Abstract
Summary: Cogan syndrome is an uncommon disorder of unknown etiology characterized by vestibuloauditory dysfunction and nonsyphilitic interstitial keratitis. To our knowledge, the case herein is the first report to demonstrate the cerebral angiographic findings of a patient with this syndrome.
Cogan syndrome is an uncommon disease characterized by nonsyphilitic interstitial keratitis and vestibuloauditory dysfunction. Typically, it presents with episodes of interstitial keratitis associated with periods of auditory dysfunction that resemble Menière disease and eventually lead to bilateral hearing loss (1, 2). Recent reports, however, consider Cogan syndrome to be a multisystem disease with a wide clinical spectrum (1–3). Approximately 12% to 15% of patients develop vasculitis involving vessels of all sizes in various organ systems (4). Even though autopsy reports of intracranial vasculitis have been reported in the literature (5), to our knowledge, there are no existing reports that directly demonstrate through angiography the intracranial vascular abnormalities associated with this disorder.
Case Report
In 1992, a 57-year-old man presented with progressive bilateral hearing loss, dizziness, and difficulty maintaining balance when walking. Subsequently, he developed bilateral nongranulomatous anterior uveitis that was treated with corticosteroid eye drops. By 1994, the patient was completely deaf with considerably impaired vestibular function. Owing to his bilateral vestibulopathy, his gait grew increasingly worse. At this time, the patient was hospitalized for chronic anemia and gastrointestinal hemorrhage. He experienced two strokes, one in 1996 and one in 1997, when he was diagnosed with Cogan syndrome. By 1999, the patient could no longer ambulate and became bedridden. He became progressively less communicative and experienced significant dysarthria and oropharyngeal dysphagia as a result of the two strokes. In June 2000, the patient died of sepsis after several episodes of recurrent aspiration pneumonia.
The patient had no history of hypertension, heart disease, lipid profile abnormality, or diabetes mellitus. Prior to his strokes, he had no history of headache or transient ischemic attack. He did not have a history of syphilis, and a fluorescent treponemal antibody absorption test for syphilis was negative. Blood tests conducted on several occasions during the period between 1992 and 1999 revealed anemia and a gradually increasing elevated erythrocyte sedimentation rate (ESR). In the years between 1992 and 1999, the patient's ESR increased from rates in the low 60s to the most recent rate of 125. Analysis of the cerebrospinal fluid revealed a glucose level of 61 mg/dL, a total protein level of 62 mg/dL, and a white blood cell count of 30 (polymorphonuclear 12%, mononuclear 88%). Results for lyme disease, cryptococcus, tumor cells, and angiotensin converting enzyme were negative. An audiometric test showed complete bilateral sensorineural hearing loss. Colonoscopy, endoscopy, and other diagnostic tests were unable to locate the source of the patient's gastrointestinal hemorrhage, which was presumed to be secondary to vasculitis. MR imaging of the brain performed in 1994 revealed a small lacunar infarction in the right putamen. An MR examination in 1997 revealed an area of infarction in the posterior right parietotemporal lobe and right basal ganglia from the patient's previous stroke (Fig 1A). It also showed a more recently developed subacute infarction in the left basal ganglia (Fig 1B). MR angiography revealed a possible aneurysm measuring 8 mm in diameter at the vertebrobasilar junction and mild stenosis of the mid basilar artery. There was focal stenosis of the M1 segment of the right middle cerebral artery (MCA) and dilatation of the M1 segment of the left MCA with mild focal stenosis distal to the area of dilatation.
