Abstract
BACKGROUND AND PURPOSE: Among the several reports of elastase-induced aneurysm models, only the rabbit common carotid artery (CCA) model has been used for testing endovascular occlusion devices. Our purpose was to study the growth characteristics of an elastase-induced aneurysm model in rabbits for the purpose of determining whether delayed aneurysm enlargement occurs after creation.
METHODS: Nine New Zealand White rabbits (3–4 kg) were used in this study. All study animals underwent surgery to isolate the right CCA. In three control animals, the lumen was incubated with saline and iodinated contrast material for 20 minutes. In six test animals, the lumen of the CCA was incubated with porcine elastase for 20 minutes. In all study animals, the distal right CCA was ligated. IV digital subtraction angiography was performed on postprocedural days 3, 5, 7, 14, 28, 35, 56, 84, and 112. Using an external sizing reference, the width and height of patent arterial segments at the right CCA origin were measured by two observers. For test animals, aneurysm dimensions were compared between early and late time points by using the Student's t test.
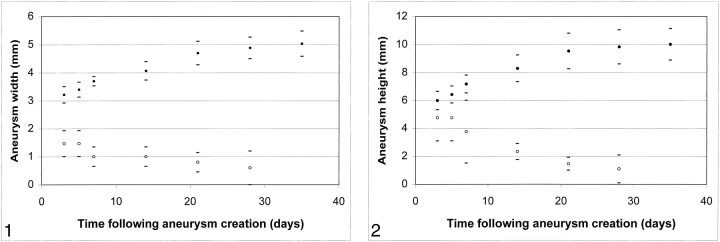
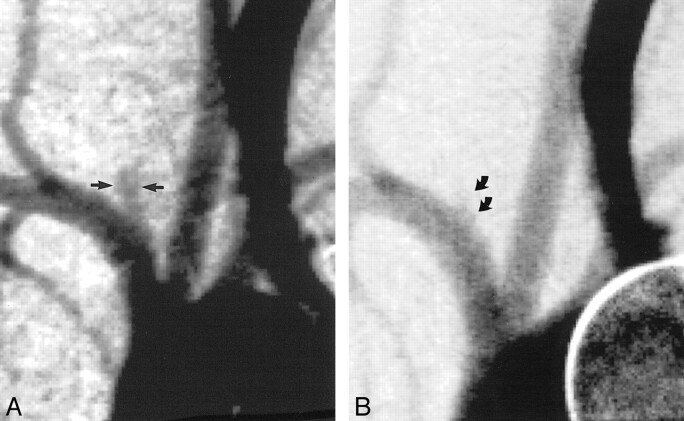
RESULTS: In the control (no elastase) animals, slitlike cavities at the origin of the right CCA decreased in size over time to become nearly obliterated by 21 days. Conversely, a short segment of the proximal CCA remained widely patent in all six test animals. With the exception of a single time point in one test animal, all “aneurysm” cavities in the test animals were dilated as compared with the normal diameter of the CCA. On day 3 after surgery, the mean width and height of the aneurysm cavities in the test animals were 3.2 ± 0.6 and 6.0 ± 1.3 mm, respectively. Compared with dimensions at day 3, aneurysms in test animals were larger at day 14, with mean width and height of 4.1 ± 1.7 and 8.3 ± 1.9 mm, respectively (P = .02). Aneurysms in test animals had increased further at 21 days compared with 14 days (P = .01). Compared with measurements obtained at 21 days, dimensions remained essentially unchanged at 28 and 35 days. Thirty-five days after surgery, mean width and height were 5.0 ± 0.9 and 10.0 ± 2.2 mm, respectively. Follow-up imaging performed ≤4 months after aneurysm creation showed no further change in aneurysm dimensions.
CONCLUSION: Elastase incubation and vessel ligation results in patent aneurysmally dilated arterial segments at the origin of the right CCA in rabbits. These aneurysms show progressive increases in diameter over time, finally stabilizing at approximately 1 month. Our data, which show early progressive aneurysm enlargement, suggest that this model may be used for the study of systemic therapies aimed at diminishing aneurysm rest regrowth and also indicate that embolization of these model aneurysms should be delayed at least 21 days after aneurysm creation.
Previous descriptions of animal models of saccular aneurysms encompass numerous species, techniques of construction, and applications (1–3). Rats, rabbits, dogs, swine, sheep, and nonhuman primates have been used for saccular aneurysm creations (1–10). Vein-patch aneurysms comprise the most common form of saccular aneurysm construction in animals, but several recent reports have detailed the use of elastase, either topical or endoluminal, for induction of saccular aneurysms (5, 6, 8–10). Among the several reports of elastase-induced aneurysm models, only the rabbit common carotid artery (CCA) model has been used for testing of endovascular occlusion devices (6, 8).
