Abstract
BACKGROUND AND PUPOSE: Tuberculous lymphadenitis and metastatic nodes from nasopharyngeal carcinoma are common in Asians and are often indistinguishable clinically. Because their treatment depends on prompt diagnosis, we undertook this study to evaluate if power Doppler sonography could distinguish these two pathologic abnormalities. The intranodal vascular appearances of tuberculous neck nodes are compared with benign reactive neck nodes and metastatic nodes from nasopharyngeal carcinoma.
METHODS: The appearances of power Doppler sonograms of 42 tuberculous nodes were compared with 28 metastatic nodes from nasopharyngeal carcinoma and 27 benign reactive nodes. The intranodal distribution of vessels and the intranodal vascular resistance of vessels were compared among these three groups. All examinations were performed by the same sonologist (A.A.), who had more than 3 years' scanning experience, and all data analysis was performed by the same investigator (M.Y.).
RESULTS: The intranodal vascular distribution in tuberculous nodes was varied and simulated both benign and malignant disease. Avascularity of nodes and displacement of hilar vascularity were frequent in tuberculous nodes. Metastatic nodes from nasopharyngeal carcinoma (resistive index [RI], 0.81±0.09; pulsatile index [PI], 1.91±0.81) had a higher vascular resistance than did tuberculous nodes (RI, 0.71±0.11; PI, 1.34±0.55). Tuberculous nodes had a higher vascular resistance than did reactive nodes (RI, 0.66±0.09; PI, 1.10±0.26).
CONCLUSION: Avascularity, displaced hilar vessels, and low intranodal vascular resistance are clues that may suggest the tuberculous nature of neck nodes. However, there is overlap of appearance between tuberculous nodes, benign reactive neck nodes, and metastatic nodes. Thus, histologic analysis is often required for a definitive diagnosis.
Tuberculosis is common in Southeast Asia, and with the spread of AIDS, it is now becoming increasingly common in developed countries. Despite advances in diagnosis and treatment of tuberculosis, it continues to remain a diagnostic dilemma owing to its remarkable ability to mimic other benign and malignant diseases.
Nasopharyngeal carcinoma is also common in Southeast Asia, and the clinical presentation of metastatic nodes arising from nasopharyngeal carcinoma is similar to that of tuberculous lymphadenitis. Patients with either pathologic abnormality may present with palpable cervical lymph nodes, and clinical examination alone cannot differentiate the two.
As an initial investigation of choice, the role of gray scale sonography of neck lymph nodes is well established (1, 2). When combined with fine-needle aspiration cytology, sonography is more accurate than is clinical examination because of its high sensitivity and specificity (3). The advent of power Doppler sonography has further increased the amount of information that can be obtained during sonography of cervical nodes. The presence and distribution of intranodal vascularity and estimates of intravascular resistance may be evaluated with power Doppler sonography. Recent reports suggest that, on the basis of this distribution of intranodal vascularity and intranodal resistance, it is now possible to differentiate benign from malignant nodes with a high degree of accuracy (4–8). We, therefore, undertook to 1) document the intranodal vascular appearances of tuberculous nodes in the neck and identify their diagnostic features, if any, and 2) compare the intranodal vascular appearances of tuberculous neck nodes with benign reactive neck nodes and metastatic nodes from nasopharyngeal carcinoma.
Methods
We evaluated power Doppler sonograms of 42 tuberculous nodes and compared their appearances with 28 metastatic nodes from nasopharyngeal carcinoma and 27 benign reactive nodes. All the patients presented with palpable lymphadenopathy. Forty-nine of the patients studied were male and 48 were female and ages ranges from 18 to 80 years.
At the time of sonographic examination, none of the patients with metastatic nodes from nasopharyngeal carcinoma had undergone radiation therapy for the primary tumor or the nodes. All patients with tuberculous and benign reactive nodes presented with palpable, persistent, painless cervical lymphadenopathy. In all these patients, no primary tumor was detected on physical examination and panendoscopic findings were normal. Patients with proven tuberculous nodes were placed on anti-tuberculous drugs and patients with benign reactive nodes were followed up in the clinic. At routine follow-up, no primary tumor was detected in any of these patients.
Gray scale and power Doppler sonography were performed on the palpable node. Among patients with more than one node detected sonographically, fine-needle aspiration cytology was performed on the node that showed the most vascularity on power Doppler sonograms. The diagnoses of all nodes included in this study were confirmed by fine-needle aspiration cytology.
