Abstract
Background and aim:
Seasonal allergic rhinitis (SAR) is a common disease in childhood that is characterized by bothersome symptoms and impaired quality of life (QoL). As histamine is the pivotal pathogenic mediator in SAR, antihistamines are the first-line option in the treatment. Cetirizine is a well-known effective antihistamine. This real-life pilot study aimed to investigate the effectiveness of a 4-week continuous cetirizine treatment in a group of Italian children with SAR.
Methods:
Total symptom score (TSS) and the Pediatric Rhinoconjunctivitis Quality of Life Questionnaire (PRQLQ) were assessed at baseline and the end of the treatment.
Results:
Cetirizine significantly improved QoL (in all domains) and symptom severity (p<0.001 for both).
Conclusions:
The present preliminary study showed that a 4-week cetirizine treatment was able to improve QoL significantly. Cetirizine treatment also significantly reduced symptom severity in Italian children with SAR and was safe.
Keywords: seasonal allergic rhinitis, children, quality of life, symptoms, cetirizine
Introduction
Allergic rhinitis (AR) is a common disease as it may affect up to 30% of children worldwide (1). Type 2 inflammation, characterized by T helper 2 polarization, allergen-specific production, and eosinophilic mucosal infiltration, leads AR (2). Inflammatory events cause the occurrence of typical symptoms, including nasal and ocular symptoms, including itching, sneezing, watery rhinorrhoea, lacrimation, and obstruction, which is the most troublesome complaint and closely correlates with allergic inflammation (3). All these symptoms are sustained by the release, from activated mast cells, of histamine (4). Therefore, histamine is the pivotal mediator involved in AR, and its antagonism represents a first-line therapeutic option (5). Moreover, AR symptoms are usually very bothersome and irritant in children and consequently affect their quality of life (QoL) in a significant manner (6).
From a clinical point of view, AR is usually classified as seasonal (SAR) or perennial (PAR), considering the symptom duration and the causal allergen. SAR significantly affects nasal function and is burdened by relevant comorbidity (6,7).
SAR treatment consists mostly of antihistamines and intranasal corticosteroids, even though antihistamines are favorite in childhood (1). In this regard, cetirizine is a second-generation antihistamine that is effective and safe in clinical practice from 30 years (8). Also, long-term cetirizine therapy exerts optimal outcomes in children (9). There is evidence that cetirizine is effective in children with SAR (10) and improves QoL in children and adults (11,12). However, these studies were conducted in the United States. Thus, the characteristics of the patients are different from the national ones, mainly concerning the type of allergens, the duration of pollination, the socio-cultural issues, and dietetic behaviors that may affect gut and airway microbioma (13). Therefore, the current multicentric study was designed to evaluate the effects of long-term cetirizine treatment on symptoms and QoL in a group of Italian children with SAR.
Methods
The present observational retrospective open study was a 4-week polycentric trial, including 62 patients suffering from SAR. SAR diagnosis was performed, according to validated criteria (14), such as if nasal symptom history was consistent with documented sensitization.
Inclusion criteria were: age range 6-12 years, SAR diagnosis, Total Symptoms Score (TSS) ≥ 15, written informed consent of patients and parents or legal guardians. Exclusion criteria were: PAR, uncontrolled asthma, secondary rhinitis to other causes, concomitant acute or chronic rhinosinusitis, nasal polyps, current use of topical or systemic corticosteroids, antihistamines, antileukotrienes, inadequate washout of them, nasal anatomic defect, respiratory infections in the last two weeks, participation in other clinical studies in the last month, documented hypersensitivity to the study product or its excipients, and trip planned outside of the study area.
The study was conducted in five third-level pediatrics department. Both parents of each child gave informed written consent. Each Ethics Committee approved the study procedure (ClinicalTrials.gov ID NCT03365648).
The treatment consisted of the administration of cetirizine 5 mg/twice daily for four weeks.
