Abstract
BACKGROUND AND PURPOSE: Proton MR spectroscopy (MRS) is still in the early stages in the evaluation of epilepsy, and comparisons with MR imaging and positron emission tomography (PET) in the same patients have rarely been documented. The purpose of this study was to evaluate the lateralizing ability of single-voxel MRS in comparison with MR imaging and PET in patients with hippocampal sclerosis.
METHODS: Thirty-three patients with intractable temporal lobe epilepsy whose MR imaging diagnosis was unilateral hippocampal sclerosis and who underwent anterior temporal lobectomy and had good postsurgical outcome over 1-year follow-up were included in the study. MR spectra were obtained from the hippocampus bilaterally, using the point-resolved spectroscopy sequence. Metabolite ratios of NAA/Cho and NAA/Cr were calculated from the relative peak height measurements. An NAA/Cho ratio of 0.8 or less and an NAA/Cr ratio of 1.0 or less were regarded as abnormal. The MRS results were compared retrospectively with those of MR imaging and PET as to the ability to lateralize the epileptogenic focus.
RESULTS: The sensitivity of MRS and PET (concordance with MR imaging) was 85% each in the lateralization of the ipsilateral lesion side. Bilateral abnormalities were seen in 30% of the patients. False-lateralization rates for MRS and PET were 3% and 6%, respectively. The concordance rate of MRS and PET was 73%, when comparing the results of the ipsilateral lesion side.
CONCLUSION: MRS may be used as an adjunct tool in the evaluation of hippocampal sclerosis, like PET, although its sensitivity has to be improved and the clinical significance of bilateral abnormality is still to be determined.
Hippocampal sclerosis is the most common substrate in patients with medically intractable temporal lobe epilepsy (1, 2). Although the pathophysiology of hippocampal sclerosis is not completely understood, it is histologically characterized by neuronal loss and gliosis of the hippocampus (1). Most patients with hippocampal sclerosis have persistent seizures despite medical treatment and may benefit from surgery. In fact, surgical resection of the hippocampus and anterior temporal lobe can cure epilepsy in as many as 90% of these patients (3–5). Therefore, accurate preoperative localization of the seizure focus is essential to the success of seizure surgery (6). Sleep-deprivation electroencephalography (EEG) and video-surface EEG monitoring are the primary laboratory methods for localizing ictal activity, but the success rate of EEG methods ranges from 60% to 90% (7). Currently, MR imaging is considered the imaging method of choice for localizing the epileptogenic focus. Single-photon emission CT (SPECT), positron emission tomography (PET), proton MR spectroscopy (MRS), and invasive studies are complementary tools, particularly for patients with inconclusive lateralization on video-EEG and MR imaging (8–12).
SPECT and PET have been studied extensively in the localization of epileptogenic foci, but MRS is still in the early stage of clinical application in patients with epilepsy. To our knowledge, studies comparing MRS with MR imaging and PET are scarce (13), presumably because of the limited availability of the techniques and the continuing advances in technology.
The purpose of our study was to evaluate the ability of single-voxel proton MRS to lateralize the seizure focus in patients with hippocampal sclerosis as compared with that of MR imaging and PET.
Methods
Subjects
Our study included 33 patients with temporal lobe epilepsy (21 men and 12 women; age range, 17 to 46 years; mean age, 28 years) who had MR imaging findings of unilateral hippocampal sclerosis, underwent anterior temporal lobectomy, and had a good postsurgical outcome (Engel class I or II) during a follow-up period of least 12 months after surgery. From July 1997 through March 1999, a total of 79 patients underwent surgery for medically intractable temporal lobe epilepsy in our institution. The presurgical diagnoses included unilateral hippocampal sclerosis on MR imaging (n = 55), neoplasm (n = 6), cavernous angioma (n = 2), cortical dysplasia (n = 2), and others (n = 14). Preoperatively, it was our intention that the patients with hippocampal sclerosis were to have MR imaging, MRS, and PET. In fact, although MR imaging was performed in all the patients, owing to equipment or technical problems, unavailable radioactive tracers, or the high cost of PET, only 40 patients had MRS and 39 patients had PET. Among the 39 patients who underwent MR imaging, MRS, and PET, 33 had a good postsurgical outcome (Engel class I or II) and four had a poor postsurgical outcome (Engel class III or IV). The remaining two patients were lost to follow-up. The postoperative outcome at the latest follow-up was classified according to Engel's four categories (14) by an epileptologist. In this scheme, class I (seizure-free) indicates an absence of seizure activity since surgery, regardless of medication; class II indicates rare seizures, meaning a few seizures in a year; class III indicates worthwhile improvement, meaning at least a 75% improvement in seizure frequency compared with preoperative status; and class IV denotes no worthwhile improvement.
