Abstract
Summary: Left vocal cord paralysis in association with patent ductus arteriosus is unusual. We report a patient with long-standing patent ductus arteriosus (PDA) in whom CT studies obtained before and after paralysis developed showed an interval increase in size of the pulmonary trunk. The pathogenesis of left vocal cord paralysis in association with long-standing PDA is discussed.
Patent ductus arteriosus (PDA) is a congenital cardiovascular malformation usually diagnosed in childhood. Left vocal cord paralysis in association with long-standing PDA is uncommon (1–3). We report a case of left vocal cord paralysis in a 76-year-old woman with PDA in whom CT was able to depict the disorder.
Case Report
A 76-year-old woman presented with 10-day history of hoarseness and a 10-year history of shortness of breath. Two years previously, at age 74, she had undergone a CT examination of the chest for dyspnea (Fig 1A and B). The CT scans showed a dilated pulmonary trunk, but the PDA remained undiagnosed. Seven months before the current presentation, the patient's symptoms had worsened, and at that time, at age 75, she was admitted to another hospital, where auscultation over the third left sternal border showed a grade 2/6 continuous murmur. Transthoracic or transesophageal echocardiography was nondisclosive, but cardiac catheterization successfully revealed PDA (Fig 1C), showing pulmonary hypertension and no reversal shunt (aortic pressure of 185/67 mm Hg, pulmonary artery pressure of 55/26 mm Hg, and right ventricular pressure of 48/8 mm Hg). Because of her age and refusal to undergo any surgical intervention, the patient was treated with diuretics and improved.
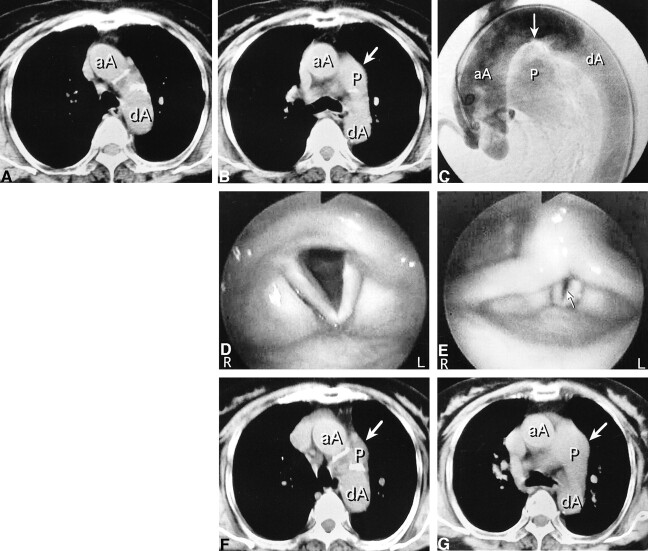
fig 1.
76-year-old woman with long-standing PDA.
A and B, Axial CT studies at age 74, obtained for dyspnea, before left vocal cord paralysis developed. The pulmonary trunk is not present at the level of the lower border of the aortic arch (A). At the level of the carina (B), the main pulmonary trunk (arrow) is larger than normal.
C, Digital subtraction aortogram, lateral view, at age 75, shows flow into the pulmonary artery through the PDA (arrow).
D and E, At age 76, endoscopic views of larynx during quiet respiration (D) and phonation (E) show a large glottic gap (arrow) due to bowing of the paralyzed left vocal cord.
F and G, Axial CT scans at age 76, obtained after paralysis developed. At the level of the lower border of the aortic arch (F), the pulmonary trunk (arrow) is present in the same section in which it was not seen before (A). There are no other mediastinal lesions. At the level of the carina (G), an enlarged and upwardly displaced pulmonary trunk (arrow) is seen adjacent to the expected course of the left recurrent laryngeal nerve.
aA indicates ascending aorta; dA, descending aorta; P, pulmonary trunk.
A sudden onset of hoarseness caused her to be referred to our institution. Until then, she had had no history of dysphonia. Laryngeal examination revealed paralysis of the left vocal cord (Fig 1D and E). The remainder of the larynx, hypopharynx, and oropharynx was normal. A CT study of the neck was performed to identify the cause of this paralysis, but there were no significant findings related to the paralysis. Esophagoscopy disclosed no pathologic lesion. A CT scan of the chest obtained 2 weeks after the onset of hoarseness (Fig 1F and G) showed that the pulmonary trunk had enlarged relative to the CT findings 2 years earlier. There was no mediastinal mass or enlarged hilar lymph node, and it was believed that the enlarged and upwardly displaced pulmonary trunk was responsible for the compression of the left recurrent laryngeal nerve and the left vocal cord paralysis.
Discussion
The left recurrent laryngeal nerve arises from the vagus nerve on the arch of the aorta and passes through the triangle formed by the aortic arch, the ligamentum arteriosum, and the pulmonary artery. Because the distance between the aorta and the left pulmonary artery is only 4.0 mm at this site, an area known as the aortic triangle, the nerve is vulnerable at this point.
