Abstract
Summary: Spinal cord astrocytomas are rare neoplasms that can result in alteration of the spinal cord structural integrity, which can be assessed by using diffusion tensor imaging methods. Our objective was to visualize the deformation of the posterior spinal cord lemniscal and corticospinal tracts in 5 patients with low-grade astrocytomas compared with 10 healthy volunteers by using 3D fiber-tracking reconstructions.
Intrinsic tumors of the spinal cord (approximately 15%–20% of all central nervous system [CNS] tumors) are rare neoplasms (incidence of 1.1/100,000 persons),1 among which astrocytomas are the most frequent type (29%). Preliminary studies2-4 previously assessed the feasibility of MR spinal cord imaging by using diffusion tensor imaging (DTI), an MR technique that evaluates the translation of extracellular water molecules within the white matter fibers5 and enables reconstruction of 3D images in the brain6 and spinal cord 4,7 of white matter tracts by using specialized fiber-tracking (FT) algorithms. We applied this technique in 5 patients with spinal cord astrocytomas to visualize the spinal cord tumor extension inside the white matter fibers.
Description of the Technique
Five patients (all women; mean age, 35.4 years) with low-grade astrocytomas of various locations (diagnosed on MR imaging and presurgical biopsy; Table) referred to our institution from our neurosurgical emergency unit were prospectively selected between November 2003 and April 2005. Only 2 patients had solid mass tumor (patients 1 and 3); the other 3 had cysts around the masses. As a control group, we enrolled 10 fully informed healthy volunteers (3 men and 7 women; mean age, 32.3 years) without neurologic disease.
Imaging was performed on a 1.5T MR imaging system with actively shielded magnetic field gradients (Gmax, 40 mT/m). The protocol began with the acquisition of a sagittal T2-weighted fast spin-echo (FSE) sequence (field of view, 39.9 × 39.9 cm; image matrix, 640 × 384; section thickness, 3 mm; TR/TE, 5030/118 milliseconds; echo train length, 23). Subsequently a sagittal spin-echo single-shot echoplanar parallel Grappa diffusion-weighted imaging sequence with acceleration factor 2 and 25 noncollinear gradient directions was applied with 2 b values (b = 0 and 500 seconds/mm2; field of view, 17.9 × 17.9 cm; image matrix, 128 × 128; 12 sections with section thickness of 3 mm; nominal voxel size, 1.4 × 1.4 × 3 mm; TR/TE, 4600/83 milliseconds). The DTI sequence duration was 3 minutes 10 seconds.
Fractional anisotropy (FA) values were calculated on a voxel-by-voxel basis by using dedicated software (http://fmritools.hd.free.fr). In healthy volunteers, FA measurements were made at 3 different levels (cervical, C2–C5; high thoracic, T1–T6; and low thoracic, T7–T12) by using regions of interest (averaged surface, 20 mm2; 10 voxels) located on the spinal cord by using the most accurate b0 image then reported to FA maps. In patients, the FA measurements were made at the tumor site (within the mass and avoiding cysts) by using small regions of interest of 20 mm2 (10 voxels) that were exactly located inside the spinal cord on the most accurate b0 image. These regions of interest were then reported to FA maps to avoid partial volume effects, magnetic susceptibility effects, and motion artifacts. The regions of interest in patients and the healthy volunteers were matched in size and spinal cord level.
In addition to the 2D parametric FA color maps, 3D white matter fiber tracts were created by using the principal diffusion directions method,8-10 where the eigenvector corresponding to the largest eigenvalue is extracted from the diffusion tensor field generated from the DTI datasets in the region where the diffusion was linear, typically for a FA thresholding value of 0.18, and an angulation threshold of 45° (to prevent fibers from sudden transition and to keep tracking based on the connectivity of the neighborhood) as described elsewhere.4,8-10
Discussion
All patients had abnormal T2-weighted areas at the site of tumor.
FA parameter performs a complete evaluation of the water diffusivity by using scalar properties of the DTI sequence. In the spinal cord, the white matter fibers have a craniacaudal orientation and are very anisotropic. In volunteers, FA values were identical at the different spinal cord levels (FA, 0.74 ± 0.04). Previous studies performed in healthy volunteers2-4 reported FA values similar to those we found. All patients had decreased FA values in the mass tumor site (FA, 0.48 ± 0.02), which suggests either local extracellular edema or decreased number of fibers increasing the extracellular space, or both, as reported elsewhere in brain diseases.11
Fiber tracking performed on the spinal cord in all healthy volunteers showed the main posterior white matter tracts (posterolateral corticospinal tracts and posterior lemniscal tracts; Fig 1). In patients, the FT 3D reconstructions of the spinal cord involvement showed the boundaries of the lesion matching those seen on the T2-weighted imaging in case of solid tumors (eg, without cyst and without visible edema; 40% of patients, patients 1 and 3), with warped or destroyed spinal cord fibers (Fig 2). Unfortunately FT did not assess these boundaries in patients with cystic tumor components (all other patients). FT algorithm is based on the principal diffusion direction method, which is used to reconstruct fiber tracts by using thresholding FA values of 0.18, where the diffusion is very linear.4,8-10 In the case of cysts, FA values are <0.18, and the FT algorithm is unable to link voxels inside the same tract because of the increased isotropic diffusivity of extracellular water, contrary to solid-state lesions, where FA values are about 0.48. Furthermore, the algorithm is unable to distinguish between extracellular edema and destroyed white matter tracts by tumor cell involvement. Decreasing the FA thresholding value to try detect tracts among edema or tumor cells yielded numerous seemingly unrelated and multidirectional tracks. Increasing the FA thresholding value decreased the regular tracts that were seen on volunteers. Consequently, the relevant FA value to be used in spinal cord FT was assessed at 0.18. All of these data suggest that fiber tracking may be used to visualize the warped white matter tracts in the solid state astrocytomas but lacks sensitivity in cases of cystic and/or vasogenic edema tumors. Larger studies are necessary to confirm these findings.