MR Imaging
Standard MR imaging was performed on a clinical 1.5-T MR unit. The temporal lobes and hippocampi were evaluated with 2D T2-weighted fast spin-echo sequences with 3-mm-thick sections and with T1-weighted 3D magnetization-prepared rapid acquisition (MP-RAGE) gradient-echo sequences with 1.5-mm-thick sections in the oblique coronal plane perpendicular to the long axis of the hippocampus. Spatial resolution was approximately 1.0 × 1.0 mm (matrix, 256 × 256; field of view, 25 cm). The criteria for a diagnosis of hippocampal sclerosis on MR images included the presence of unilateral atrophy and high T2 signal of the hippocampus.
Proton MRS
Single-voxel proton MRS was carried out on the same day or within 1 week after MR imaging on the same 1.5-T unit as MR imaging. The MR spectra were obtained from the volume of interest (VOI) positioned in the medial temporal lobes, including most of the head and body of the hippocampus and a part of the amygdala, the parahippocampal gyrus, and CSF of the temporal horn, bilaterally. The VOI size was approximately 2.25 cm3 (1.5 × 1.5 × 1.0 cm) in most patients. The VOI placement in this study avoided potential magnetic susceptibility artifacts from the skull base and sphenoidal sinus and contamination from fat in the skull base. The spectra were obtained using a point-resolved spin-echo spectroscopy (PRESS) sequence with parameters of 1500–2000/144, 288 (TR/TE) and 128 scans in all subjects. Before spectroscopic measurements were obtained, the field homogeneity was optimized over the selected VOI by observing the MR signal of tissue water with the spatially selective PRESS sequence. Typical full widths at half maximum of 4 to 8 Hz were achieved in most examinations. The water signal was suppressed by a frequency-selective saturation pulse at the water resonance. A sweep width of 1000 Hz was used with data size of 1024 points. Only the second half of the echo was acquired. Following the zero-filling of 4096 points in all free-induction-decay data, exponential line broadening (center, 0 msec; half-time, 150 msec) was done before Fourier transformation. A zero-order phase correction was applied to all spectra.
The MR spectra were evaluated with a focus on the metabolite ratios of N-acetylaspartate (NAA) at 2 ppm, creatine–phosphocreatine couple (Cr) at 3 ppm, and choline–containing compounds (Cho) at 3.2 ppm. The metabolite ratios of NAA/Cho and NAA/Cr were calculated from the relative peak height measurement. The MRS results were interpreted as abnormal when the NAA/Cho ratio was less than 0.8 or the NAA/Cr ratio was less than 1.0, or both, in accordance with the results of a previous study (15).
PET
PET was performed with an IV injection of 370 MBq(10 mCi) of 18F-fluorodeoxyglucose (FDG) during the interictal period on an ECAT EXACT 47 scanner. Acquisition time was approximately 20 minutes. Axial and coronal images were reconstructed with a Shepp-Logan filter (cut off, 0.30 cycles per pixel) as matrices of 128 × 128 × 47, with a size of 2.1 × 2.1 × 3.4 mm. The criterion for abnormality was the presence of an asymmetric decrease in FDG uptake (asymmetry of 15% or more).
Comparative Evaluation of MR Imaging, MRS, and PET
The original MRS results were compared with those of MR imaging and PET without retrospective reinterpretation with regard to the concordance rate for lateralization of the epileptogenic focus, the sensitivity of MRS with the use of MR imaging as the standard of reference, the false-negative rate, and the rate of bilateral abnormalities. The results of PET were also assessed with the focus on the concordance rate with MR imaging for lateralization of the epileptic focus and on the false-negative and false-positive rates.
