Abstract
BACKGROUND AND PURPOSE: Long-term patency in untreated experimental aneurysms represents a critical attribute of any system proposed for the testing of aneurysm occlusion devices. Our purpose was to evaluate the long-term patency in elastase-induced saccular aneurysm models in rabbits.
METHODS: Serial intravenous digital subtractive angiography (IVDSA) was performed in 20 elastase-induced saccular aneurysm models in rabbits 1, 3, 6, 9, 12, and 24 months after creation. Aneurysm dimensions, including neck diameter, width, and height, were measured and calculated from IVDSA images. Comparisons of the aneurysm sizes across time were performed by using the paired Wilcoxon signed rank test and the Friedman test.
RESULTS: None of the 20 aneurysms showed spontaneous thrombosis at any time point. Mean dimensions did not change over time for any parameter. Mean aneurysm neck, width, and height were not significantly changed at interval evaluations of 1, 3, 6, 9, 12, and 24 months (P = .210; P = .413, and P = .405, respectively).
CONCLUSION: Long-term patency in elastase-induced saccular aneurysm models in rabbits is excellent. Aneurysm dimensions remain stable for as long as 2 years following creation.
Long-term patency in untreated experimental aneurysms represents a critical attribute of any system proposed for the testing of aneurysm occlusion devices; models that undergo spontaneous thrombosis are very limited in evaluating the effectiveness of new occlusion devices. Natural history study showing long-term patency of untreated aneurysms has rarely been performed for previously described aneurysm models. Two small series of swine aneurysms noted spontaneous thrombosis in all cases.1,2 Our group reported a series of untreated canine aneurysms in which we followed 18 sidewall aneurysms for as long as 9 months.3
The rabbit elastase-induced aneurysm model has been adopted by multiple groups for study of imaging and intervention.4–16 This model may offer benefit over other aneurysm models, in light of its morphologic and hemodynamic similarity to human saccular aneurysms. Early data suggested strong potential for the use of the elastase-induced aneurysm model in long-term studies, because none of these aneurysms demonstrated spontaneous thrombosis in as long as 6 months of follow-up after creation.17 To further validate this model, we performed a large-scale, systemic evaluation of the long-term patency rates.
Methods
Aneurysm Creation
Elastase-induced, saccular aneurysms were created in 20 New Zealand white rabbits (body weight, 3–4 kg) by using the rabbit elastase model. The institutional animal care and use committee at our institution approved all procedures. Detailed procedures for aneurysm creation have been described elsewhere.18 In brief, anesthesia was induced with an intramuscular injection of ketamine, xylazine, and acepromazine (75 mg/kg, 5 mg/kg, and 1 mg/kg, respectively). By using a sterile technique, the right common carotid artery (RCCA) was exposed and ligated distally. A 1–2-mm beveled arteriotomy was made and a 5F vascular sheath (Cordis Endovascular, Miami Lakes, Fla) was advanced retrograde in the RCCA to a point approximately 3 cm cephalad to the origin of RCCA. A roadmap image was obtained by injection of contrast through the sheath retrograde in the RCCA, to identify the junction between the RCCA and the subclavian and brachiocephalic arteries (Advantx; General Electric Company, Milwaukee, Wisc). A 3F Fogarty balloon (Baxter Healthcare Corporation, Irvine, Calif) was advanced through the sheath to the level of the origin of the RCCA with fluoroscopic guidance and was inflated with iodinated contrast material. Porcine elastase (5.23 μ/mgP, 40.1 mgP/mL, approximately 200 U/mL; Worthington Biochemical Corporation, Lakewood, NJ) was incubated within the lumen of the common carotid above the inflated balloon for 20 minutes, after which the catheter, balloon, and sheath were removed, and the RCCA was ligated below the sheath entry site.
