Abstract
BACKGROUND AND PURPOSE: Cytogenetic abnormalities, especially chromosome 13 deletion, are high-risk factors for multiple myeloma. Attaining the highest detection rates of cytogenetic abnormalities is important to provide accurate prognostic information to the referring oncologist. The purpose of this study was to use CT-guided percutaneous fine-needle aspiration bone biopsy (CT-guided FNA) of MR-detected focal lesions in patients with multiple myeloma to increase identification of abnormal cytogenetics.
METHODS: Patients enrolled in two clinical trials for myeloma therapy underwent MR imaging of the entire spine and pelvis. CT-guided FNA biopsy samples obtained from MR-detected focal lesions in these patients were sent for cytogenetic analysis. FNA results were then compared with random bone marrow sampling of the iliac crest done at or near the same time as the FNA to provide the data revealed in this study.
RESULTS: Forty-one patients (47 lesions) in one of the trials and 37 patients (38 lesions) in the other trial had biopsies performed. CT-guided FNA revealed cytogenetic abnormalities in 21% of the total patient population and new information in nearly 10% of the patients in one trial and in 20% of those in the other trial.
CONCLUSION: CT-guided biopsy of MR-detected focal lesions is a safe technique that can provide important cytogenetic information in a significant number of patients with multiple myeloma not identified during random marrow sampling.
Multiple myeloma is a plasma cell tumor that consists primarily of differentiated B-cells. While survival can range from a few months to many years, median survival is approximately 3 years with conventional chemotherapy (1). Because of the great variation in survival, identification of prognostic factors is crucial in planning appropriate treatment. Initially, the Durie-Salmon staging system was used to estimate prognosis by determining the plasma cell mass (2). While increasing stage generally indicated poorer survival, it did not help predict which patients should undergo more aggressive therapy (3). The search for better predictors of event-free survival and overall survival identified low β-2-microglobulin, low C-reactive protein levels, short duration of standard dose therapy, and normal cytogenetics as the most important predictors of a good prognosis. Multivariate analysis of laboratory data has shown that abnormal cytogenetics is the single most important factor affecting a poor prognosis (4).
In this study, biopsies of MR-detected focal lesions were performed with the use of CT guidance. Patients with multiple myeloma who enrolled in either of two NIH-sponsored protocols, total therapy II (TT II), for newly diagnosed patients, and DTPACE, for previously treated patients, were investigated to determine if more cytogenetic abnormalities could be identified than by random marrow screening alone. TT II is a phase III study for patients with newly diagnosed multiple myeloma to evaluate antiangiogenesis with thalidomide and posttransplant consolidation chemotherapy. DTPACE (acronym for dexamethasone, cyclophosphamide, etoposide, and cisplatin with adriamycin and thalidomide) is a phase III study evaluating DTPACE versus high-dose melphalan and autologous transplant in patients with previously treated multiple myeloma.
Methods
Patients enrolled in either of two treatment protocols for multiple myeloma, TT II or DTPACE, were included in the study group. All patients underwent survey MR imaging of the axial skeleton and pelvis. Sagittal T1-weighted (300/15/2 [TR/TE/excitations]) and short-tau inversion-recovery (STIR) (2000/20/2, TI = 150) sequences in three separate slabs were used to evaluate the cervical, thoracic, and lumbar spine. Coronal T1-weighted (300/15/2) and STIR (2000/20/2, TI = 150) sequences were obtained to assess the pelvis. Focal disease was identified in 41 of the 84 patients enrolled in the TT II trial and in 37 of the 67 patients enrolled in the DTPACE trial (Figs 1 and 2). These patients were referred for CT-guided fine-needle aspiration biopsy (CT-FNA).
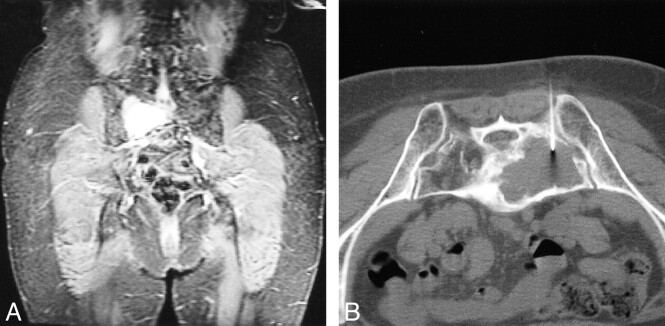
fig 1.