Results
Preoperatively, MRS was concordant with MR imaging on both sides in 18 (55%) of 33 patients. When comparing the results on only the ipsilateral side, regardless of the results on the contralateral side, MRS was concordant with MR imaging in 28 (85%) of the patients (Fig 1). Among these 28 patients, both the NAA/Cho and NAA/Cr ratios were abnormal in 13 patients, only the NAA/Cho ratio was abnormal in seven, and only the NAA/Cr ratio was abnormal in eight. The false-negative rate of MRS on the ipsilateral side was 15% (five of 33 patients) (Fig 2). Among these five patients with false-negative results, four had no abnormality in either hippocampus and one had abnormal MRS findings in the contralateral hippocampus, which appeared normal on MR images (false-positive result) (Fig 2 and Table 1). When comparing the results for the contralateral (normal) side, MRS was concordant with MR imaging in 22 (67%) of the 33 patients. Abnormal MRS findings were noted on the normal-appearing contralateral side on MR images in 11 (33%) of the 33 patients. Bilateral abnormalities at MRS were seen in 10 (30%) of the 33 patients, in whom MR images showed unilateral hippocampal sclerosis (Fig 3). Of the 11 patients with abnormal MRS findings on the contralateral side (10 bilateral abnormal MRS findings and one unilateral false-positive MRS finding), four had abnormalities in both the NAA/Cho and NAA/Cr ratios, five had an abnormality in only the NAA/Cho ratio, and two had an abnormality in only the NAA/Cr ratio.
fig 1.
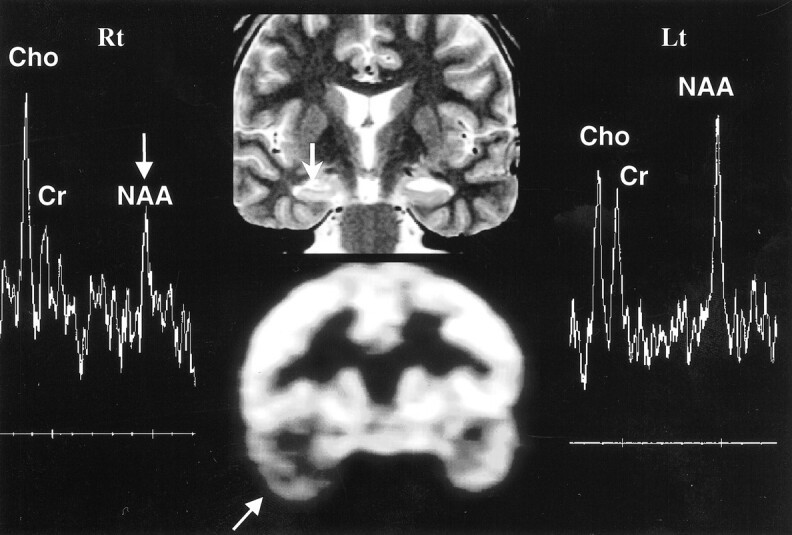
Concordance among MR imaging, MRS, and PET in right hippocampal sclerosis. Oblique coronal T2-weighted MR image (top row, middle) shows right hippocampal sclerosis (arrow), in concordance with PET scan (bottom row, middle), which shows decreased metabolism in right temporal lobe (arrow). The MR spectrum from the right hippocampus (Rt) shows marked decrease in NAA/Cho ratio (arrow). The MR spectrum from left hippocampus is normal (Lt)
fig 2.