For numerous reasons, the rabbit CCA elastase-induced aneurysm represents a valuable tool for radiologists involved in preclinical evaluation of aneurysm occlusion devices. First, the aneurysms are easily constructed, requiring less than 1 hour of operative time, and the technique can be readily mastered, even by practitioners with little or no surgical training. Second, the long-term patency of the model has been established for up to 1 year. Third, the aneurysms occur either at an arterial bifurcation or along a vessel curve, similar to locations typical for berry aneurysms in humans. Last, standard catheter systems can be used when embolizing the aneurysms, rendering the model realistic for training physicians or testing devices.
Even though our previous work established the usefulness of the rabbit CCA elastase-induced aneurysm model for testing endovascular devices, we had never reported detailed observations of the early period of formation of these aneurysms. Other types of elastase-induced aneurysms, specifically the rat abdominal aortic aneurysm model, show delayed ongoing enlargement over a period of 2 weeks after elastase injury (11). Detailed study of the enlarging abdominal aneurysms has shed light on the factors leading to abdominal aneurysm formation, including but not limited to the role of endogenous proteases (12–15).
Documentation of delayed enlargement in the rabbit CCA saccular aneurysm model would have both immediate practical importance and long-range ramifications. The practical considerations include obligatory delay of embolization after creation, to allow stabilization of aneurysm size before placement of embolic devices. In addition, detailed study of the biology of the aneurysm wall during enlargement may allow elucidation of the processes contributing to saccular aneurysm formation and enlargement, especially because the proteases implicated in abdominal aortic aneurysm formation have also been implicated in cerebral aneurysm formation (16–18).
In the current study, we report a detailed evaluation of the rate and duration of aneurysm growth in the days, weeks, and months after elastase-induced aneurysm creation in New Zealand White rabbits. Our purpose was to document the presence or absence of delayed aneurysm enlargement.
Methods
Aneurysm Creation
All animal experimentation was approved by the Animal Review Committee at our institution. Nine New Zealand White rabbits (3–4) kg were used in this study: six test and three control animals. Anesthesia was induced with an intramuscular injection of ketamine/xylazine (80/8 mg/kg, respectively), and maintenance anesthesia was IV administered with pentobarbital. Using a sterile technique, surgical exposure of the right carotid sheath was performed. The right CCA was isolated, and proximal and distal control of the vessel was obtained using 3–0 silk suture. A 1- to 2-mm bevelled arteriotomy was made, and a 5F vascular sheath (Cordis Endovascular Systems, Miami Lakes, FL) was passed retrograde to the mid portion of the CCA. A 3F Fogarty balloon (Baxter Healthcare Corporation, Irvine, CA) was advanced through the sheath to the level of the CCA origin and inflated with iodinated contrast material. In the three control animals, saline and contrast material were mixed in a 50:50 concentration and incubated in the lumen of the CCA, above the inflated balloon, for 20 minutes. In the six test animals, porcine elastase (Sigma, St. Louis, MO) was incubated in the lumen of the CCA, above the balloon, for 20 minutes. After deflation of the balloon, the catheter system was removed and the vessel ligated in its mid portion. The skin was closed with a running suture.
Follow-up Imaging
All study animals underwent imaging on days 1, 3, 5, 7, 14, 21, and 35 after surgery. Test animals underwent imaging at months 1, 2, 3, and 4 after aneurysm creation, but these delayed imaging sessions were not performed in the control animals because cavities had essentially disappeared by day 35 (see details below). We used IV digital subtraction angiography (DSA) for follow-up imaging. Because serial angiography was necessary and catheter angiography in the rabbit typically requires cutdown and subsequent ligation of the femoral arteries, alternative imaging was required. The animals were anesthetized as described above. A 24-g angiocatheter was placed in a right or left ear vein. During two frames per second DSA imaging, 5 mL of iodinated contrast material was infused into the ear vein. Filming was performed into the arterial phase. An external sizing device was in place during IV DSA. The width and height of the aneurysm cavities were determined in reference to the external sizing device. The width of the aneurysm cavity was determined at its point of maximum width, whereas the height was measured from the dome to the line connecting the proximal and distal aneurysm neck. Widths and heights were compared between each time point and the next earliest time point using the Student's t test.