Sacral plasmacytoma in a patient in the TT II trial. A, Coronal STIR T1-weighted image shows a large hyperintense plasmacytoma in the right sacral ala. B, CT-guided FNA of a large lytic lesion shows cytogenetic abnormalities, including chromosome 13 deletion
The lesions selected for CT-FNA were identified on STIR sequences. They were the largest or most accessible lesions that could be safely approached. A new or enlarging lesion was selected from follow-up MR studies. Biopsy sites included the thoracic spine, lumbar spine, and bony pelvis (Table 1). Biopsies were performed under local anesthesia using lidocaine. Depending on the clinical situation, meperidine hydrochloride or promethazine hydrochloride was given intramuscularly for sedation. Biopsies were performed using a coaxial technique with 11- and 13-gauge Manon (Cook, Bloomington, IN), 15-gauge Geremia (Cook), 17-gauge Percucut (E-Z-EM, Westbury, NY), 18-gauge Franseen (Cook), and 18-gauge Spinal (Becton Dickinson, Franklin Lakes, NJ) guide needles. Samples were obtained using 20- and 22-gauge Spinal (Becton Dickinson) and Chiba (Cook) needles and 18- and 20-gauge Franseen (Cook) needles. Three to four passes were performed for each lesion and the specimens were sent for cytogenetic analysis. Core biopsies were not done at this time. Random marrow sampling (RMS) was performed under local anesthesia from the iliac crest with the use of a 15-gauge Illinois needle (Baxter Healthcare, Cherry Hill, NJ). The RMS and FNA specimens were placed in a culture medium containing RPMI 1640 supplemented with 20% fetal bovine serum (Gibco BRL, Grand Island, NY). The cytogenetic results from the CT-FNA and the RMS obtained at or near the same time as the CT-FNA were compared (Tables 2 and 3). Cytogenetic results were considered abnormal if 20 cells in metaphase were present and two or more cells contained the same chromosomal duplication or translocation or if three or more cells contained the same chromosomal deletion. When 20 metaphase cells were not present or not enough cells had the same abnormality, the sample was classified as no growth or as not evaluable.
TABLE 1:
Biopsy sites
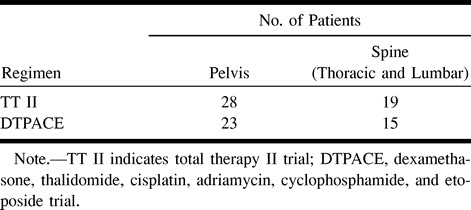
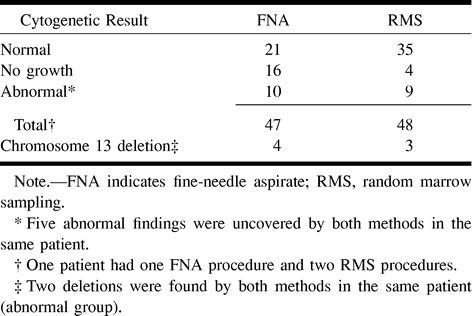
TABLE 2:
TT II trial: cytogenetic results from CT-guided fine-needle aspiration biopsy and random marrow sampling
Results
All biopsies were performed without complication. Between the two protocols, 51 biopsies were performed in the bony pelvis and 34 in the thoracic and lumbar spine. The CT-FNA and RMS results of the patients in the TT II and DTPACE trials are listed in Tables 2 and 3, respectively. In the TT II group, CT-FNA identified 10 abnormal karyotypes with discovery of four chromosome 13 deletions. RMS, meanwhile, identified nine abnormal karyotypes and three chromosome 13 deletions. The identification of similar abnormal karyotypes by both methods occurred in five patients, including two chromosome 13 deletions. New cytogenetic information was revealed in four patients (9.5%) by CT-FNA, including two patients (5%) with an unsuspected chromosome 13 deletion.