Systemic or intranasal corticosteroids, leukotriene antagonists, and sodium cromoglicate were prohibited during the study. Two visits were performed: Visit 1 at baseline (W0) and Visit 2 after 4 weeks, i.e., end of treatment (W4).
The primary endpoint of this study was the QoL change from the baseline to the end of the treatment (4 weeks). The secondary objective was the total symptom score (TSS) change from W0 to W4.
The validated Pediatric Rhinoconjunctivitis Quality of Life Questionnaire (PRQLQ) consists of 23 questions in 5 domains (nasal symptoms, ocular symptoms, practical issues, limitation of activities, other symptoms), that are answered on a 7-point scale (0-6), where 0 represents the absence of problems and 6 the most significant symptom distress (15). Children will complete the questionnaire together with a parent at baseline and Visit 2. A Total Score was calculated as the mean of the five domains.
TSS was the sum of 3 domains:
Nasal symptoms (TNSS) included itching, sneezing, rhinorrhea, nasal congestion.
Ocular symptoms (TOSS): itching, hyperemia of the conjunctiva, tearing
Throat symptoms (TTSS): itching, coughing.
With the help of their parents, patients scored symptoms severity on a 4-point scale: 0 = absent or irrelevant, 1 = mild, 2 = moderate, 3 = severe.
Safety will be assessed on the incidence of adverse events for each treatment and physical examination.
The analysis was performed using a t-test for independent samples.
Results
Globally, 62 children (34 males and 28 females, aged 6-12 years, mean age 9.1 ± 1.88) were enrolled. Demographic and clinical data, including the type of pollen allergy, are reported in Table 1.
Table 1.
Demographic and clinical characteristics of the patients.
| Mean age (years) | 9.1 ± 1.88 |
| Males | 34 |
| Females | 28 |
| Allergy to Gramineae | 40 |
| Allergy to Parietaria | 32 |
| Allergy to Betulaceae | 19 |
| Allergy to Oleaceae | 8 |
| Allergy to Compositeae | 4 |
The total score of QoL was 2.0 (± 0.88) at baseline and 1.3 (± 0.87) at W4; there was a significant (p<0.001) difference between the two visits (Figure 1). The data concerning the single domains are reported in Table 2; there was a significant reduction for all domains (p<0.001 for all).
Figure 1.
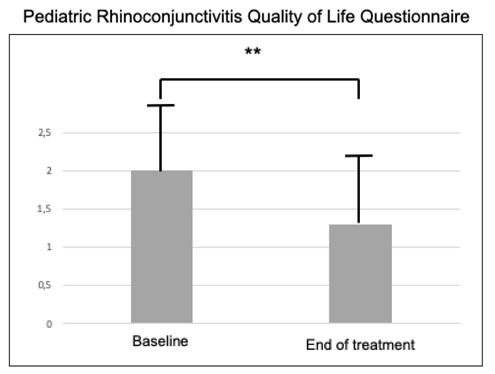
Total Score of QoL at baseline and after the treatment.
** = p<0.001
Table 2.
Domains of the Pediatric Rhinoconjunctivitis Quality of Life Questionnaire (PRQLQ) at the baseline and the end of treatment.
| Domain | Baseline | End of treatment |
| Nose symptoms | 2.91 ± 1.12 | 1.84 ± 1.32** |
| Eye symptoms | 1.52 ± 1.35 | 0.74 ± 1.03** |
| Practical problems | 2.24 ± 1.02 | 1.56 ± 1.04** |
| Other symptoms | 1.68 ± 1.18 | 1.18 ± 1.08** |
| Activity limitations | 1.79 ± 1.32 | 1.18 ± 1.16** |
** p<0.001
The TSS at baseline was 16.1 (± 1.2). After treatment, TSS significantly (p<0.001) diminished to 6.39 (± 4.38), as reported in Figure 2. The data concerning the single domains are reported in Table 3; there was a significant reduction for all domains (p<0.001 for all).
Figure 2.