All examinations were performed by the same sonologist (A.A.) using a 7- to 10-MHz transducer (ATL; HDI 3000, Bothell, WA). The vascular patterns and intranodal resistance were evaluated during real-time scanning. In all patients, power Doppler sonography was performed using standardized parameters (9). Settings of power Doppler sonography were set for high sensitivity with a low wall filter to allow detection of vessels with low blood flow. Pulsed repetition frequency was 700 Hz and medium persistance was used. The color gain was increased until background noise appeared and then reduced until the noise was suppressed, thus ensuring maximum sensitivity (10, 11). When consistent Doppler signals were obtained, the color map was used to guide the placement of the pulsed Doppler gate and tracings of the arterial signal recorded by using a sample volume of 1 mm. The vascular resistance was evaluated at random sites within three vessels that consistently demonstrated three consecutive Doppler spectral waveforms. The mean resistive index (RI) and pulsatile index (PI) were estimated.
The vascular patterns of lymph nodes were classified into three main categories according to the location of the vascularity:
1) Hilar: flow signals branching radially from the hilus, regardless of whether the signals originate from the central region of from the periphery (Fig 1).
fig 1.
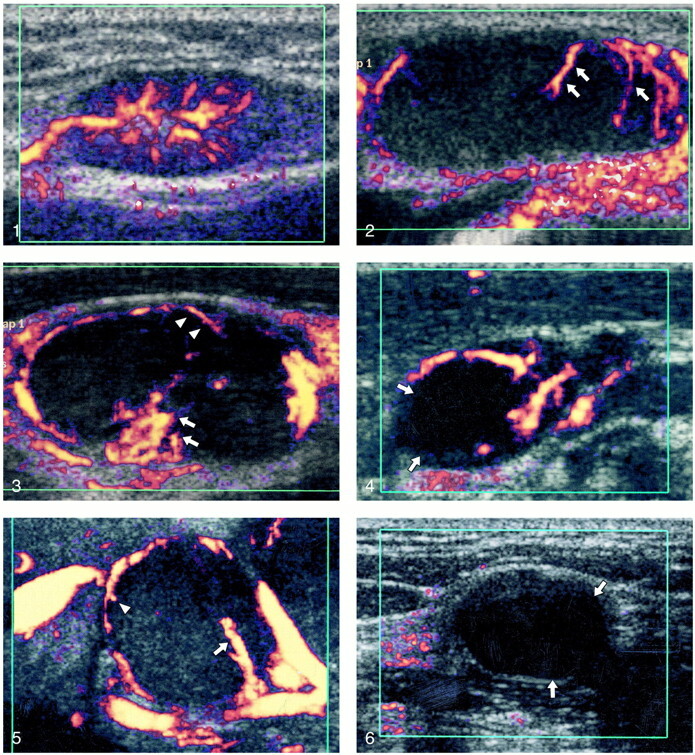
Power Doppler sonogram of a reactive node showing hilar vascularity.fig 2. Power Doppler sonogram of a malignant node showing capsular vascularity (arrows).fig 3. Power Doppler sonogram of a malignant node showing hilar (arrows) and capsular (arrowheads) vascularity.fig 4. Power Doppler sonogram of a tuberculous node showing displaced hilar vessels. Note the adjacent area of cystic necrosis (arrows).fig 5. Power Doppler sonogram of a tuberculous node showing hilar (arrows) and capsular vascularity (arrowheads).fig 6. Power Doppler sonogram of an avascular tuberculous node. Note areas of cystic necrosis (arrows)
2) Capsular (or peripheral): flow signals along the periphery of the lymph nodes, with branches perforating the periphery of the node and not arising from the hilar vessels (Fig 2).
3) Mixed: presence of hilar and capsular flow (Fig 3).
For the vascular resistance, two cut off points were evaluated: RI (0.7 and 0.8) and PI (1.5 and 1.6). These cut off points were selected because they were previously reported to be useful in differentiating benign from malignant nodes (RI, sensitivity = 47%–80% and specificity = 94%–100%; PI, sensitivity = 55%–94% and specificity = 97%–100%) (6, 12).
Because tuberculous lymphadenitis is treated with anti-tuberculous drugs, without resorting to excision of the nodes, the histologic confirmation of intranodal vascular processes could not be confirmed in this study.
Fisher's exact test, the χ2 test, and an unpaired t test were used to calculate significance of the difference in vascular patterns and vascular resistance between tuberculous and metastatic nodes and between tuberculous and reactive nodes.