Fig 1.
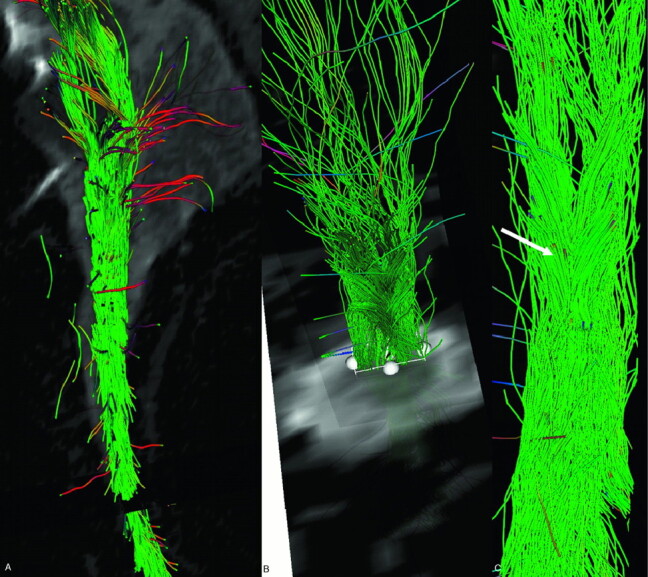
Fiber tracking performed on a volunteer’s cervical spinal cord focused on the posterior lemniscal tracts. Sagittal (A), axial (B), and coronal (C) views show tracts reconstructed over the b0 sequence. Note the decussating fibers (arrow).
Fig 2.
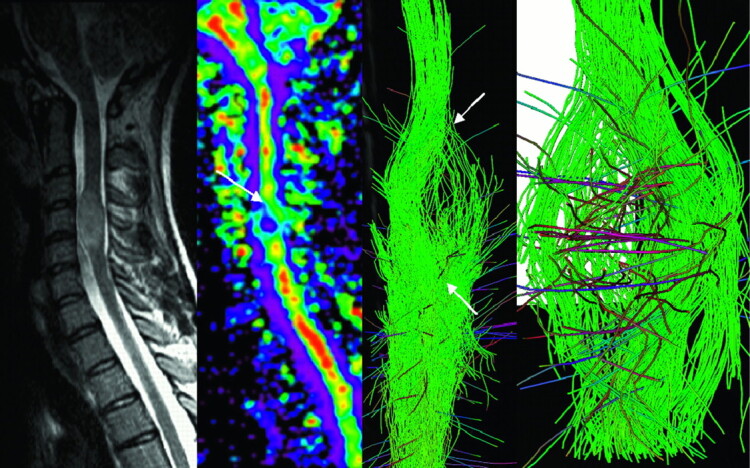
MR imaging of a spinal cord involvement due to a solid state astrocytoma. FA map and fiber tracking over b0 image show warped fibers around the tumor. Neither the vasogenic edema nor the cystic portion of the tumor was visible on the T2-weighted image, and boundaries of the lesion visible on both the FA map and 3D FT reconstructions (arrow) matched those on T2-weighted imaging.
Patients’ data with estimates of FA on tumor level
| Age (y)/Sex | Tumor | Extension | Location | Mass FA Mass |
|---|---|---|---|---|
| 23/F | Astrocytoma | C3C5 | C4 | 0.5 ± 0.08 |
| 44/F | Astrocytoma | C1C6 | C3 | 0.49 ± 0.08 |
| 40/F | Astrocytoma | C1C5 | C3 | 0.48 ± 0.12 |
| 32/F | Astrocytoma | T8T11 | T9 | 0.45 ± 0.08 |
| 38/F | Astrocytoma | C3T3 | C6 | 0.48 ± 0.08 |
References
- 1.Stein BM, McCormick PC: Spinal intradural tumors. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York: McGrawHill;1996. :1769–89
- 2.Holder C, Muthupillai R, Mukundan S, et al. Diffusion-weighted MR imaging of the normal human spinal cord in vivo. AJNR Am J Neuroradiol 2000;21:1799–1806 [PMC free article] [PubMed] [Google Scholar]
- 3.Ries M, Jones R, Dousset V, et al. Diffusion tensor MRI of the spinal cord. Magn Reson Med 2000;44:884–92 [DOI] [PubMed] [Google Scholar]
- 4.Facon D, Ozanne A, Fillard P, et al. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol 2005;26:1587–94 [PMC free article] [PubMed] [Google Scholar]
- 5.Basser P, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor. MRI. J Magn Reson 1996;111:209–19 [DOI] [PubMed] [Google Scholar]
- 6.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A 1999;96:10422–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler Kingshott C, Hickman S, Parker G, et al. Investigating cervical spinal cord structure using axial diffusion tensor imaging. Neuroimage 2002;16:93–102 [DOI] [PubMed] [Google Scholar]
- 8.Xu D, Mori S, Solaiyappan M, et al. A framework for callosal fiber distribution analysis. Neuroimage 2002;17:113–43 [DOI] [PubMed] [Google Scholar]
- 9.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–69 [DOI] [PubMed] [Google Scholar]
- 10.Westin CF, Maier SE, Mamata H, et al. Processing and visualization for diffusion tensor MRI. Med Image Anal 2002;6:93–108 [DOI] [PubMed] [Google Scholar]
- 11.Jones DK, Simmons A, Williams SCR, et al. Noninvasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med 1999;42:37–41 [DOI] [PubMed] [Google Scholar]