Results
The results are shown in Figures 1 and 2. Representative images are presented as Figures 3 through 6.
fig 1.
Mean aneurysm width versus time after aneurysm creation. Control animals (no elastase) showed progressive decrease in width over time (open circles). Test animals (elastase injured) showed progressive increase in aneurysm width over time, ≤21 days after aneurysm creation (solid circles).
fig 2. Mean aneurysm height versus time after creation. Control (no elastase) animals showed progressive decrease in height over time (open circles). Test animals (elastase injured) showed progressive increase in aneurysm height over time, ≤21 days after aneurysm creation (solid circles). Upper error bars for control animals at 3 and 7 days were removed to avoid confusion.
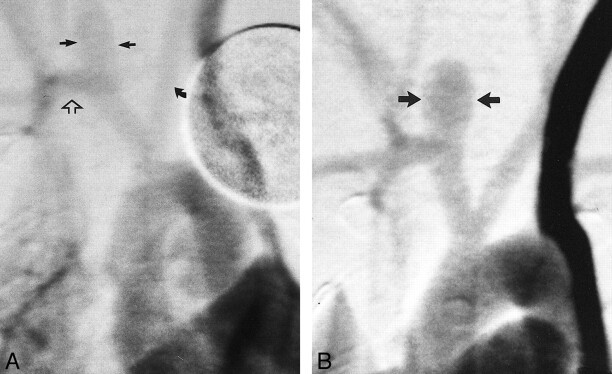
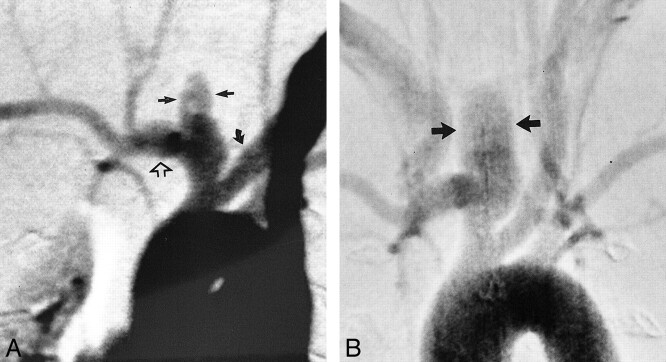
fig 3.
Angiograms of control animal 3 (no elastase).
A, Anteroposterior view IV DSA obtained 3 days after surgery. There is a small, slitlike opening at the stump of the right CCA (straight arrow).
B, Anteroposterior view IV DSA obtained 4 weeks after surgery. There has been progressive decrease in the size of the stump of the CCA (curved arrow).
Control Animals
In the three rabbits used as control animals for which no elastase was used but all other surgical procedures were identical to those used in the test animals, dilated segments of artery failed to form. In early imaging sessions, slitlike cavities were present at the right CCA origin, and these cavities decreased in size over time (Figs 1–3). The mean diameter of the stump decreased from 1.2 mm at early time points to <1.0 mm at later time points. At no time after aneurysm creation did any control animal show dilation of the stump of the CCA.
Test Animals
A short segment of the proximal CCA remained patent in all six test animals. Representative images are shown in Figures 4 through 6. The normal diameter of the CCA ranges from 2 to 2.5 mm. In our study animals, with the exception of a single test animal at the 3-day time point, all patent artery segments were aneurysmally dilated at all time points.
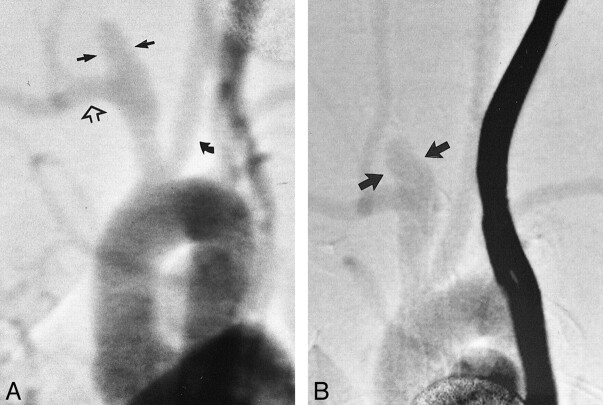
fig 4.
Angiograms of test animal 1.
A, Anteroposterior view IV DSA obtained 3 days after creation of aneurysm at origin of right CCA. Brachiocephalic arteries are opacified, including the left CCA (curved arrow), right subclavian artery (open arrow), and “aneurysm cavity” (ie, the aneurysmal dilation of right CCA origin) (straight solid arrows). The aneurysm cavity measures 3.5 mm in width, in reference to the external sizing device.