Results
A total of 42 tuberculous nodes, 28 metastatic nodes, and 27 reactive nodes were included in the study. They ranged in size from 6 mm to 30 mm in maximum transverse diameter. There were 17 tuberculous nodes, 14 metastatic nodes, and 20 reactive nodes with a maximum transverse diameter less than 10 mm. Tuberculous nodes were found at Level I (n = 2), Level II (n = 4), Level IV (n = 1), and Level V (n = 35). In the metastatic group, all 28 nodes were found at Level II. In the reactive group, lymph nodes were found at Level I (n = 5), Level II (n = 16), and Level V (n = 6).
Metastatic nodes from nasopharyngeal carcinoma (RI, 0.81±0.09; PI, 1.91±0.81) had a higher vascular resistance than tuberculous nodes (RI, 0.71±0.11; PI, 1.34±0.55) (P<.05). Tuberculous nodes had a higher vascular resistance than reactive nodes (RI, 0.66±0.99; PI, 1.10±0.26) (P<.05).
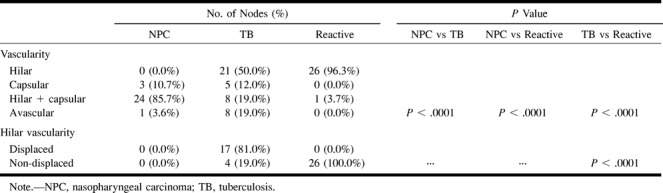
The Table compares the vascular patterns of metastatic nodes from nasopharyngeal carcinoma, tuberculous nodes, and reactive nodes. In metastatic nodes, most showed mixed vascularity (hilar and capsular) (86%) and none of them had hilar vascularity only. In tuberculous lymphadenitis, the majority showed hilar (50%) vascularity, and most of the hilar vessels were displaced (81%). Most of the reactive nodes showed non-displaced hilar vascularity (96%). There is a significant difference in vascular pattern among metastatic nodes from nasopharyngeal carcinoma, tuberculous lymphadenitis, and reactive nodes (P<.0001).
Discussion
In clinical practice, patients with tuberculous adenitis or benign reactive cervical lymphadenitis often present with discrete, non-tender nodes in the posterior triangle. However, 50% of patients with nasopharyngeal carcinoma, a major cancer occurring among Cantonese Chinese, also present with clinically palpable neck nodes, and this percentage is even higher among patients younger than 21 years (13). Although their clinical presentation is similar, it is important to differentiate these lesions, as their prognosis and treatment are different.
In Europe and Asia, gray scale sonography (combined with fine-needle aspiration cytology) with its high sensitivity (92%) and specificity (97%) is often considered as the initial investigation of choice for differentiating benign from malignant nodes (3, 14). It evaluates the nodal distribution, shape, size, and internal architecture. However, on the basis of these features alone, it is not possible to differentiate tuberculous from metastatic nodes (15). The gray scale sonographic features that help in differentiating the two are the presence of nodal matting and adjacent soft-tissue edema, which are common in patients with tuberculous nodes (15).
There are numerous reports of the role of power Doppler sonography in differentiating benign from malignant nodes (5–8, 16, 17). Although tuberculous nodes are included in these studies, their numbers are limited (three to six lymph nodes) and, to our knowledge, there are only two reports (6, 7) with more than 15 nodes (17–29 lymph nodes) in their study. We were therefore interested to see whether power Doppler sonography could help in differentiating tuberculous from metastatic nasopharyngeal carcinomatous nodes, as both these conditions are common in Southeast Asia. In this study, all the examinations were performed by the same sonologist, which may eliminate intraobserver variation. The sonologist performing the examination must have adequate experience and meticulous technique during the examination. The scanning parameters (as mentioned in the Methods section) must be standardized and applied carefully during the examination. Patient cooperation is also essential, as movement and swallowing produce artifact and adversely affect evaluation of the small intranodal vessels.
Intranodal Vascular Distribution
The presence of neovascularity in malignant nodes and the patterns of vascular distribution within nodes have been previously described (4, 8, 18). In reactive nodal disease, the diffuse nature of the histologic process occurring within the node preserves the normal vascular pattern, and reactive nodes therefore tend to have prominent hilar vascularity (6, 18) owing to increased vessel diameter and blood flow (8).