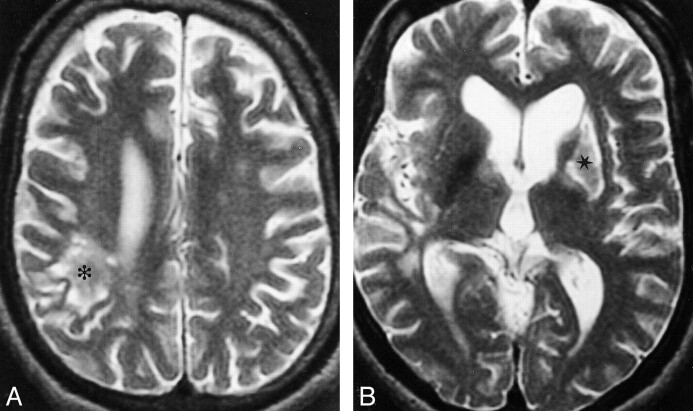
fig 1.A and B, MR images obtained in 1997 after the patient's cerebrovascular accidents in 1996 and 1997. Axial T2-weighted images (4000/100/2) reveal a subacute infarction in the left basal ganglia (asterisk) and a chronic infarction in the posterior right parietal lobe (star)
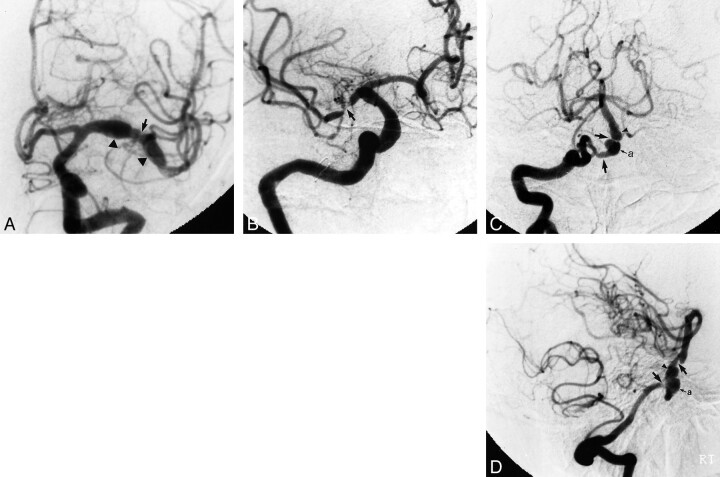
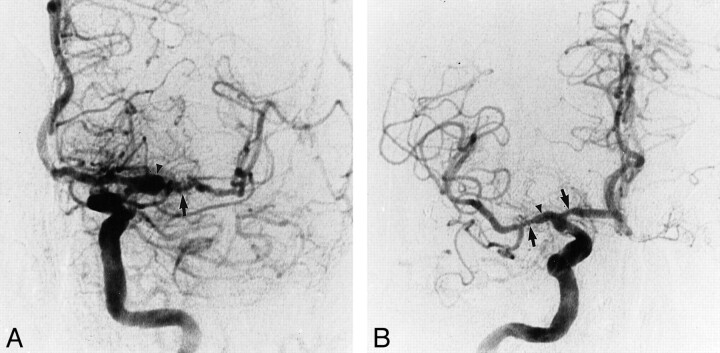
The first digital substraction angiography was performed in 1997. The ascending aorta, the origin of the great vessels, and aortic arch were found to be normal. Mild stenosis of the petrous segment of the left internal carotid artery was noted. Alternating areas of prominent ectasia and minimal stenosis were found in the M1 segment of the left MCA (Fig 2A). Occlusion, stenosis, and contour irregularity were also seen in the proximal portion and distal branches of the anterior segment of the MCA. Injection of the right internal carotid artery revealed mild stenosis affecting the petrous segment and a rather ectatic cavernous segment. M1 segments of the right MCA were affected by severe prethrombotic focal stenosis (Fig 2B). The anterior temporal artery displayed contour irregularity. On the posterior circulation, bilateral stenosis of the V4 segment of the vertebral artery was noted, and an aneurysm measuring approximately 8 mm in diameter was found at the vertebrobasilar junction. The course of the proximal and mid portions of the basilar artery alternated between areas of prominent stenosis and mild dilatation (Fig 2 C and D). After corticosteroid and immunosuppressant medical therapy, a second angiography was performed in 1999. There were no changes noted in the vertebral or basilar arteries. The ectasia of the proximal portion of the left MCA had become more prominent since 1994 and the stenosis seen at the middle of the M1 segment had progressed further into severe occlusive disease in the left MCA and its branches (Fig 3A). The parenchymal phase of the angiography demonstrated a number of areas of arterioarterial anastomoses secondary to occlusive disease in the MCA branches. Interestingly, the prethrombotic stenosis observed in the M1 segment of the right MCA in 1994 had improved, and in 1999, was only mildly stenotic (Fig 3B).
fig 2.
Cerebral angiographic images obtained in 1997.
A and B, An anteroposterior projection of the left internal carotid artery (A) and a 20° left posterior oblique projection (B) of the right internal carotid artery also reveal alternating areas of stenosis (arrow) and ectasia (arrowheads). Note stenotic areas in the course of the anterior temporal artery. No evidence of aneurysm was noted in the internal carotid artery distributions.
C and D, Anteroposterior projection (C) and lateral projection (D) of the selected injection of the right vertebral artery show alternating areas of ectasia (arrowheads) and stenosis (arrow) involving the distal segment of the right vertebral artery and the proximal segment of the basilar artery. A small aneurysm (a) is present at the vertebrobasilar junction.
fig 3.