In the DTPACE group, FNA identified eight abnormal karyotypes. Chromosome 13 deletions were found in four patients. RMS identified nine abnormal karyotypes and one chromosome 13 deletion. All chromosome 13 deletions were uncovered in different patients. The identification of similar abnormal karyotypes by both methods occurred in one patient. Thus, FNA provided new cytogenetic information in seven patients (19%), including four (11%) with an unsuspected chromosome 13 deletion.
Discussion
No specific causative cytogenetic abnormality has been associated with multiple myeloma. Identification of cytogenetic abnormalities is important, however, because they are affiliated with differing rates of event-free survival, overall survival, complete remission, and sustained complete remission (4). Not all abnormal karyotypes signify a poor prognosis. Multivariate analysis of the various chromosomal abnormalities identified deletions of chromosome 13 or 11q, and any translocation, as poor prognostic factors. Other chromosome abnormalities did not predict negative outcomes (5). [In a recent study comparing overall survival and 5-year event-free survival in patients with multiple myeloma receiving high-dose therapy, Desikan et al (4) reported a 52% rate of 5-year continuous complete remission in 112 patients without chromosome 13 abnormalities with β-2-microglobulin (<2.5 mg/L), C-reactive protein (<4 mg/L), and on standard chemotherapy (<12 months). In 390 patients without chromosome 13 abnormalities in complete remission after high-dose therapy, 35% had a 5-year continuous complete remission whereas none of 54 patients with chromosome 13 abnormalities who were in complete remission sustained a 5-year continuous complete remission. In an overall analysis of all treated patients, the presence of chromosome 13 abnormalities reduced the 5-year event-free survival from 20% to 0% and overall survival from 44% to 16% (4).
Cytogenetic evaluation requires the presence of tumor cells in metaphase. These cells are often found in low numbers in patients without aggressive or advanced disease, since the plasma cells have low mitotic activity. Thus, obtaining necessary cytogenetic information can be extremely difficult. Studies have shown that approximately 30% to 50% of patients have cytogenetic abnormalities (6, 7). Abnormalities are more commonly discovered in previously treated patients than in those with newly diagnosed disease (6). Sawyer et al (7) found that chromosome 13 deletion was the most common chromosomal loss. This abnormality was identified in 9% of all patients and in 29% of patients with abnormal cytogenetics; however, using more advanced techniques, such as fluorescence in situ hybridization, cytogenetic abnormalities can be seen in 80% to 90% of patients with myeloma (8, 9).
CT-guided biopsy is commonly used to diagnose both primary and metastatic tumors of the axial skeleton. CT-guided spinal biopsy is a safe technique with little morbidity and provides adequate tissue for diagnostic purposes in 95% of cases (10, 11). The diagnosis of multiple myeloma in this setting occurs when the biopsy is performed to exclude other diagnostic possibilities, if it was not considered before the procedure. Since the diagnosis of multiple myeloma is most often made with routine laboratory evaluations, biopsy of lytic lesions is not necessary for diagnosis and is not indicated in these situations (12). In our review of the literature, however, no CT-FNA studies have been performed in patients with multiple myeloma to obtain specimens primarily for cytogenetic analysis rather than for diagnostic indicators.
Because all patients with multiple myeloma will have had RMS during their initial diagnostic evaluation and during serial follow-up studies, CT-FNA can only be useful if it provides information not already known to the clinician. CT-FNA identified abnormal karyotypes in approximately 21% of samples in each treatment protocol. This was similar to the number identified by RMS. More significantly, CT-FNA discovered unsuspected cytogenetic abnormalities missed on RMS in nearly 10% of patients in the TT II trial and in 20% of those in the DTPACE trial. These findings include new identification of chromosome 13 deletion in 5% of the patients in the TT II group and in 10% of those in the DTPACE group. This information altered the prognosis and treatment in patients with multiple myeloma (4). Further therapy with a nonmyeloablative matched mini-allotransplant was considered in this group of patients.