Malignant nodes have a more variable vascular pattern and show at least one of the four abnormal vascular patterns previously described: avascular areas, displacement of vessels, increased peripheral vessels, and aberrant course of hilar vessels (19). The increase in peripheral nodal vascularity is secondary to the initial deposition of carcinomatous cells in the marginal and medullary sinuses. Neovascularization by infiltrating tumor induces aberrant feeding vessels in the periphery of the tumor rests by tumor angiogenesis and sinusoid tumor vascularity within the tumor rests (4). The capsule and perinodal tissues may be invaded at a later stage (4). As the node is progressively involved with tumor, increased vascularity is seen both in the central as well as the peripheral zones of the nodes.
Power Doppler sonography is able to detect this abnormal vascularity, evaluate the distribution of vessels, and estimate the intranodal vascular resistance, thus differentiating histologically proven benign from malignant nodes with a high degree of accuracy (sensitivity, 83%–89%; specificity, 76%–98%) (4, 7, 8). In this study, tuberculous nodes demonstrated three common intranodal vascular patterns on power Doppler sonograms.
Hilar Vascularity
In this study, 50% of tuberculous nodes showed only hilar vascularity, compared with 41% in Wu et al's (7) series. Hilar vascularity was seen in 96% of reactive nodes but in none of the metastatic nodes.
Both tuberculous nodes and benign reactive nodes frequently demonstrated hilar vascularity. Further evaluation of the tuberculous nodes showed that in 81% there was obvious mass effect and displacement of the hilar vessels (Fig 4), which was not seen in benign reactive nodes. In all cases, the displacement of these vessels was attributable to focal areas of necrosis (Fig 4) that could be seen with gray scale sonography. Therefore, the presence of only displaced hilar vascularity appears to be a useful feature in differentiating tuberculous nodes from metastatic nodes.
Mixed (Capsular and Hilar) Vascularity
In this study, 19% of tuberculous nodes showed a mixed (capsular and hilar [Fig 5]) vascularity compared with 53% in Wu et al's (7) study and 76% in Na et al's (6). A similarly mixed vascularity was seen in 4% of reactive nodes in this study, and 86% of metastatic nodes from nasopharyngeal carcinoma. Therefore, the presence of mixed vascularity may not consistently help to differentiate between a tuberculous and metastatic node.
Avascular Nodes
Absent flow signals on power Doppler sonograms do not necessarily mean perfusion is absent. The reasons for not seeing the intranodal vessels may be attributed to the following (20):
1) The relatively low number of back-scattering erythrocytes in the tiny peripheral vessels decreases the signal intensity, which may not surpass the noise level.
2) Low-flow velocities result in a smaller Doppler shift, which may be suppressed by a high wall filter.
3) Postprocessing functions that suppress flow signals in echogenic tissues to reduce motion artifacts may also suppress the signal emanating from the nodes.
In addition, avascularity in tuberculous nodes may be due to the necrotizing granulomatous lesions that may obliterate intranodal vessels (21). Avascularity of tuberculous nodes may also reflect later stages of the disease, when healing has begun, and fibrosis and hyalinization cause compression and obliteration of intranodal vessels.
In this study, in 19% of tuberculous nodes, no vascular signal could be detected by power Doppler sonography (Fig 6) compared with 41% reported in the study by Wu et al (7) and the 6% reported in Na et al's (6) series. Of the eight avascular tuberculous nodes in this study, seven (88%) showed large areas of intranodal cystic necrosis on gray scale imaging (cystic necrosis >50% of the nodal cross-sectional area). The avascularity of tuberculous nodes associated with intranodal necrosis may, therefore, help to differentiate it from other malignant and reactive nodes.
Capsular Vascularity
In metastatic nodes, destruction of hilar vascularity may result in the induction of vascular supply from the peripheral preexisting vessels or from vessels in the perinodal soft tissues. A similar angiogenesis can occur in tuberculous nodes (6), as central necrosis may destroy hilar vascularity, resulting in vascular supply from the periphery, particulary from the inflamed perinodal soft tissues. Deformed vessels arising from the hilus can also mimic peripheral vascularity (6).
In this study, 12% of tuberculous nodes showed only capsular vascularity compared with 11% in Wu et al's (7) series and 24% in Na et al's (6) series. Eleven percent of metastatic nodes from nasopharyngeal carcinoma and none of the benign reactive neck nodes in this study showed a similar capsular vascularity. Thus, capsular vascularity without hilar vascularity does not appear to help in distinguishing tuberculous nodes from malignant nodes.