A and B, Selective injections of the internal carotid artery distributions performed in 1999 after immunosuppresant/corticosteroid therapy.
Selected anteroposterior projection of the left internal carotid artery (A) and anteroposterior projection of the selected right internal carotid artery injection (B) show prominent ectasia (arrowheads) within the M1 segment of left MCA. Severe occlusive disease (arrow) is noted within the left MCA distribution. Compared with the angiographic images of 1997, the ectasis in the left M1 segment of the MCA is more prominent as is the stenotic portion of this vessel. The stenosis of the M1 segment of the right MCA has improved (arrow).
Discussion
Cogan syndrome is a rare, multisystem disease characterized by nonsyphilitic interstitial keratitis and audiovestibular dysfunction. Other ocular manifestations of the disease include granolomatous uveitis, episcleritis, scleritis, macular involvement, coroneal ulceration, optic neuritis, central venous occlusion, glaucoma, and retinal vasculitis (1). The etiology and pathogenesis of Cogan syndrome are unknown. Initially, the disease was thought to be caused by infection; however, Cogan syndrome is currently believed to be an autoimmune disorder (1, 2). Approximately half of the patients affected by Cogan syndrome manifest nonspecific systemic symptoms. Constitutional symptoms such as fever, chills, weight loss, arthralgia, and myalgia present commonly. Respiratory symptoms include dyspnea, hemoptysia, and pleuritic chest pain. Gastrointestinal symptoms include nausea, dyspepsia, melana, diarrhea, and hepatosplenomegaly (1, 2). Neurologic symptoms of Cogan syndrome present in approximately 50% of cases; among these symptoms are headache, psychosis, coma, convulsion, neuropathy, and stroke (6, 7).
Two of the most serious systemic manifestations of Cogan syndrome are aortic insufficiency and vasculitis. The prevalence of vasculitis onset in Cogan syndrome patients is reported at 12% to 15%. (1, 4) Vollertsen et al (4) reported the median interval from the diagnosis of Cogan syndrome until the onset of vasculitis to be approximately 7 months (range, 3 weeks to 8 years), and two thirds of the patients who developed vasculitis did so within 1 year. Clinically, vasculitis has been reported to affect the skin, kidneys, subcutaneous nodules, distal coronary arteries, and muscles. Autopsies have revealed vasculitis in the dura, brain, gastrointestinal system, kidneys, spleen, aorta, and the coronary arteries (4, 8, 9).
Pathologic findings associated with Cogan syndrome include acute vasculitis and fibrosis that may affect any artery or vein. Pathologic examinations of the proximal portion of the aorta in patients with Cogan syndrome have shown generalized dilatation and narrowing of the coronary ostia in the region of the valve (5). Microscopic examination of the aorta revealed neutrophils, mononuclear cells, giant cells, destruction of the internal elastic membrane, neovascularization, necrosis, scarring, and fibrotic hypertrophy. Similar findings, including inflammation, necrosis, and fibrosis, were noted in other vessels as well. The infiltrate may consist of neutrophils, mononuclear cells, plasma cells, eosinophils, and giant cells. Intima proliferation with disruption of the internal elastic membrane may occur, in addition to intimal thickening and fibrosis. This type of involvement may extend into the perivascular tissues (1, 5). Autopsy findings reported by H.G. Thomas (10) included numerous aneurysmal endothelial plaques affecting the entire aorta and surrounding ostia, as well as a bilateral carotid bifurcation aneurysm.
Although the intracranial vascular abnormalities observed in our patient may appear to be the result of artherosclerosis, the differences observed between the angiographic findings between 1994 and 1999, as well as his medical history, suggest an inflammatory process. After the patient's first angiography in 1994, he received corticosteroids and immunosuppressant therapy. The second angiographic study showed increasing ectasis and stenosis of the M1 segment of the left MCA, with the stenotic portions becoming affected by severe occlusive disease. This study also showed the improvement of the stenotic portions of the right MCA from severe to moderate stenosis. Artherosclerosis may cause stenosis or ectasis within the cerebral arteries; however, the vascular diseases associated with artherosclerosis are unlikely to resolve or improve with time or medical treatment, as was the case with our patient. In addition, the patient has no significant risk factors predisposing him toward artherosclerosis, and some of his abnormal laboratory results indicate an inflammatory process. For these reasons, we believe that the vascular changes observed in our patient were more likely to have been the result of an inflammatory process rather than atherosclerosis.