Left recurrent laryngeal nerve paralysis is recognized as a complication of certain types of heart disease, typically mitral stenosis (4, 5), and, rarely, PDA (1–3), left ventricular failure (6), and coronary artery disease (6, 7). In 1897, Ortner (4) first described the association of paralysis of the left recurrent laryngeal nerve with mitral stenosis. According to Ortner, an enlarged left antrum can directly compress the left recurrent laryngeal nerve against the aortic arch. However, Ortner's hypothesis was refuted by the anatomic studies of Fetterolf and Norris (5) in 1911, who showed that the recurrent laryngeal nerve must be squeezed between the left pulmonary artery and the aortic arch or the ligamentum arteriosum. In a review of some of the postmortem studies in the literature, these authors concluded that even in those cases of paralysis associated with PDA, the important factor is the resulting pulmonary artery dilatation. In 1934, King et al (6) reported findings at autopsy of patients with vocal cord paralysis resulting from left ventricular failure. These investigators showed that the left recurrent laryngeal nerve degenerated at the point at which it passes between the left pulmonary artery, the arch of the aorta, and the ductus arteriosus. In 1958, when Stocker et al (7) described the cardiovocal syndrome (left vocal cord paralysis associated with heart disease), it was the consensus that the pulmonary artery plays a crucial role in the mechanism. If, however, the presence of a dilated pulmonary artery had been the only factor resulting in paralysis, the number of patients with hoarseness among those with a dilated pulmonary artery would have increased markedly. Several other etiologic factors had been proposed, including mediastinitis, pericardial effusion, obliterative pericarditis, pleural effusion, and lymphadenitis (8). The exact mechanism is still not known for certain.
Several authors (2, 7, 9) believe that hoarseness is an early symptom of cardiac decompensation, and that it may occur as an initial presenting symptom. King et al (6) suggested that their patients with arteriosclerotic heart diseases suddenly suffered left recurrent laryngeal nerve paralysis because rapid onset of left ventricular failure had produced sudden pulmonary hypertension with acute dilatation of the pulmonary vessels, which they termed dynamic dilatation. Moreover, hoarseness associated with acute cardiac exacerbation has been reported in a patient with long-standing PDA (3).
In our case, PDA had not been diagnosed and had not produced any symptoms related to the left recurrent laryngeal nerve for more than 70 years. The patient became gradually symptomatic over a period of 10 years preceding her admission, and the symptoms had worsened during the 7 months before the onset of hoarseness. This deterioration of cardiac symptoms led to the diagnosis of PDA at the age of 75 years. There was only a short period between diagnosis of the PDA and onset of hoarseness. The chest CT study disclosed enlargement of the pulmonary trunk, which corresponded to the worsening of the cardiac symptoms. Although several authors have reported that CT and MR imaging may depict the features of PDA, as well as of a dilated pulmonary artery (3, 10), unfortunately, dilatation of the PDA was not clear in our case. This is, however, the first report (to the best of our knowledge) that shows enlargement of the pulmonary trunk on CT scans after the onset of left vocal cord paralysis. In keeping with the hypothesis of King et al (6), we think that this finding suggests that dynamic dilatation of the pulmonary artery resulted in acute compression of the left recurrent laryngeal nerve in this patient with long-standing PDA.
Conclusion
We believe that rapid dilatation of the pulmonary trunk in a 76-year-old woman with long-standing PDA produced a strong squeezing effect on the left recurrent laryngeal nerve, resulting in a cardiovocal syndrome.
References
- 1.Mead KC. Persistent patency of the ductus arteriosus. JAMA 1910;50:2205-2210 [Google Scholar]
- 2.Chandrasekhar KP, Nair KR. Laryngeal nerve palsy in heart disease. Indian Heart J 1969;21:114-118 [PubMed] [Google Scholar]
- 3.Nakao M, Sawayama T, Samukawa M, et al. Left recurrent laryngeal nerve palsy associated with primary pulmonary hypertension and patent ductus arteriosus. J Am Coll Cardiol 1985;5:788-792 [DOI] [PubMed] [Google Scholar]
- 4.Ortner N. Recurrenslähmung bei Mitralstenose. Wien Klin Wochenschr 1897;10:753-755 [Google Scholar]
- 5.Fetterolf G, Norris GW. The anatomical explanation of the paralysis of the left recurrent laryngeal nerve found in certain cases of mitral stenosis. Am J Med Sci 1911;141:625-638 [Google Scholar]
- 6.King FH, Hitzig WM, Fishberg AM. Recurrent laryngeal paralysis in left ventricular failure. Am J Med Sci 1934;188:691-697 [Google Scholar]
- 7.Stocker HH, Enterline HT. Cardio-vocal syndrome: laryngeal paralysis in intrinsic heart disease. Am Heart J 1958;56:51-59 [DOI] [PubMed] [Google Scholar]
- 8.Malcomson K, Hillman LM. Ortner's syndrome. Guy's Hosp Rep 1956;105:307-319 [PubMed] [Google Scholar]
- 9.Thompson JL, Kistin AD. Hoarseness in heart disease. Ann Intern Med 1948;29:259-273 [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Mehta AC, O'Donovan PB. Computed tomography and magnetic resonance findings in long-standing patent ductus: case reports. Angiology 1996;47:393-398 [DOI] [PubMed] [Google Scholar]