Abstract
BACKGROUND AND PURPOSE: Because carotid plaque ulceration is associated with an increased risk of cerebral embolism, residual carotid plaque ulceration directly around a stent (peristent ulceration) after carotid angioplasty and stent placement (CAS) could still be a risk factor for a stroke. The purpose of this study is to understand the morphologic and clinical prognosis of peristent ulceration.
PATIENTS AND TECHNIQUES: CAS was attempted on 91 consecutive stenotic lesions (80 patients). Of these, 54 lesions (48 patients) had ulceration before CAS. Angiograms were evaluated immediately after the procedure. Peristent ulceration was found in 34 lesions (30 patients). The mean depth and length of peristent ulcers were 2.1 mm (range, 1–4.7 mm) and 8.9 mm (range, 1.5–22 mm), respectively. All patients with peristent ulceration were followed with antiplatelet therapy.
RESULTS: No ischemic event due to the lesions occurred during the mean follow-up period of 25.5 months (range, 3–48 months). Angiography on 25 lesions (21 patients) at a mean of 5.8 months (range, 1–21 months) after CAS showed that peristent ulceration disappeared in 12 lesions (48%), improved in 11 lesions (44%), and remained unchanged in 2 lesions (8%). Nine lesions (36%) showed restenosis, which were ≤30% and did not require any additional intervention. New ischemic lesions were not detected in any of the 14 patients (17 lesions) who underwent follow-up MR imaging at a mean of 9 months (range, 1–32 months) after CAS.
CONCLUSION: We conclude that peristent ulceration after CAS improves spontaneously and is not a risk factor for cerebral embolism.
Carotid angioplasty and stent placement (CAS) has been shown to be a durable and effective treatment for patients with carotid artery stenosis. Many reports have demonstrated good short- or long-term outcomes of CAS.1,2 Some of those reports suggest that morphologic features of lesions can be related to periprocedural complications of CAS.3 To know the patient’s prognosis, however, an understanding of the correlation between morphologic features of lesions after CAS and long-term complications is also important. To date, this has not been well discussed or documented.
Carotid plaque ulceration is considered to cause an increased risk of cerebral embolism because of the formation and release of microemboli on its irregular surface.4–9 Thus, CAS can prevent strokes by making the irregular surface smooth, as well as improving stenosis. When there is residual plaque ulceration around a stent (peristent ulceration), however, it could still be an embolic source because the blood may flow over the irregular surface of the ulcer through the stent mesh.
To prevent peristent ulceration, the application of a high-pressure balloon may prove effective by making the ulceration disappear due to strong compression of a stent against the carotid wall; however, strong compression often causes complications such as microemboli, hypotension, and bradycardia.10,11 In addition, it would be ineffective on calcified lesions. Therefore, peristent ulceration is often observed after CAS and should be analyzed as a risk factor for further thromboembolic events.1 In this study, we present the results from follow-up of patients with peristent ulceration, to assess its morphologic changes and possible contribution to embolic stroke after CAS.
Patients and Techniques
Angioplasty Procedure
Ninety-one CAS procedures were performed in 80 patients (68 men and 12 women) with carotid stenosis at our institute between February 2001 and May 2004. All patients received aspirin (81 mg/day) and ticlopidine (200 mg/day) for ≥3 days before CAS. With patients under general anesthesia, CAS was performed with a cerebral protection device (Naviballoon, Silascon, Kaneka Medics; PeucuSurge, Medtronic). Heparin (80 IU/Kg) was given only once just before the procedure and was not reversed after CAS. A self-expandable stent ([n = 73] Wallstent, Boston Scientific; [n = 18] SMART stent, Cordis) was applied after predilation with an adequate angioplasty balloon. Atropine sulfate (0.5 mg) was given intravenously just before balloon inflation. The stent was selected to be 1–1.2 times the diameter of the proximal reference vessel. Postdilation was used only to dilate the residual stenosis, which was >20%, but not to treat any peristent ulceration or to make the stent contact the carotid wall tightly. The lesions were evaluated with angiography in anteroposterior and lateral directions immediately before and after CAS. Peristent ulceration was defined in this report as carotid wall irregularity around a stent, which is covered by, but not attached to, a stent and which was an irregular surface of the carotid plaque before CAS. Percent stenosis was determined according to the North American Symptomatic Carotid Trial.