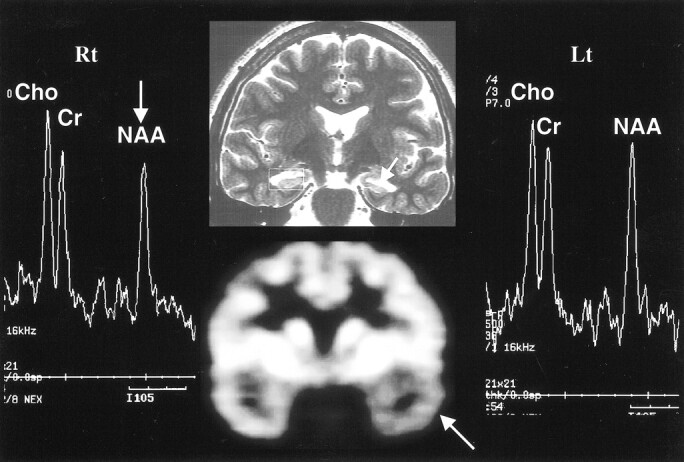
Discordant MRS with MR imaging and PET in left hippocampal sclerosis. Oblique coronal T2-weighted MR image (top row, middle) shows left hippocampal atrophy (arrow), in concordance with PET scan (bottom row, middle), which shows decreased metabolism in left temporal lobe (arrow). However, the MR spectrum from the left hippocampus (Lt) appears within normal range (false-negative finding), based on the abnormal criteria of an NAA/Cho ratio of 0.8 or less or an NAA/Cr ratio of 1.0 or less. The MR spectrum from the left hippocampus (Lt) shows a decrease in NAA/Cho and NAA/Cr ratios (arrow) on right side (Rt) (false-positive finding)
Summary of MRS and PET results in lateralization of hippocampal sclerosis (n = 33)

fig 3.
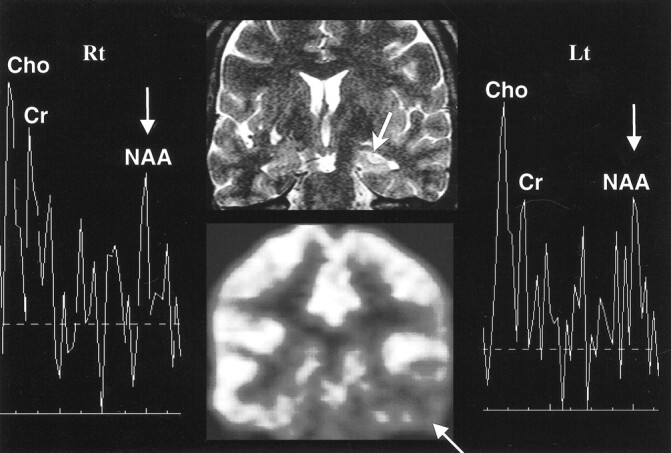
Bilateral abnormality on MRS in left hippocampal sclerosis. Oblique coronal T2-weighted MR image (top row, middle) shows left hippocampal sclerosis with increased signal intensity (arrow), in concordance with PET scan (bottom row, middle), which shows decreased metabolism (arrow) in left temporal lobe. The MR spectra (Rt and Lt) show bilateral abnormalities (arrows), decreased NAA/Cho ratios bilaterally, and decreased NAA/Cr ratio on right side
PET was concordant with MR imaging in 28 (85%) of the 33 patients (Figs 1 and 2). Among the five patients with discordance between PET and MR imaging, three (9%) had no abnormality on PET studies on either side. In the other two patients (6%), the PET results were opposite to the results of MR imaging. With MR imaging as the standard of reference, false-negative and false-positive rates of PET were 15% (five of 33) and 6% (two of 33), respectively.
MRS of both sides was concordant with PET in 14 (42%) of the 33 patients. When comparing MRS with PET on only the ipsilateral lesion side, regardless of the results on the contralateral side, MRS was concordant with PET in 24 (73%) of the 33 patients. Among the nine patients with discordance between MRS and PET (27%), MRS correctly lateralized the lesion side (concordant with MR imaging) in four patients, whereas PET correctly lateralized the lesion side in the other four patients. In the remaining patient, both MRS and PET were discordant with MR imaging. When comparing MRS with PET results on the contralateral (normal) side as seen on MR images, MRS was concordant with PET in 20 (61%) of the 33 patients. Of the 13 patients with discordance between MRS and PET findings, MRS was concordant with MR imaging in two patients, whereas PET was concordant with MR imaging in the other 11 patients.