B, Anteroposterior view IV DSA obtained 21 days after creation of aneurysm. The morphology of the aneurysm cavity has changed as compared with that seen on day 3 in that the aneurysm cavity is more bulbous in shape (arrows). The aneurysm cavity measures 5.4 mm in width.
On day 3 after surgery, the mean width of the aneurysm cavities in test animals was 3.2 ± 0.6 mm. Compared with dimensions at day 3, aneurysms cavities in these same animals were wider at day 14, with a mean width of 4.1 ± 1.7 mm (P = .02). Aneurysm width had increased further at 21 days, as compared with measurements obtained at 14 days (P = .01). Compared with measurements obtained at 21 days, the mean aneurysm width remained essentially unchanged at 28 days and at all subsequent follow-up term points, with a final average width of 5.0 ± 0.9 mm.
On day 3 after surgery, the mean height of the aneurysm cavities in the test animals was 6.0 ± 1.3 mm. Compared with dimensions measured at day 3, aneurysm cavities in these same animals had increased in height at day 14, with a mean height of 8.3 ± 1.9 mm (P = .02). Aneurysms had increased further in height at 21 days, as compared with 14 days (P = .03). Compared with measurements obtained at 21 days, the mean aneurysm height remained essentially unchanged at 28 days and at all subsequent follow-up term points, with a final average height of 10.0 ± 2.2 mm.
Discussion
The data presented herein add useful information regarding the natural history of elastase-induced, saccular aneurysms in rabbits. Our previously published work focused primarily on the technique of construction, histology, and long-term stability of the model aneurysms (6, 8). The current data offer additional detail regarding rate and duration of progressive enlargement of the aneurysms. Specifically, mild dilation of the proximal CCA was noted immediately after surgery. Subsequently, the aneurysms exhibited progressive enlargement in both width and height over a period of approximately 3 weeks after aneurysm creation. After that time period, there was no change in aneurysm size. These data indicate that when using the model to test endovascular occlusion devices, at least 21 days should elapse between aneurysm creation and embolization. If aneurysm embolization is performed before that time, the efficacy of the occlusion device may be affected by ongoing expansion of the aneurysm cavity.
Our previously published reports of series of aneurysms created in New Zealand White rabbits in the right and left CCA using elastase injury (6, 8) focused primarily on the mode of construction of the aneurysms, using endovascular occlusion in the initial study and surgical ligation in more recent reports. The data previously presented were insufficient to determine whether saccular aneurysms in rabbits showed progressive aneurysm enlargement because follow-up data were collected at time points that were too infrequent and too delayed to uncover this progressive enlargement. If not for the corresponding data published for the rat abdominal aortic aneurysms and the occasional observation that early IV DSA seemed to markedly underestimate the aneurysm size noted at the time of delayed embolization procedures, we might never have chosen to study the early growth characteristics of the model.
Although the practical ramifications of progressive aneurysm enlargement are obvious, we think that future, more detailed study of the expanding aneurysms may prove useful in characterizing the biology of saccular aneurysm formation. This stems from the similarities between the behavior of our saccular aneurysm model and that of the model on which it was based, the rat model of abdominal aortic aneurysms (11). In 1990, Anidjar et al (11) reported aneurysmal enlargement of rat abdominal aortae after luminal perfusion with porcine elastase, with lack of enlargement in control saline-perfused vessels. As in our model of elastase-induced saccular aneurysms in rabbits, the rat abdominal aortic aneurysms showed early mild dilation and then progressive enlargement over a period of 14 days. Subsequent studies have confirmed that the delayed enlargement of the rat abdominal aneurysms is caused by production of endogenous proteases, specifically matrix metalloproteases 2 and 9 (12, 13, 15).
The correlation of increased metalloprotease activity with abdominal aneurysm formation in the rat model has met with considerable interest in the vascular surgery community, because these same endogenous proteins are elevated in patients with abdominal aortic aneurysms (14). Furthermore, numerous compounds, including doxycycline and indomethacin, have been shown to inhibit metalloprotease activity in vivo, and administration of these drugs has been shown to inhibit aneurysm growth in the rat model (2, 13–15, 19). Metalloproteases have been associated with numerous intracranial lesions, including aneurysms. Tissue samples from intracranial aneurysms have been shown to express elevated levels of metalloproteases, especially after aneurysm rupture (6, 18).