Patients Selection and Follow-Up
An irregular surface of the carotid plaque, which can be defined as plaque ulceration, was observed in 54 lesions (48 patients) before CAS.12 Among these patients, peristent ulceration was detected in 34 lesions identified in 30 patients. All patients were followed up with antiplatelet therapy (81 mg/day of aspirin and 200 mg/day of ticlopidine). Follow-up neurologic examinations were performed by any one of us in our clinic and were supplemented by telephone interviews. Angiography and MR imaging were scheduled during the follow-up period, when possible.
All images were evaluated by 2 neurosurgeons (S.K. and K.K.). After angiograms were entered into a computer, the size of ulcers and the vessel diameter were measured in comparison with the diameter of a guiding catheter by using the NIH Image analysis program (version1.62 [http://rsb.info.nih.gov/nih-image/]).
Results
The clinical characteristics and follow-up findings in these 30 patients are summarized in Tables 1 and 2. The mean depth and length of peristent ulcers were 2.1 mm (range, 1–4.7 mm) and 8.9 mm (range, 1.5–22 mm), respectively. All of the 30 patients with peristent ulceration were followed for a mean time of 25.5 months (range, 3–48 months). Three patients died during follow-up: one from interstitial pneumonia at 25 months (patient 4), one from myocardial infarction at 31 months (patient 7), and one from brain stem hemorrhage at 3 months (patient 20). No ipsilateral neurologic symptoms appeared in any of the patients during the follow-up period. Follow-up angiography, which was performed on 25 lesions (21 patients) a mean of 5.8 months (range, 1–21 months) after CAS, showed that peristent ulceration disappeared in 12 lesions (48%), improved in 11 lesions (44%), and remained unchanged in 2 lesions (8%). Although neointimal hyperplasia was observed in 9 lesions (36%), restenosis rate was ≤30% and no additional intervention was required. New ischemic lesions were not detected in any of the 14 patients (17 lesions) who underwent MR imaging a mean of 9 months (range, 1–32 months) after CAS.
Table 1:
Clinical characteristics and follow-up results in 30 patients (34 lesions) with residual plaque ulceration
| Patient No./Age (y)/Sex | Side | Stenosis (%) | Symptoms* before CAS | Size of Residual Ulcer† (mm) | Follow-up Angiography |
Symptoms* during Follow-up | Follow-up Period (mo) | ||
|---|---|---|---|---|---|---|---|---|---|
| Period (mo) | Ulcer | Restenosis | |||||||
| 1/74/M | L | 48 | + | 2.3 × 6 | 7 | Improved | None | − | 48 |
| 2/59/M | L | 43 | + | 1.5 × 8 | NA | − | 47 | ||
| 3/64/M | L | 60 | − | 1.5 × 8 | NA | − | 45 | ||
| 4/68/M | L | 62 | − | 2 × 6 | 12 | Disappeared | 20% | − | 25‡ |
| 5/53/M | L | 73 | + | 2.6 × 3 | NA | − | 41 | ||
| 6/71/M | L | 75 | + | 2.2 × 6 | 4 | Disappeared | None | − | 34 |
| R | 62 | + | 2.8 × 11 | 21 | Improved | None | − | 33 | |
| 7/75/M | R | 62 | + | 1.8 × 8 | NA | − | 31‡ | ||
| 8/76/M | R | 87 | + | 1.2 × 2 | 6 | Improved | 30% | − | 36 |
| 9/73/M | L | 81 | + | 1.5 × 6 | 12 | Disappeared | 20% | − | 32 |