Discussion
In the management of temporal lobe epilepsy, exact lateralization/localization of seizure focus with a noninvasive study is crucial, because surgical resection of the epileptogenic lesion results in good outcome (Engel class I or II) and a failure to lateralize the focus with noninvasive examination may lead to an invasive study or to additional surgery for placement of intracranial electrodes, which may have potential risks (16, 17). However, many sources of temporal lobe epilepsy, including hippocampal sclerosis and developmental anomalies, may not be recognized, even by a variety of MR imaging techniques, including MR volumetry and measurement of T2 relaxation time, SPECT, and PET (8–12, 18–26).
Recent preliminary reports on proton MRS have noted that while MRS is promising it provides variable results in the evaluation of mesial temporal lobe epilepsy (27–33). Most MRS studies in patients with temporal lobe epilepsy have shown a decrease in NAA, NAA/Cr, and/or NAA/Cho+Cr (28–31). Some studies have shown a decrease in only NAA without changes in Cr or Cho (32, 33), whereas others have reported an increase in Cr and Cho (27, 28). Common to all studies is the decrease of NAA in the affected temporal lobe of patients with epilepsy as compared with control subjects. In our previous study (15), NAA/Cho and NAA/Cr ratios measured in 20 healthy control subjects were greater than 0.8 and 1.0, respectively, with a 95% confidence level (2 SD). These results were based on the determination of abnormal criteria of NAA/Cho and NAA/Cr ratios in the present study. In general, our results agree with those of others in showing decreased NAA/Cho or NAA/Cr in the hippocampus of patients with hippocampal sclerosis (28–31). A reduction in NAA/Cho or NAA/Cr may be interpreted as a decrease of NAA, reflecting neuronal loss, an increase in Cho or Cr, presumably reflecting gliosis (27, 34), or both.
In the present study, MRS revealed an abnormality in the affected hippocampal region in only 28 (85%) of the 33 patients with hippocampal sclerosis. The reason for the false-negative rate of 15% is uncertain, although it might be attributable in large part to the partial volume effect. MR spectra in the present study were obtained from the VOI, including the hippocampal head and body and parts of the normal parenchyma of the temporal lobe and CSF of the temporal horn. In the cases of severe hippocampal atrophy, a larger part of the normal temporal lobe included in the VOI might have caused the false-negative results. To minimize the potential partial volume effect, we reduced the size of the VOI to 1.5 × 1.5 × 1.0 cm.
The results from the contralateral hippocampus in the present study differ somewhat from those reported by other investigators (27, 35). One study found that the contralateral hippocampus of patients with temporal lobe epilepsy showed MRS features similar to those in control subjects (35). However, Conelly et al (27) reported that levels of NAA, Cho, and NAA/Cho+Cr ratio were significantly lower in the contralateral hippocampus of patients with temporal lobe epilepsy than in those of healthy control subjects. These investigators also found bilateral abnormalities in 10 (40%) of 25 patients studied with MRS. In our study, bilateral abnormalities were found in 10 (30%) of 33 patients. It is believed that bilateral abnormalities indicate the existence of real bilateral disease rather than a false-positive result on the contralateral side, even though the clinical significance of MRS abnormality on the contralateral side has yet to be determined. These results are consistent with the finding of bilateral disease in up to 50% of patients with temporal lobe epilepsy in one study (36).
Several FDG-PET studies in patients with temporal lobe epilepsy have shown a 60% to 90% prevalence of hypometabolism in the temporal lobe (8, 9, 37–41). In the present study, PET showed hypometabolism on the lesion side in 28 (85%) of 33 patients, which was concordant with MR imaging findings. Achten et al (13) reported comparable findings in a comparative study of MRS and PET for lateralization of refractory temporal lobe epilepsy. In their study, MRS and PET lateralized the seizure focus in 76% and 76% of the patients, respectively, when EEG findings were regarded as the standard of reference. The reasons why both PET and MRS showed higher rates of correct lateralization in our study are uncertain. Several factors, including a different subject population, different imaging techniques, and a different standard of reference, might account for this discrepancy.