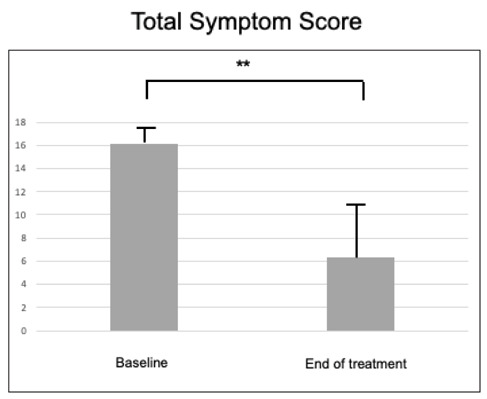
Total Symptom Score at baseline and after the treatment.
** = p<0.001
Table 3.
Domains of the single and total symptom score domains at the baseline and the end of treatment.
| Domain | Baseline | End of treatment |
| TNSS | 10.6 ± 0.5 | 3.98 ± 2.76 ** |
| TOSS | 3.7 ± 0.3 | 1.38 ± 1.02 ** |
| TTSS | 1.8 ± 0.4 | 1.03 ± 0.6 ** |
| TSS | 16.1 ± 1.2 | 6.39 ± 4.38 ** |
** = p<0.001
The cetirizine treatment was well tolerated, and no clinically-relevant adverse event was reported.
Discussion
SAR’s clinical relevance depends on several issues, including bothersome complaints, negative impact on personal and social quality of life, frequent comorbidities, familiar involvement, and the socio-economic burden, including the medicine costs and school absenteeism.
The current study showed that a 4-week continuous cetirizine treatment was able to improve QoL and reduce symptom severity significantly. In particular, cetirizine significantly affected all QoL domains. This finding is particularly interesting if contextualized in the global concept of QoL that embraces all the aspects of daily life. Moreover, the treatment was safe and well-tolerated.
These outcomes are consistent with the previous studies that demonstrated the effectiveness of cetirizine in improving SAR symptoms (10) and QoL (11,12). The present study adds the novelty that has been conducted in more Italian pediatric allergy centers and in a real-life setting, such as enrolling consecutive outpatients.
The present study has some limitations, including the open design, the assessment of clinical data alone, the lack of mediators’ assessment, and the sample size was not calculated for statistical purposes. The lack of a control group introduced an important bias, so the results should be considered only preliminary. On the other hand, the current study was performed in a real-world setting. More and more attention is paid to the real-world studies as they may provide information more adherent to the daily practice that randomized controlled trial that involves selected patient populations that rarely mirror the real situation (16,17).
In conclusion, the present pilot study showed that a 4-week cetirizine treatment was able to significantly improve QoL and reduce symptom severity in Italian children with SAR and was safe.
Conflict of interest:
all authors declare that have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Financial disclosure:
All the authors state that there is no conflict of interest. There was no sponsorship. Dompé Farmaceutici SpA, Milan, Italy, provided an unrestrictional grant to cover the costs of publication.
