1103. [DOI] [PubMed] [Google Scholar]
- 16.Mattei G, De Vogli R, Ferrari S, Pingani L, Rigatelli M, Galeazzi GM. Impact of the economic crisis on health-related behaviors in Italy. Int J Soc Psychiatry. 2017 Aug 1;20764017726097 doi: 10.1177/0020764017726097. [DOI] [PubMed] [Google Scholar]
- 17.Miettunen J, Lauronen E, Kantojärvi L, Veijola J, Joukamaa M. Inter-correlations between Cloninger’s temperament dimensions— a meta-analysis. Psychiatry Res. 2008 Jul 15;160(1):106–14. doi: 10.1016/j.psychres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Sutin AR, Costa PT, Uda M, Ferrucci L, Schlessinger D, Terracciano A. Personality and metabolic syndrome. Age. 2010 Dec;32(4):513–9. doi: 10.1007/s11357-010-9153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