Intravenous Digital Subtraction Angiography (IVDSA)
All animals underwent follow-up angiography at each time point (1, 3, 6, 9, 12, and 24 months after creation). All rabbits survived for the full duration of the study. At each time point, as mentioned above, anesthesia was induced as that for creation and a 24-gauge angiocatheter was placed in the left ear vein. Radiopaque sizing markers, consisting of metallic spheres ranging from 2 to 11 mm in diameter, were placed over the chest, as close as possible to the brachiocephalic artery but not obscuring the region of the aneurysm. Technical details can be found as reported elsewhere.17 DSA, at 2 frames per second, was performed with a hand injection of 7 mL of iodinated contrast material (Omnipaque 300, GE Healthcare, Rochester, Minn) into the left ear-vein catheter as quickly as possible. Filming was carried into the arterial phase. Following IVDSA, magnified views of the aneurysm were transferred to film from the IVDSA runs. Animals were allowed to recover for use in further studies.
Image Interpretation
Two experienced observers—an experienced neuroradiologist (D.F.K.) and a trained research fellow (Y.H.D.)—evaluated aneurysm morphology. The IVDSA images for any given subject were viewed separately. Readers were blinded to measurements obtained from the other reading session. To assess intrareader variability, IVDSA images were interpreted twice by both readers. To avoid recall bias, reading sessions were separated by at least 2 weeks.
The width, height, and neck diameters of the aneurysm cavities were determined by employing the external sizing device as a reference. The width of the aneurysm cavity was determined at its point of maximum measurement, while the height was measured from the aneurysm dome to the midportion of a line connecting the proximal and distal portions of the aneurysm neck. Reduction in aneurysm width or height of >1.5 mm was considered evidence of interval partial thrombosis.
Statistical Analysis
Aneurysm sizes (neck, width, and height) were compared within different time points by using the Friedman test. Differences of aneurysm sizes (neck, width, and height) between each time point were compared by using the paired Wilcoxon signed rank test. The differences of the above were evaluated by using an upper 95% confidence limit.
Results
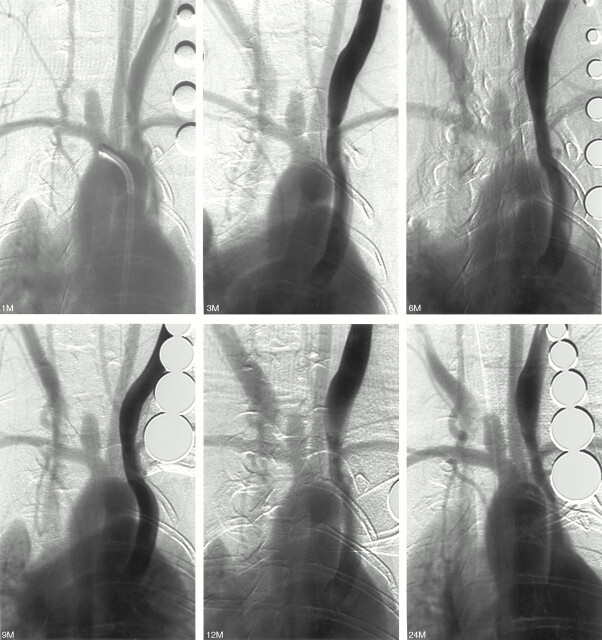
None of the 20 aneurysms showed spontaneous thrombosis from angiography up to 2 years following creation (Fig 1). Representative images are shown in Fig 1. Mean aneurysm sizes after different periods of creation are shown in Table 1.
Fig 1.
Rabbit 7. IVDSA 1, 3, 6, 9, 12, and 24 months after creation. The aneurysm remains patent and stable radiographically.