| 10/73/M | R | 78 | − | 2.4 × 6 | 4 | Improved | None | − | 30 |
| 11/71/M | R | 80 | − | 1 × 6 | NA | − | 30 | ||
| 12/72/M | R | 86 | + | 1.2 × 6 | 5 | Disappeared | None | − | 28 |
| L | 75 | + | 2 × 5 | 3 | No change | 20% | − | 26 | |
| 13/66/F | R | 40 | + | 2.8 × 13 | 3 | Disappeared | None | − | 28 |
| 14/50/M | R | 48 | + | 1 × 11 | NA | − | 28 | ||
| 15/69/M | L | 90 | + | 1.2 × 8 | 2 | Improved | None | − | 26 |
| R | 33 | + | 1.8 × 2.5 | NA | − | 24 | |||
| 16/74/M | R | 52 | − | 1.8 × 8 | 4 | Improved | None | − | 25 |
| 17/75/M | R | 56 | + | 1.8 × 12 | NA | − | 23 | ||
| 18/67/M | L | 30 | + | 2.8 × 15 | 10 | Improved | 20% | − | 22 |
| 19/60/M | L | 88 | + | 2 × 10 | 5 | Improved | 20% | − | 22 |
| 20/79/M | L | 78 | + | 2 × 8 | NA | − | 3‡ | ||
| 21/70/M | R | 33 | + | 3.3 × 12 | 1 | Disappeared | None | − | 21 |
| L | 36 | + | 2.8 × 9 | 1 | Disappeared | None | − | 20 | |
| 22/75/F | L | 64 | − | 2.4 × 11 | 4 | Improved | None | − | 20 |
| 23/65/M | R | 52 | + | 3.8 × 14 | 9 | Disappeared | None | − | 20 |
| 24/79/M | L | 50 | − | 1.5 × 6 | 4 | Disappeared | None | − | 15 |
| 25/81/M | R | 45 | + | 4.7 × 11 | 4 | Disappeared | 20% | − | 16 |
| 26/77/F | R | 32 | + | 1 × 18 | 4 | Improved | None | − | 15 |
| 27/80/M | L | 62 | + | 2.6 × 10 | 5 | No change | None | − | 15 |
| 28/81/F | R | 95 | + | 1.8 × 12 | 4 | Improved | 20% | − | 14 |
| 29/72/F | R | 70 | + | 1.2 × 22 | 6 | Disappeared | None | − | 14 |
| 30/76/M | L | 71 | + | 1.5 × 1.5 | 4 | Disappeared | 30% | − | 9 |
Note:—CAS indicates carotid artery stenting; NA, not applicable.
Ipsilateral ischemic neurologic symptoms.
Depth × length.
These patients died: patient 4 from interstitial pneumonia, patient 7 from myocardial infarction, and patient 20 from brain stem hemorrhage.
Table 2:
Clinical data of patients with residual plaque ulceration
| Men | 25 (83%) |
| 29 lesions (85%) | |
| Women | 5 (17%) |
| 5 lesions (15%) | |
| Age (y) | |
| Mean ± SD | 71 ± 7.3 |
| Range | 50–81 |
| Side | |
| Right | 17 |
| Left | 17 |
| Symptomatic lesion | 27 (79%) |
| Size of ulcer (mm; mean ± SD) | |
| Depth | 2.1 ± 0.8 |
| Length | 8.9 ± 4.4 |
| Follow-up angiography (n = 25) | |
| Period (mo, mean ± SD) | 5.8 ± 4.3 |
| Ulcer | |
| Disappeared | 12 (48%) |
| Improved | 11 (44%) |
| No change | 2 (8%) |
| Restenosis | |
| 30% | 2 (8%) |
| 20% | 7 (28%) |
| None | 16 (64%) |
| Follow-up period (mo) | |
| Mean ± SD | 25.5 ± 10.4 |
| Range | 3–48 |
| Symptoms* during follow-up | 0 (0%) |
Ipsilateral ischemic neurologic symptoms.
Discussion
In our series, peristent ulceration disappeared or improved in 92% of the lesions that were treated angiographically within a mean duration of 5.8 months after CAS. Ischemic events due to the lesions did not occur in any of the 30 patients, for a mean follow-up time of 21.8 months. We did not classify the lesions in this report, though there were various shapes and sizes of peristent ulcers (Figs 1–5). We made this decision in light of the fact that all had good prognosis and, consequently, classification did not seem to be necessary.
Fig 1.