In our patient, both petrous segments of the internal carotid artery, the right MCA, and both right and left vertebral arteries were affected by scattered stenosis, peculiar diffuse irregular narrowing, occlusion, and stenosis. Other studies of Cogan syndrome describe extracranial vessels affected by these phenomena as well. The angiographic findings associated with vasculitis resulting from Cogan syndrome are scattered stenosis, peculiar diffuse irregular narrowing, and tortuosity of affected vessels (4). In the literature, these findings were seen in the aorta, celiac, renal, mesenteric, iliac, femoral, popliteal, and trifurcation arteries (1, 7, 11).
Because of both the extracranial angiographic and pathologic findings described in several other cases of vasculitis associated with Cogan syndrome, as well as the intracranial findings of our own case, we believe that the alternating pattern of stenosis and ectasis as well as a tendency toward aneurysm formation is characteristic of the disorder. The angiographic images of the renal artery and the aorta presented by two authors (10, 12) show evidence of both ectasis and the stenotic/ectatic pattern; however, neither feature is noted in the text of the articles. Gross examinations of the aorta in patients with Cogan syndrome have shown generalized dilatation and narrowing of the coronary ostia; this also suggests the pattern of alternating stenosis and ectasis (1, 5). Aneurysm formation associated with vasculitis seen in Cogan syndrome is suggested by autopsy findings reported by H.G. Thomas (10), which included numerous aneurysmal endothelial plaques affecting the entire aorta and surrounding ostia, as well as a bilateral carotid bifurcation aneurysm.
Conclusion
We present the angiographic findings of a patient with intracranial vasculitis resulting from Cogan syndrome. Other authors have described stenosis and peculiar diffuse irregular narrowing within extracranial arteries; we found these changes to be present intracranially as well. In addition, we discovered a pattern of alternating ectasia and stenosis, as well as an associated aneurysm.
Acknowledgments
We would like to thank Angela Balzé for her assistance in preparing the manuscript.
Footnotes
Address reprint requests to S. James Zinreich, M.D., Neuroradiology/Phipps B-100, 600 North Wolfe Street, Baltimore, MD 21287.
References
- 1.Vollertsen RS, Mc Donald TJ, Younge BR, Banks PM, Stanson AW, Ilstrup DM. Cogan's syndrome: 18 cases and a review of the literature. Mayo Clin Proc 1986;61:344-361 [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS. Cogan's syndrome: studies in thirteen patients long-term follow-up, and a review of the literature. Medicine 1980;59:426-440 [PubMed] [Google Scholar]
- 3.Cheson BD, Bluming AZ, Alroy J. Cogan's syndrome: A systemic vasculitis. Am J Med 1976;60:549-555 [DOI] [PubMed] [Google Scholar]
- 4.Vollertsen RS. Vasculitis and Cogan's syndrome. Rheum Dis Clin North Am 1990;16:433-438 [PubMed] [Google Scholar]
- 5.Ho AC, Roat MI, Venbrux A, Hellmann DB. Cogan's syndrome with refractory abdominal aortitis and mesenteric vasculitis. J Rheumatol 1999;26:1404-1407 [PubMed] [Google Scholar]
- 6.Bicknell JM, Holland JV. Neurological manifestations of Cogan's syndrome. Neurology 1978;28:278-281 [DOI] [PubMed] [Google Scholar]
- 7.Chynn EW, Jacobiec FA. Cogan's syndrome: ophthalmic, audiovestibular, and systemic manifestations and therapy. Int Opthalmol Clin 1996;36:61-72 [DOI] [PubMed] [Google Scholar]
- 8.Crawford WJ. Cogan's syndrome associated with polyarteritis nodosa: a report of three cases. Pa Med 1957;60:835-838 [PubMed] [Google Scholar]
- 9.Fisher ER, Hellstrom HR. Cogan's syndrome and systemic vascular disease. Arch Pathol 1961;72:572-592 [PubMed] [Google Scholar]
- 10. Thomas HG. Case report: clinical and radiological features of Cogan's syndrome—non-syphilitic interstitial keratitis, audiovestibular symptoms, and systemic manifestations. Clin Radiol 1992;45:418-421 [DOI] [PubMed] [Google Scholar]
- 11.Bastug DE, Dominc A, Ortiz O, Di Bartolomeo G, Kotzan JM, Abraham FM. Popliteal artery thrombosis in a patient with Cogan's syndrome: treatment with thrombolysis and percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol 1997;20:57-59 [DOI] [PubMed] [Google Scholar]
- 12.Vella JP, Callaghan JO, Hockey D, Walse JJ. Renal artery stenosis complicating Cogan's syndrome. Letter. Clin Nephrol 1992;470:407-408 [PubMed] [Google Scholar]