One must note that, despite the benign nature of tuberculous nodes, the intranodal vascular distribution within tuberculous nodes (displaced hilar; mixed, avascular areas; capsular vascularity) mimics features that have previously been described for malignant nodes (19). However, selective hilar vascularity and avascular nodes are more common in tuberculous nodes.
Intranodal Vascular Resistance (RI, PI)
Previous reports (6, 12, 16, 22) have suggested that benign nodes can be differentiated from malignant nodes by estimating the intranodal resistance. Malignant nodes tend to have higher intranodal vascular resistance compared with benign lymphadenopathy (6, 12, 22).
Metastatic nodes from nasopharyngeal carcinoma tended to show a high RI and PI. All of the studied metastatic nodes from nasopharyngeal carcinoma had an RI ≥ 0.7, and 67% of them had an RI ≥ 0.8. Seventy-eight percent of metastatic nodes had a PI ≥ 1.5 and 59% of them had a PI ≥ 1.6. For tuberculous lymphadenitis, 56% of nodes had an RI ≥ 0.7 and 18% had an RI ≥ 0.8. Approximately 21% of tuberculous nodes had a PI ≥ 1.5 and 12% of them had a PI ≥ 1.6. For reactive nodes, 33% had an RI ≥ 0.7 and 7% had an RI ≥ 0.8. Approximately 7% of reactive nodes had a PI ≥ 1.5 and 4% had a PI ≥ 1.6.
There is a significant difference in both RI and PI between metastatic nodes and tuberculous nodes (P < .05) and between metastatic nodes and reactive nodes (P < .05). However, there is no significant difference in RI and PI between tuberculous nodes and reactive nodes (P > .05).
In this study, malignant nodes showed higher intravascular resistance compared with tuberculous nodes and benign reactive nodes. As reported by Na et al (6), tuberculous nodes in this study also had significantly higher intranodal vascular resistance compared with reactive nodes.
Conclusion
On power Doppler sonograms, avascularity, displaced hilar vessels, and low intranodal vascular resistance are clues that may suggest the tuberculous nature of neck nodes.
However, although these differences in the vascular patterns and intranodal resistance are statistically different between tuberculous nodes and metastatic nodes, there is an overlap of appearances between the two. Tuberculous nodes also demonstrate features of benign reactive neck nodes. Thus, despite advances in sonography, in clinical practice, tuberculosis remains a great mimic, and aspiration cytology is still necessary and is more reliable for a definitive diagnosis.
Vascular patterns of metastatic tuberculous and reactive nodes
Footnotes
Address reprint requests to Anil T. Ahuja, Department of Diagnostic Radiology and Organ Imaging, Prince of Wales Hospital, Shatin N.T., Hong Kong, China.
References
- 1.Ahuja A, Ying M, King W, Metreweli C. A practical approach to ultrasound of cervical lymph nodes. J Laryngol Otol 1997;111:245-256 [DOI] [PubMed] [Google Scholar]
- 2.Ying M, Ahuja AT, Evans R, King W, Metreweli C. Cervical lymphadenopathy: sonographic differentiation between tuberculous nodes and nodal metastases from non-head and neck carcinomas. J Clin Ultrasound 1998;26:383-389 [DOI] [PubMed] [Google Scholar]
- 3.Baatenburg de Jong RJ, Rongen RJ, Verwoerd CD, van Overhagen H, Lameris JS, Knegt P. Ultrasound-guided fine-needle aspiration biopsy of neck nodes. Arch Otolaryngol Head Neck Surg 1991;117:402-404 [DOI] [PubMed] [Google Scholar]
- 4.Ariji Y, Kimura Y, Hayashi N, et al. Power Doppler sonography of cervical lymph nodes in patients with head and neck cancer. AJNR Am J Neuroradiol 1998;19:303-307 [PMC free article] [PubMed] [Google Scholar]
- 5.Steinkamp HJ, Meuffelmann M, Bock JC, Thiel T, Kenzel P, Felix R. Differential diagnosis of lymph node lesions: a semiquantitative approach with colour Doppler ultrasound. Br J Radiol 1998;71:828-833 [DOI] [PubMed] [Google Scholar]