Although the progressive increase in the width of the saccular aneurysms is easily explained by ongoing damage by endogenous proteins, the increase in aneurysm height is more difficult to explain. During surgery for creation of aneurysms, the carotid artery is ligated approximately 4 cm cephalad to the vessel origin. Very rapidly after surgery, the carotid artery undergoes retrograde thrombosis, leaving an aneurysm stump approximately 6 mm in height. Within 2 weeks, the intraluminal thrombus organizes to leave an atretic fibrotic lumen above an endothelial-lined aneurysm dome (6, 8). It is unlikely that the dome of the aneurysm, capped with fibrotic tissue, can expand. Rather, the increase in aneurysm height probably reflects ongoing elongation of the lateral walls of the aneurysm cavity.
Although we remain convinced that the data presented herein indicate compelling evidence for progressive aneurysm enlargement in our model, this study did suffer several limitations. First, IV DSA rather than conventional DSA was used to follow the aneurysm sizes. Conventional DSA would have been impossible because of the need to ligate the femoral artery in the rabbit after catheter placement, and multiple time points were needed in all study animals. IV DSA can be performed repeatedly without loss of catheter access sites. We think that measurements from IV DSA are comparable with conventional DSA, but the signal-to-noise ratio is lowered with the former technique as compared with the latter.
Intraaneurysmal pressure differences throughout the cardiac cycle theoretically could affect aneurysm diameter and height. In the current study, we did not correlate size with pressure. However, during hundreds of embolization sessions using this model, we have not seen demonstrable differences in aneurysm size relative to the cardiac cycle, as evidenced by changes in diameter during intraarterial injections. Furthermore, because cardiac cycle variations would be random regarding our filming, they would only serve to mask any progressive enlargement. Because we noted significant enlargement even with this potential limitation, we are unconcerned about cardiac cycle variability in our model.
It remains possible that differences in projection from one imaging session to another may account for some differences in aneurysm size. This error, however, would be random and thus would only serve to mask differences in aneurysm size over time. Because we showed compelling evidence for enlargement even with this possible error, projection differences are considered relatively unimportant in this study. Furthermore, changes in right anterior oblique and/or left anterior oblique as well as craniocaudal angulation would have essentially no effect on the measured width of the aneurysm cavities.
Another limitation of this study is the lack of histologic correlation. Because this was a longitudinal study and multiple time points were necessary for each study animal, histology was not obtained. Our group has extensive data regarding the histology of the model aneurysms, and relevant features have been published previously (6, 8).
Some investigators may disagree with our interpretation of the usefulness of the model regarding the ideal time after creation for embolization. Specifically, we choose to embolize aneurysms after they have achieved stable size to remove as many variables as possible when comparing one embolic device with another; as such, we wait >21 days to embolize. It is perfectly acceptable to us if other investigators, who are evaluating devices that preclude aneurysm growth, embolize earlier. However, the latter approach would add problems related to unorganized thrombus in the aneurysm dome.
It is our intent to model future studies of the saccular rabbit aneurysms on the basis of techniques used for rat abdominal aortic aneurysms. Specifically, we will test the hypothesis that metalloprotease activity is elevated in the rabbit aneurysms, and that administration of metalloprotease inhibitors can block progressive aneurysm enlargement. This information is considered valuable for all types of physicians who treat intracranial aneurysms, including interventional neuroradiologists.
fig 5.
Angiograms of test animal 2.
A, Anteroposterior view IV DSA obtained 3 days after creation of aneurysm at origin of right CCA. Brachiocephalic arteries are opacified, including the left CCA (curved arrow), right subclavian artery (open arrow), and aneurysm cavity (straight solid arrows). The aneurysm cavity measures 2.8 mm in width.
B, Anteroposterior view IV DSA obtained 17 weeks after creation of aneurysm. The aneurysm cavity measures 4.0 mm in width (arrows).
fig 6.
Angiograms of test animal 4.
A, Anteroposterior view IV DSA obtained 3 days after creation of aneurysm at origin of right CCA. Brachiocephalic arteries are opacified, including the left CCA (curved arrow), right subclavian artery (open arrow), and aneurysm cavity (straight solid arrows). The aneurysm cavity measures 4.0 mm in width.
B, Anteroposterior view IV DSA obtained 4 months after creation of aneurysm. The aneurysm cavity has markedly enlarged in size as compared with the earlier image, with the aneurysm cavity measuring 6.0 mm in width.