CT-FNA should not be performed without appropriate imaging evaluation. Although nearly all patients will have lytic lesions in the spine or pelvis by CT, morphologic determination of which lesion will most likely yield cells with abnormal cytogenetics is very difficult. MR identification of focal lesions has been extensively studied in multiple myeloma (13–16). In our patients, we selected focal lesions that were detected on STIR images that corresponded to a lytic lesion on CT scans, as these may indicate sites with increased plasma cells, more aggressive cells, or different clonal elements. Complications are least likely to occur when biopsies are done in the pelvis. The biopsy site was selected on the basis of the most accessible lesion in the pelvis, then in the lumbar spine, and, last, in the thoracic spine. Other selection criteria included the development of a new lesion or an increase in size of a previously identified lesion as compared with previous MR studies. Lesions occurring in a known radiation therapy site in the pelvis or spine were excluded. Other exclusion criteria were recent surgery, proximity of the surgical and biopsy sites, presence of surgical hardware at the biopsy site, and presence of prosthetic devices, such as braces and ostomy bags.
The yield of abnormal karyotypes by CT-FNA was less than previously reported using RMS. Technical factors account for some of the discrepancy. First, the needle gauge used for FNA sampling is smaller than that used in RMS. We have revised our method of obtaining CT-FNA samples to include five needle passes into the biopsy site. A 20-gauge Chiba needle is used for the first two passes to obtained a core sample of 2 to 3 mm. These are used for cytogenetic analysis. The last three passes were done using 22-gauge Chiba needles and the specimens were sent for other laboratory tests. More passes are made if additional tissue is needed. Our initial analysis showed better cytogenetic results. Second, owing to the delicate nature of the cell specimen, no delay should occur in placing the specimens in the appropriate culture medium for cytogenetic testing. Otherwise, decreased rates of cell growth will lead to increased numbers of specimens that are not evaluable. We have revised our method of handling the cytogenetic specimen to include immediate transport to the laboratory so the specimen can be placed in the appropriate culture medium.
Conclusion
Cytogenetic information is invaluable in determining a prognosis for patients with multiple myeloma. CT-FNA is a safe technique that, when used in conjunction with an MR survey of the axial skeleton, may identify cytogenetic abnormalities not seen during RMS.
TABLE 3:
DTPACE trial: cytogenetic results from CT-guided fine-needle aspiration biopsy and random marrow sampling
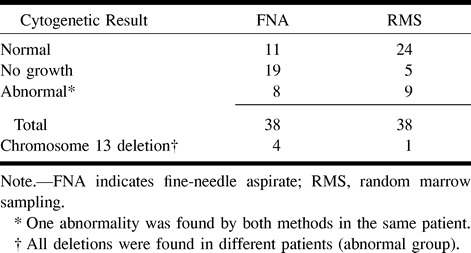
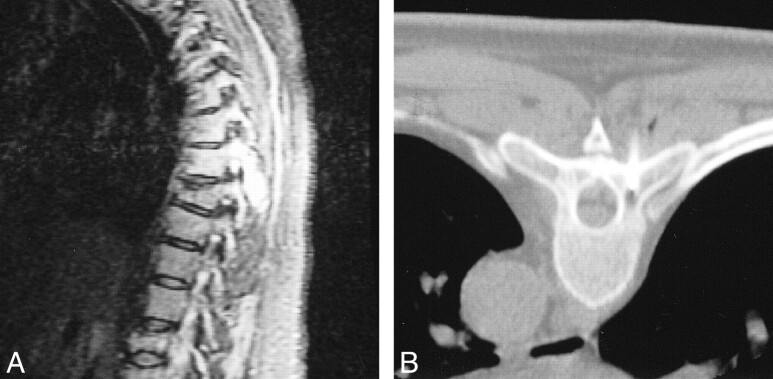
fig 2.
T6 plasmacytoma of the posterior elements in a patient in the DTPACE trial. A, Sagittal STIR T1-weighted image shows a large hyperintense lesion involving the spinous process and right lamina. B, CT scan shows only a small lytic component, for which biopsy revealed chromosome 13 deletion.