Aneurysm sizes over time
| 1 Month | 3 Months | 6 Months | 9 Months | 1 Year | 2 Years | |
|---|---|---|---|---|---|---|
| Neck width (mean ± SD, mm) | 3.5 ± .7 | 3.3 ± .9 | 3.4 ± .7 | 3.4 ± .8 | 3.5 ± .8 | 3.4 ± .8 |
| Aneurysm width (mean ± SD, mm) | 3.8 ± .8 | 3.9 ± .9 | 4.1 ± 1.0 | 4.0 ± 1.1 | 4.1 ± 1.0 | 4.0 ± .9 |
| Aneurysm height (mean ± SD, mm) | 8.7 ± 1.7 | 8.4 ± 1.5 | 8.7 ± 2.0 | 8.8 ± 1.7 | 8.6 ± 1.6 | 8.5 ± 1.7 |
Aneurysm Neck
Mean neck diameters 1, 3, 6, 9, 12, and 24 months after creation were 3.5 ± 0.7 mm, 3.3 ± 0.9 mm, 3.4 ± 0.7 mm, 3.4 ± 0.8 mm, 3.5 ± 0.8 mm, and 3.4 ± 0.8 mm, respectively. There were no significant differences among these neck diameters (P = .210).
Aneurysm Width
Mean aneurysm widths at 1, 3, 6, 9, 12, and 24 months were 3.8 ± 0.8 mm, 3.9 ± 0.9 mm, 4.1 ± 1 mm, 4.0 ± 1.1 mm, 4.1 ± 1.0 mm, and 4.0 ± 0.9 mm, respectively. There were no significant differences among these widths (P = .413).
Aneurysm Height
Mean aneurysm heights at 1, 3, 6, 9, 12, and 24 months after creation were 8.7 ± 1.7 mm, 8.4 ± 1.5 mm, 8.7 ± 2.0 mm, 8.8 ± 1.7 mm, 8.6 ± 1.6 mm, and 8.5 ± 1.7 mm, respectively. There were no significant differences among these heights (P = .405).
Discussion
This study confirms excellent long-term patency of elastase-induced, saccular aneurysms in rabbits. In no case did we document spontaneous thrombosis. Aneurysm dimensions remained stable throughout the follow-up period, as long as 2 years following creation. These data provide further support for the application of this model for the study of saccular aneurysms.
Data regarding long-term aneurysm patency in untreated subjects are considered critical if the model is to be disseminated for imaging and endovascular therapy applications.19 Spontaneous aneurysm thrombosis may render an animal model unsuitable for testing of new devices for endovascular embolization. This study showed that all aneurysms remained patent 2 years after creation. No thrombosis was found on angiography at any time point.
We have previously demonstrated early growth of elastase-induced aneurysms, as long as 3 weeks following creation.17 This early growth may reflect either ongoing injury to the vessel wall or ongoing interplay between aneurysm hemodynamics and mechanical integrity. In any event, the experimental aneurysms tend to grow early and then stabilize. The exact relevance of this versus human aneurysms remains unclear.
Spontaneous thrombosis of human intracranial aneurysms is rare.20–22 Our model mimics this attribute of human aneurysms, with low rates of spontaneous thrombosis.
Conclusion
Elastase-induced saccular aneurysm models in rabbits remain patent and stable for as long as 2 years after creation, which is desirable for testing modified coils for aneurysm embolization.
Footnotes
This study was supported by National Institutes of Health grant NS42646.
These data were presented at the 43rd annual meeting of the American Society of Neuroradiology, Toronto, Ontario, Canada, May 21–27, 2005.