In our study, PET results on the lesion side were concordant with MRS findings in 24 (73%) of the 33 patients. In four (27%) of the nine patients in whom PET and MRS showed discordant findings, MRS correctly lateralized the lesion side, but PET did not. The cause of this discordance between PET and MRS is unknown. Achten et al (13) postulated that glucose hypometabolism might represent a more advanced state of neuronal change than does low NAA. The false lateralization rates (false-positive result on the contralateral side and false-negative result on the ipsilateral side) for MRS and PET were 3% (one in 33 patients) and 6% (two in 33 patients), respectively.
The discrepancies among the different imaging techniques may be explained by the fact that they measure different aspects of the epileptic process; that is, MR imaging depicts only gross anatomic alterations associated with epilepsy whereas PET has the unique ability to image cerebral metabolism and MRS measures the metabolites of cellular chemical components associated with epilepsy. MR imaging and PET have an advantage over MRS in visualizing the whole brain; they not only depict the lesion in the medial temporal lobe but can also show abnormalities in other regions, such as an asymmetric, small, ipsilateral mamillary body and fornix on MR images in a patient with hippocampal sclerosis (42), and an asymmetric hypometabolism of the thalamus on PET scans in a patient with mesial temporal lobe epilepsy (43). MRS, on the other hand, is a multifocal technique that only samples selected areas.
Precise localization and removal of the epileptogenic focus is the ultimate treatment goal in patients with medically intractable epilepsy. Ideally, the probability of cutting the primary seizure focus out of the brain must approach 100% before an irreversible lobectomy is performed. However, until now, no single diagnostic test has proved sufficient in localizing the site to be surgically resected. At our institution, the presurgical evaluation protocol includes interictal EEG, MR imaging, ictal video-EEG monitoring with scalp electrodes, interictal PET, ictal SPECT, if possible, neuropsychological testing, and Wada test. When the lateralization or localization is inconclusive, with some discrepancy among the multiple techniques, an invasive study, usually subdural electrodes, is performed. In the present study, when we exclude the two patients who were lost to follow-up, the good postsurgical outcome rate reached 89% (33 of 37 patients).
Our results indicate that MRS is similar to PET in the ability to lateralize hippocampal sclerosis and is superior to PET in its ability to detect bilateral abnormalities. Because the results of MR imaging and postsurgical outcome were concordant with MRS in some patients and were concordant with PET in others, MRS may be useful as an adjunct for lateralization of seizure focus in the evaluation of hippocampal sclerosis. However, further studies are required for patients in whom lateralization of the seizure focus is inconclusive.
Conclusion
When using both MR imaging findings of unilateral hippocampal sclerosis and good postsurgical outcome as the standards of reference, we found that MRS had a sensitivity of 85% in the detection of ipsilateral abnormality. PET was also concordant with MR imaging in 85% of cases. The concordance rate of MRS and PET was 73% when comparing the results of the ipsilateral lesion side. The rate of bilateral abnormality with MRS was 30% (10 of 33 patients). The false-lateralization rates of MRS and PET were 3% and 6%, respectively.
MRS may be useful as an adjunct in the evaluation of hippocampal sclerosis, like PET, although its sensitivity has to be improved and the clinical significance of bilateral abnormalities remains to be determined. Further investigation is needed to ascertain the practical role of MRS in the evaluation of patients with inconclusive lateralization of the seizure focus before MRS is widely used in the presurgical workup of patients in clinical practice.
Footnotes
Supported by a grant from the Ministry of Health & Welfare, Korea (HMP-99-N-02-0003).
Address reprint requests to Kee-Hyun Chang, MD, Department of Diagnostic Radiology, Seoul National University Hospital, 28 Yeongon-dong, Chongno-gu, Seoul 110-744, Korea.