- 6.Na DG, Lim HK, Byun HS, Kim HD, Ko YH, Baek JH. Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. AJR Am J Roentgenol 1997;168:1311-1316 [DOI] [PubMed] [Google Scholar]
- 7.Wu CH, Chang YL, Hsu WC, Ko JY, Sheen TS, Hsieh FJ. Usefulness of Doppler spectral analysis and power Doppler sonography in the differentiation of cervical lymphadenopathies. AJR Am J Roentgenol 1998;171:503-509 [DOI] [PubMed] [Google Scholar]
- 8.Wu CH, Shih JC, Chang YL, Lee SY, Hsieh FJ. Two dimensional and three dimensional power Doppler sonographic classification of vascular patterns in cervical lymphadenopathies. J Ultrasound Med 1998;17:459-464 [DOI] [PubMed] [Google Scholar]
- 9.Ahuja AT, Ho SSY, Leung SF, Kew J, Metreweli C. Metastatic adenopathy from nasopharyngeal carcinoma: successful response to radiation therapy assessed by color duplex sonography. AJNR Am J Neuroradiol 1999;20:151-156 [PubMed] [Google Scholar]
- 10.Cosgrove DO, Rajendra PK, Bamber JC, et al. Breast disease: color Doppler US in differential diagnosis. Radiology 1993;189:99-104 [DOI] [PubMed] [Google Scholar]
- 11.McNicholas MM, Mercer PM, Miller JC, McDermott EW, O'Higgins NJ, MacErlean DP. Color Doppler sonography in the evaluation of palpable breast masses. AJR Am J Roentgenol 1993;161:765-771 [DOI] [PubMed] [Google Scholar]
- 12.Steinkamp HJ, Mayrer J, Cornehl M, Knobber D, Hettwer H, Felix R. Recurrent cervical lymphadenopathy: differential diagnosis with color-duplex sonography. Eur Arch Otorhinolaryngol 1994;251:404-409 [DOI] [PubMed] [Google Scholar]
- 13.Skinner DW, van Hasselt A. Symptomatology. In: van Hasselt A, Gibb A, eds. Nasopharyngeal Carcinoma. Hong Kong: The Chinese University Press 1991;85-92
- 14.Bruneton JN, Roux P, Caramella E, Dermard F, Vallicioni J, Chauvel P. Ear, nose, and throat cancer: ultrasound diagnosis of metastasis to cervical lymph nodes. Radiology 1984;152:771-773 [DOI] [PubMed] [Google Scholar]
- 15.Ahuja A, Ying M, Evans R, King W, Metreweli C. The application of ultrasound criteria for malignancy in differentiating tuberculous cervical adenitis from metastatic nasopharyngeal carcinoma. Clin Radiol 1995;50:391-395 [DOI] [PubMed] [Google Scholar]
- 16.Chang DB, Yuan A, Yu CJ, Luh KT, Kuo SH, Yang PC. Differentiation of benign and malignant cervical lymph nodes with color Doppler sonography. AJR Am J Roentgenol 1994;162:965-968 [DOI] [PubMed] [Google Scholar]
- 17.Dragoni F, Cartoni C, Pescarmona E, et al. The role of high resolution pulsed and color Doppler ultrasound in the differential diagnosis of benign and malignant lymphadenopathy. Results of multivariate analysis. Cancer 1999;85:2485-2490 [DOI] [PubMed] [Google Scholar]
- 18.Giovagnorio F, Caiazzo R, Avitto A. Evaluation of vascular patterns of cervical lymph nodes with power Doppler sonography. J Clin Ultrasound 1997;25:71-76 [DOI] [PubMed] [Google Scholar]
- 19.Castenholz A. Architecture of the lymph node with regard to its function. In: Grundmann E, Vollmer E, eds. Reaction Patterns of the Lymph Node. New York: Springer Verlag 1990;1-32 [DOI] [PubMed]
- 20.Tschammler A, Wirkner H, Ott G, Hahn D. Vascular patterns in reactive and malignant lymphadenopathy. Eur Radiol 1996;6:473-480 [DOI] [PubMed] [Google Scholar]
- 21.Ioachim HL. Metastatic nasopharyngeal carcinoma. In: Ioachim HL, ed. Lymph Node Pathology. 2nd ed. Philadelphia: J.B. Lippencott Co. 1994;613-618
- 22.Choi MY, Lee JW, Jang KJ. Distinction between benign and malignant causes of cervical axillary, and inguinal lymphadenopathy: value of Doppler spectral waveform analysis. AJR Am J Roentgenol 1995;165:981-984 [DOI] [PubMed] [Google Scholar]