References
- 1.Byrne JV, Hope JK, Hubbard N, et al. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. AJNR Am J Neuroradiol 1997;18:29–33 [PMC free article] [PubMed] [Google Scholar]
- 2.Guglielmi G, Ji C, Massoud TF, et al. Experimental saccular aneurysms. II. A new model in swine. Neuroradiology 1994;36:547–50 [DOI] [PubMed] [Google Scholar]
- 3.Kallmes DF, Altes TA, Vincent DA, et al. Experimental side-wall aneurysms: a natural history study. Neuroradiology 1999;41:338–41 [DOI] [PubMed] [Google Scholar]
- 4.Cloft HJ, Altes TA, Marx WF, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology 1999;213:223–28 [DOI] [PubMed] [Google Scholar]
- 5.Short JG, Fujiwara NH, Marx WF, et al. Elastase- induced saccular aneurysms in rabbits: comparison of geometric features with those of human aneurysms. AJNR Am J Neuroradiol 2001;22:1833–37 [PMC free article] [PubMed] [Google Scholar]
- 6.Kallmes DF, Fujiwara NH, Berr SS, et al. Elastase-induced saccular aneurysms in rabbits: a dose-escalation study. AJNR Am J Neuroradiol 2002;23:295–98 [PMC free article] [PubMed] [Google Scholar]
- 7.Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol 2002;23:1580–88 [PMC free article] [PubMed] [Google Scholar]
- 8.Kallmes DF, Fujiwara NH, Yuen D, et al. A collagen- based coil for embolization of saccular aneurysms in a New Zealand white rabbit model. AJNR Am J Neuroradiol 2003;24:591–96 [PMC free article] [PubMed] [Google Scholar]
- 9.Kallmes DF, Williams AD, Cloft HJ, et al. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology 1998;207:519–23 [DOI] [PubMed] [Google Scholar]
- 10.De Gast AN, Altes TA, Marx WF, et al. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery 2001;49:690–94 [DOI] [PubMed] [Google Scholar]
- 11.Assar OS, Fujiwara NH, Marx WF, et al. Aneurysm growth, elastinolysis, and attempted doxycycline inhibition of elastase-induced aneurysms in rabbits. J Vasc Interv Radiol 2003;14:1427–32 [DOI] [PubMed] [Google Scholar]
- 12.Thiex R, Moller-Hartmann W, Hans FJ, et al. Are the configuration and neck morphology of experimental aneurysms predictable? A technical approach. Neuroradiology 2004;46:571–76 [DOI] [PubMed] [Google Scholar]
- 13.Hoh BL, Rabinov JD, Pryor JC, et al. A modified technique for using elastase to create saccular aneurysms in animals that histologically and hemodynamically resemble aneurysms in human. Acta Neurochir (Wien) 2004;146:705–11 [DOI] [PubMed] [Google Scholar]
- 14.Hans FJ, Krings T, Moller-Hartmann W, et al. Endovascular treatment of experimentally induced aneurysms in rabbits using stents: a feasibility study. Neuroradiology 2003;45:430–34 [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara NH, Kallmes DF. Healing response in elastase-induced rabbit aneurysms after embolization with a new platinum coil system. AJNR Am J Neuroradiol 2002;23:1137–44 [PMC free article] [PubMed] [Google Scholar]
- 16.Krings T, Hans FJ, Moller-Hartmann W, et al. Time-of-flight-, phase contrast and contrast enhanced magnetic resonance angiography for pre-interventional determination of aneurysm size, configuration, and neck morphology in an aneurysm model in rabbits. Neurosci Lett 2002;326:46–50 [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara NH, Cloft HJ, Marx WF, et al. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. AJNR Am J Neuroradiol 2001;22:698–703 [PMC free article] [PubMed] [Google Scholar]
- 18.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award: creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. AJR Am J Roentgenol 2000;174:349–54 [DOI] [PubMed] [Google Scholar]
- 19.Raymond J, Salazkin I, Metcalfe A, et al. Lingual artery bifurcation aneurysms for training and evaluation of neurovascular devices. AJNR Am J Neuroradiol 2004;25:1387–90 [PMC free article] [PubMed] [Google Scholar]
- 20.Brownlee RD, Tranmer BI, Sevick RJ, et al. Spontaneous thrombosis of an unruptured anterior communicating artery aneurysm: an unusual cause of ischemic stroke. Stroke 1995;26:1945–49 [DOI] [PubMed] [Google Scholar]
- 21.Hamilton MG, Dold ON. Spontaneous disappearance of an intracranial aneurysm after subarachnoid hemorrhage. Can J Neurol Sci 1992;19:389–91 [PubMed] [Google Scholar]
- 22.Tanabe M, Inoue Y, Hori T. Spontaneous thrombosis of an aneurysm of the middle cerebral artery with subarachnoid haemorrhage in a 6-year-old child: case report. Neurol Res 1991;13:202–04 [DOI] [PubMed] [Google Scholar]