References
- 1.Babb T, Brown WJ. Pathological findings in epilepsy. In: Engel J Jr, ed. Surgical Treatment of the Epilepsies. New York: Raven; 1987;511-540
- 2.Bruton CJ. The Neuropathology of Temporal Lobe Epilepsy.. Oxford: Oxford University Press 1988;1-158
- 3.Jack CR, Sharbrough FW, Casino GD, Hirschorn KA, O'Brien PC, Marsh WR. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol 1992;31:138-146 [DOI] [PubMed] [Google Scholar]
- 4.Jack CR. Epilepsy: surgery and imaging. Radiology 1993;189:635-646 [DOI] [PubMed] [Google Scholar]
- 5.Bronen RA. Epilepsy: the role of MR imaging. AJR Am J Roentgenol 1992;159:1165-1174 [DOI] [PubMed] [Google Scholar]
- 6.Jayakar P, Duchonwny M, Resnick JJ, Aivarey LA. Localization of seizure foci: pitfalls and caveats. J Clin Neurophysiol 1991;8:414-431 [DOI] [PubMed] [Google Scholar]
- 7.Schomer DJ. Current concepts in neurology: partial epilepsy. N Engl J Med 1983;309:536-539 [DOI] [PubMed] [Google Scholar]
- 8.Spencer SS. The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia 1994;35:S72-S89 [DOI] [PubMed] [Google Scholar]
- 9.Duncan JS. Imaging and epilepsy. Brain 1997;120:339-377 [DOI] [PubMed] [Google Scholar]
- 10.Stefan H, Pawlik G, Bocher-Schwarz HG, et al. Functional and morphological abnormalities in temporal lobe epilepsy: a comparison of interictal and ictal EEG, CT, SPECT and PET. J Neurol 1987;234:377-384 [DOI] [PubMed] [Google Scholar]
- 11.Coubes P, Awad IA, Antar M, et al. Comparison and spatial correlation of interictal HMPAO-SPECT and FDG-PET in intractable temporal lobe epilepsy. Neurol Res 1993;15:160-167 [DOI] [PubMed] [Google Scholar]
- 12.Won HJ, Chang KH, Cheon JE, et al. Comparison of MR imaging with PET and ictal SPECT in 118 patients with intractable epilepsy. AJNR Am J Neuroradiol 1999;20:593-599 [PMC free article] [PubMed] [Google Scholar]
- 13.Achten E, Santens P, Boon P, et al. Single-voxel proton MR spectroscopy and positron emission tomography for lateralization of refractory temporal lobe epilepsy. AJNR Am J Neuroradiol 1998;19:1-8 [PMC free article] [PubMed] [Google Scholar]
- 14.Engel J Jr. Outcome with respect to seizures. In: Surgical Treatment of the Epilepsies. New York: Raven 1987;553-571
- 15.Chang KH, Kim HD, Park SW, et al. Usefulness of single voxel proton MR spectroscopy in the evaluation of hippocampal sclerosis. Korean J Radiol 2000;1:25-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson F. Identification of Candidates for Surgical Treatment of the Epilepsies. New York: Raven; 1987;51-70
- 17.Awad IA, Rosenfeld J, Ahl J, Hahn JF, Luders H. Intractable epilepsy and structural lesions of the brain: mapping, resection strategies, and seizure outcome. Epilepsia 1991;32:179-186 [DOI] [PubMed] [Google Scholar]
- 18.Baulac H, Granat O, Gao X, Laplane D. Hippocampal sclerosis and temporal lobe epilepsy: a MRI study. Epilepsia 1991;32:2-3 [Google Scholar]
- 19.Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GCA, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology 1990;40:1869-1875 [DOI] [PubMed] [Google Scholar]
- 20.Lencz T, McCarthy G, Bronen RA, et al. Qualitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol 1992;31:629-637 [DOI] [PubMed] [Google Scholar]
- 21.Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphologic studies in frontal and temporal lobe epilepsy. Brain 1992;115:1001-1015 [DOI] [PubMed] [Google Scholar]
- 22.Ronen RA, Cheung G, Charles JT, et al. Imaging findings in hippocampal sclerosis: correlation with pathology. AJNR Am J Neuroradiol 1991;12:933-940 [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson GD, Berkovic SF, Duncan JS, Conelly A. Optimizing the diagnosis of hippocampal sclerosis using magnetic resonance imaging. AJNR Am J Neuroradiol 1993;14:753-762 [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson GD, Conelly A, Duncan JS, Grunewald RA, Gadian DG. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative magnetic resonance T2 relaxometry. Neurology 1993;43:1793-1799 [DOI] [PubMed] [Google Scholar]