References
- 1.Licari A, Votto M, Brambilla I, Castagnoli R, Piccotti E, Olcese R, et al. Allergy and asthma in children and adolescents during the COVID outbreak: What we know and how we could prevent allergy and asthma flares. Allergy. 2020 May 17 doi: 10.1111/all.14369. 10.1111/all.14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen T, Rothenberg ME. Cell-by-cell Deciphering of T Cells in Allergic Inflammation. J Allergy Clin Immunol. 2019;144:1143–8. doi: 10.1016/j.jaci.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciprandi G, Cirillo I, Klersy C, Marseglia GL, Caimmi D. Vizzaccaro A Nasal obstruction is the key symptom in hay fever patients. Otolaryngol Head Neck Surg. 2005;133:429–35. doi: 10.1016/j.otohns.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 4.Fein MN, Fischer DA, O’Keefe AW, Sussman GL. CSACI Position Statement: Newer Generation H 1-antihistamines Are Safer Than First-Generation H 1-antihistamines and Should Be the First-Line Antihistamines for the Treatment of Allergic Rhinitis and Urticaria. Allergy Asthma Clin Immunol. 2019;15:61. doi: 10.1186/s13223-019-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciprandi G, Klersy C, Cirillo I, Marseglia GL. Quality of Life in Allergic Rhinitis: relationship with clinical, immunological, and functional aspects. Clin Exp Allergy. 2007;37:1528–35. doi: 10.1111/j.1365-2222.2007.02809.x. [DOI] [PubMed] [Google Scholar]
- 6.Ciprandi G, Cirillo I, Vizzaccaro A, Milanese M, Tosca MA. Nasal obstruction in patients with seasonal allergic rhinitis: relationships between allergic inflammation and nasal airflow. Intern Arch Allergy Immunol. 2004;134:34–40. doi: 10.1159/000077531. [DOI] [PubMed] [Google Scholar]
- 7.Cirillo I, Marseglia GL, Klersy C, Ciprandi G. Allergic patients have more numerous and prolonged respiratory infections than nonallergic subjects. Allergy. 2007;62:1087–90. doi: 10.1111/j.1398-9995.2007.01401.x. [DOI] [PubMed] [Google Scholar]
- 8.Corsico A, Leonardi S, Licari A, Marseglia GL, Miraglia del Giudice M, Peroni DG, et al. Focus on the cetirizine use in clinical practice: a reappraisal 30 years later. Multidisciplinary Respiratory Medicine. 2019;14:40. doi: 10.1186/s40248-019-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciprandi G, Tosca M. Long-term cetirizine treatment reduces allergic symptoms and drug prescriptions in children with mite allergy. Ann Allergy Asthma Immunol. 2001;87:222–6. doi: 10.1016/S1081-1206(10)62230-2. [DOI] [PubMed] [Google Scholar]
- 10.Nayak AS, Berger WE, LaForce CF, Urdaneta ER, Patel MK, Franklin KB, et al. Randomized, placebo-controlled study of cetirizine and loratadine in children with seasonal allergic rhinitis. Allergy Asthma Proc. 2017;38:222–30. doi: 10.2500/aap.2017.38.4050. [DOI] [PubMed] [Google Scholar]
- 11.Gillman SA, Blatter M, Condemi JJ, Collins M, Olufade AO, Leidy NK, et al. The health-related quality of life effects of once-daily cetirizine HCl syrup in children with seasonal allergic rhinitis. Clin Pediatr. 2002;41:687–96. doi: 10.1177/000992280204100908. [DOI] [PubMed] [Google Scholar]
- 12.Noonan MJ, Raphael GD, Nayak A, Greos L, Olufade AO, Leidy , et al. The health-related quality of life effects of once-daily cetirizine HCl in patients with seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial. Clin Exp Allergy. 2003;33:351–8. doi: 10.1046/j.1365-2222.2003.01596.x. [DOI] [PubMed] [Google Scholar]
- 13.Jares EJ, Badellino HA, Ensina LF. Registries as useful tools in characterization of allergic manifestations. Curr Opin Allergy Clin Immunol. 2016;16:250–6. doi: 10.1097/ACI.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 14.Karatzas K, Katsifarakis N, Riga M, Werchan B, Werchan M, Berger U, et al. New European Academy of Allergy and Clinical Immunology Definition on Pollen Season Mirrors Symptom Load for Grass and Birch Pollen-Induced Allergic Rhinitis. Allergy. 2018;73:1851–9. doi: 10.1111/all.13487. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;4:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 16.Use of real-world evidence to support regulatory decision-making for medical devices. Guidance for industry and Food and Drug Administration staff document issued on August 31, 2017. Bethesda: US Food and Drug Administration, US Department of Health and Human Services Food and Drug Administration, Center for Devices and Radiological Health Center for Biologics Evaluation and Research. 2017 [Google Scholar]
- 17.Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375:2293–7. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]