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, Atlanta, April 2000.
Address reprint requests to Edgardo J. Angtuaco, MD, 4301 W Markham, Slot 556, Little Rock, AR 72205.
References
- 1.Barlogie B. Plasma cell myeloma. In: Quips T, ed. William's Hematology. 5th ed. New York: McGraw-Hill 1995;1109-1126
- 2.Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer 1975;36:842-854 [DOI] [PubMed] [Google Scholar]
- 3.Kyle R. Why better prognostic factors for multiple myeloma are needed. Blood 1994;83:1713-1716 [PubMed] [Google Scholar]
- 4.Desikan R, Barlogie B, Sawyer JR, et al. Results of high dose therapy for 1,000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalities. Blood 2000;95:4008-4010 [PubMed] [Google Scholar]
- 5.Tricot G, Sawyer JR, Jagannath S, et al. Unique role of cytogenetics in the prognosis of patients with myeloma receiving high-dose therapy and autotransplants. J Clin Oncol 1997;15:2659-2666 [DOI] [PubMed] [Google Scholar]
- 6.Feinman R, Sawyer J, Hardin J, Tricot G. Cytogenetics and molecular genetics in multiple myeloma. Hematol Oncol Clin North Am 1997;11:1-25 [DOI] [PubMed] [Google Scholar]
- 7.Sawyer JR, Waldron JA, Jagannath S, Barlogie B. Cytogenetic findings in 200 patients with multiple myeloma. Cancer Genet Cytogenet 1995;82:41-49 [DOI] [PubMed] [Google Scholar]
- 8.Tabernero D, Miguel JFS, Garcia-Sanz R, et al. Incidence of chromosome numerical changes in multiple myeloma: fluorescence in situ hybridization analysis using 15 chromosome-specific probes. Am J Pathol 1996;149:153-161 [PMC free article] [PubMed] [Google Scholar]
- 9.Drach J, Schuster J, Nowotny H, et al. Multiple myeloma: high incidence of chromosomal aneuploidy as detected by interphase fluorescence in situ hybridization. Cancer Res 1995;55:3854-3859 [PubMed] [Google Scholar]
- 10.Silva P, Donahue FI, Latchaw RE. Percutaneous CT-guided biopsy of the spine: methodology to insure a high diagnostic yield and the effects on patient management. In: Proceedings of the Annual Meeting of the American Society of Neuroradiology, Atlanta, April 2000. Oak Brook, IL: American Society of Neuroradiology 2000;68-69
- 11.Mink J. Percutaneous bone biopsy in the patient with known or suspected osseous metastases. Radiology 1986;161:191-194 [DOI] [PubMed] [Google Scholar]
- 12.Grey MR, Kelsey P. Delayed diagnosis and unnecessary percutaneous biopsies in cases of myeloma presenting as chest wall tumours. Clin Lab Haematol 1998;20:259-262 [DOI] [PubMed] [Google Scholar]
- 13.Angtuaco E, Avva R, Munshi N, Peterson L, Spoon D, Barlogie B. MR skeletal survey of the axial skeleton: a predictor of patient survival in multiple myeloma? In: Proceedings of the 85th Scientific Assembly & Annual Meeting of the Radiological Society of North America, Chicago, December 1999. Oak Brook, IL: Radiological Society of North America 2000;294
- 14.Moulopoulos LA, Varma DGK, Dimopoulos MA, et al. Multiple myeloma: spinal MR imaging in patients with untreated newly diagnosed disease. Radiology 1992;185:833-840 [DOI] [PubMed] [Google Scholar]
- 15.Vande Berg BC, Lecouvet FE, Michaux L, et al. Stage I multiple myeloma: value of MR imaging of the bone marrow in the determination of prognosis. Radiology 1996;201:243-246 [DOI] [PubMed] [Google Scholar]
- 16.Lecouvet FE, Vande Berg BC, Michaux L, et al. Stage III multiple myeloma: clinical and prognostic value of spinal bone marrow MR imaging. Radiology 1998;209:653-660 [DOI] [PubMed] [Google Scholar]