- 25.Cheon JE, Chang KH, Kim HD, et al. MR of hippocampal sclerosis: comparison of qualitative and quantitative assessments. AJNR Am J Neuroradiol 1998;19:465-468 [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer SS, Theodore WH, Berkovic SF. Clinical applications: MRI, SPECT, and PET. Magn Reson Imaging 1995;13:1119-1124 [DOI] [PubMed] [Google Scholar]
- 27.Connelly A, Jackson GD, Duncan JS. King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology 1994;44:1411-1417 [DOI] [PubMed] [Google Scholar]
- 28.Gadian DG, Conelley A, Duncan JS, et al. 1H Magnetic resonance spectroscopy in the investigation of intractable epilepsy. Acta Neurol Scand 1994;152: (Suppl) 116-121 [DOI] [PubMed] [Google Scholar]
- 29.Garcia PA, Laxer KD, Ng T. Application of spectroscopic imaging in epilepsy. Magn Reson Imaging 1995;13:1181-1185 [DOI] [PubMed] [Google Scholar]
- 30.Cross JH, Conelly A, Jackson GD, et al. Proton magnetic resonance imaging in children with temporal lobe epilepsy. Ann Neurol 1996;39:107-113 [DOI] [PubMed] [Google Scholar]
- 31.Cendes F, Andermann F, Preul MC, et al. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopy images. Ann Neurol 1994;35:211-216 [DOI] [PubMed] [Google Scholar]
- 32.Hugg JW, Laxer KD, Matson GB, et al. Neuronal loss localized human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopy imaging. Ann Neurol 1993;34:788-794 [DOI] [PubMed] [Google Scholar]
- 33.Epstein CM, Boor D, Hoffman JC, et al. Evaluation of 1H magnetic resonance spectroscopic imaging as a diagnostic tool for the lateralization of epileptogenic seizure foci. Br J Radiol 1996;69:15-24 [DOI] [PubMed] [Google Scholar]
- 34.Matthews PM, Andermann F, Arnold DL. A proton magnetic resonance spectroscopy study of focal epilepsy in humans. Neurology 1990;40:985-989 [DOI] [PubMed] [Google Scholar]
- 35.Thomson JE, Castillo M, Kwock L, Walters B, Beach R. Usefulness of proton MR spectroscopy in the evaluation of temporal lobe epilepsy. AJR Am J Roentgenol 1998;170:771-776 [DOI] [PubMed] [Google Scholar]
- 36.Margerison JH, Corsellis JAN. Epilepsy and the temporal lobes. Brain 1966;89:499-530 [DOI] [PubMed] [Google Scholar]
- 37.Engel J Jr, Kuhl DE, Phelps ME, Crandall PH. Comparative localization of epileptic foci in partial epilepsy by PCT and EEG. Ann Neurol 1982;12:529-537 [DOI] [PubMed] [Google Scholar]
- 38.Gailard WD, White S, Malow B, et al. FDG-PET in children and adolescents with partial seizures: role in epilepsy surgery evaluation. Epilepsy Res 1995;20:7-84 [DOI] [PubMed] [Google Scholar]
- 39.Kuhl DE, Engel J Jr, Phelps ME, Selin C. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann Neurol 1980;8:348-360 [DOI] [PubMed] [Google Scholar]
- 40.Ryvlin P, Cinotti L, Froment JC, et al. Metabolic patterns associated with non-specific magnetic resonance imaging abnormalities in temporal lobe epilepsy. Brain 1991;114:2363-2368 [DOI] [PubMed] [Google Scholar]
- 41.Falconer MA. Mesial temporal (Anmon's horn) sclerosis as a common cause of epilepsy: aetiology, treatment and prevention. Lancet 1974;2:727-770 [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N. Clinical significance of asymmetry of the fornix and mamillary body on MR in hippocampal sclerosis. AJNR Am J Neuroradiol 1995;16:509-515 [PMC free article] [PubMed] [Google Scholar]
- 43.Rausch R, Henry TR, Ary CM, et al. Asymmetric interictal glucose hypometabolism and cognitive performance in epileptic patients. Arch Neurol 1994;51:139-144 [DOI] [PubMed] [Google Scholar]