Footnotes
Address reprint requests to Naomi H. Fujiwara, MD, Box 800170, Department of Radiology, University of Virginia Health Services, Charlottesville, VA 22908.
References
- 1.Massoud TF, Guglielmi G, Ji C, Viñuela F, Duckwiler GR. Experimental saccular aneurysms: I. review of surgically-constructed models and their laboratory applications. Neuroradiology 1994;36:537-546 [DOI] [PubMed] [Google Scholar]
- 2.Powell J. Models of arterial aneurysm: for the investigation of pathogenesis and pharmacotherapy: a review. Atherosclerosis 1991;87:93-102 [DOI] [PubMed] [Google Scholar]
- 3.Stehbens WE. Histological changes in chronic experimental aneurysms surgically fashioned in sheep. Pathology 1997;29:374-379 [DOI] [PubMed] [Google Scholar]
- 4.Abruzzo T, Shengelaia GG, Dawson RC, Owens DS, Cawley CM, Gravanis MB. Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. AJNR Am J Neuroradiol 1998;19:1309-1314 [PMC free article] [PubMed] [Google Scholar]
- 5.Cawley CM, Dawson RC, Shengelaia G, Bonner G, Barrow DL, Colohan AR. Arterial saccular aneurysm model in the rabbit. AJNR Am J Neuroradiol 1996;17:1761-1766 [PMC free article] [PubMed] [Google Scholar]
- 6.Cloft HJ, Altes TA, Marx WF, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology 1999;213:223-228 [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: part III. pathology. Surg Neurol 1979;11:299-304 [PubMed] [Google Scholar]
- 8.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999;213:217-222 [DOI] [PubMed] [Google Scholar]
- 9.Miskolczi L, Guterman LR, Flaherty JD, Szikora I, Hopkins LN. Rapid saccular aneurysm induction by elastase application in vitro. Neurosurgery 1997;41:220-229 [DOI] [PubMed] [Google Scholar]
- 10.Miskolczi L, Guterman LR, Flaherty JD, Hopkins LN. Saccular aneurysm induction by elastase digestion of the arterial wall: a new animal model. Neurosurgery 1998;43:595-600 [DOI] [PubMed] [Google Scholar]
- 11.Anidjar S, Dobrin PB, Chejfec G, Michel JB. Experimental study of determinants of aneurysmal expansion of the abdominal aorta. Ann Vasc Surg 1994;8:127-136 [DOI] [PubMed] [Google Scholar]
- 12.Miralles M, Wester W, Sicard GA, Thompson R, Reilly JM. Indomethacin inhibits expansion of experimental aortic aneurysms via inhibition of the cox2 isoform of cyclooxygenase. J Vasc Surg 1999;29:884-893 [DOI] [PubMed] [Google Scholar]
- 13.Moore G, Liao S, Curci JA, et al. Suppression of experimental abdominal aortic aneurysms by systemic treatment with a hydroxamate-based matrix metalloproteinase inhibitor (RS 132908). J Vasc Surg 1999; 29:522-532 [DOI] [PubMed] [Google Scholar]
- 14.Thompson RW, Baxter BT. MMP inhibition in abdominal aortic aneurysms. Rationale for a prospective randomized clinical trial. Ann N Y Acad Sci 1999;878:159-178 [DOI] [PubMed] [Google Scholar]
- 15.Treharne GD, Boyle JR, Goodall S, Loftus IM, Bell PR, Thompson MM. Marimastat inhibits elastin degradation and matrix metalloproteinase 2 activity in a model of aneurysm disease. Br J Surg 1999;86:1053-1058 [DOI] [PubMed] [Google Scholar]
- 16.Bruno G, Todor R, Lewis 1, Chyatte D. Vascular extracellular matrix remodeling in cerebral aneurysms. J Neurosurg 1998;89:431-440 [DOI] [PubMed] [Google Scholar]
- 17.Gaetani P, Rodriguez Tartara F, et al. Metalloproteases and intracranial vascular lesions. Neurol Res 1999;21:385-390 [DOI] [PubMed] [Google Scholar]
- 18.Peters DG, Kassam A, St Jean PL, Yonas H, Ferrell RE. Functional polymorphism in the matrix metalloproteinase-9 promoter as a potential risk factor for intracranial aneurysm. Stroke 1999;30:2612-2616 [DOI] [PubMed] [Google Scholar]
- 19.Michaelides MR, Curtin ML. Recent advances in matrix metalloproteinase inhibitors research. Curr Pharm Des 1999;5:787-819 [PubMed] [Google Scholar]