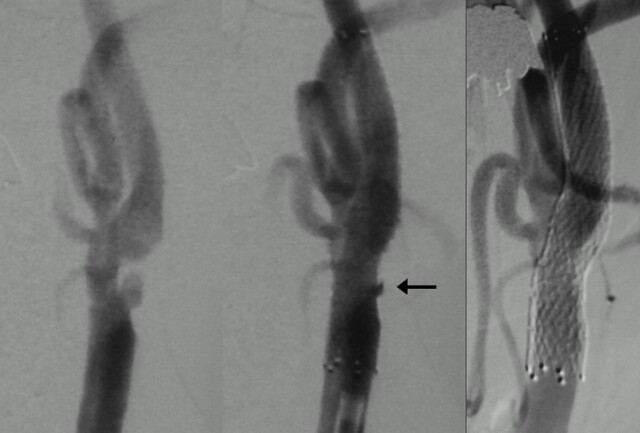
Patient 30, a 76-year-old man with left hemispheric TIAs. Left carotid angiogram (anteroposterior view) showing moderate stenosis with plaque ulceration (left). Small peristent ulceration (arrow) just after CAS (center) disappeared with expansion of a stent in 4 months (right). This is the smallest peristent ulceration in our series.
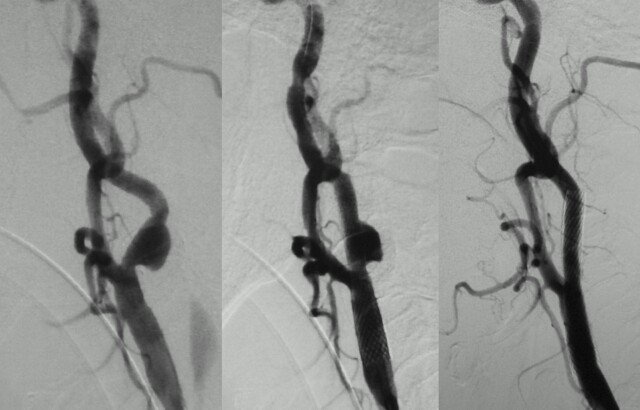
Fig 5.
Patient 19, a 60-year-old man with a recent history of left cerebral infarction. Left, Left carotid artery angiogram (anteroposterior view) revealing severe stenosis with irregular plaque surface. Center, Angiogram just after CAS showing residual stenosis and diffuse peristent ulceration because of high-grade calcification of the plaque. Right, Peristent ulceration improved after 5 months.
The main reason for improvement or disappearance of peristent ulceration is considered to be due to clot formation outside the stent. This is promoted by stasis of the blood flow in the space between the stent and the irregular surface of the ulcer, because the stent struts decrease the blood flow into and out of the space. In addition, clot formation outside a stent is also accelerated by the decreasing of turbulent flow due to the decreased and normalized blood flow velocity at the stenotic lesion by stent placement.13–15 Neointimal hyperplasia and continuous expansion of a stent after stent placement may have the effect of curing ulcers in cases of small or shallow ulceration.
Ischemic stroke might be expected due to peristent ulceration because carotid plaque surface irregularity is considered to be one of the risk factors for microemboli, which may subsequently cause ipsilateral ischemic symptoms.8 Rothwell et al studied 3007 patients with carotid plaques in the European Carotid Study Trial and concluded that angiographic plaque surface irregularity is an independent predictor of ischemic stroke at all degrees of stenosis.12 Lovett et al proved that angiographic plaque surface morphology is strongly associated with plaque instability.16 In our series, however, therewas no ischemic event observed due to peristent ulceration during the follow-up period. This is considered to be because the stent struts entrap emboli coming into the cerebral blood flow from outside the stent.
It is necessary to compress the stent strongly with a balloon against the carotid wall to prevent peristent ulceration. High-pressure postdilation, however, often causes sinus bradycardia and severe hypotension by stimulating the sinus baroreceptors, as well as cerebral ischemia due to thrombotic debris released from the plaque, even if protective devices and medications are applied.10,11 Furthermore, aggressive dilation may promote neointimal hyperplasia and subsequently restenosis after CAS.17 As a result, we performed postdilation, if necessary, with the goal only of making stenosis <20%, consequently leaving peristent ulceration in >30% of cases. This study, however, shows that peristent ulceration does not cause any complications, which leads us to the conclusion that any concerted effort to prevent peristent ulceration is not necessary.
The rate of peristent ulceration is lower with SMART stent than with Wallstent—about 5% and 45%, respectively—possibly because of their different structure.18 This study, however, cannot suggest which stent is better, because peristent ulceration would not become a complication considering the results of the study.
Antiplatelet therapy is an effective procedure to prevent ischemic strokes for patients at high risk from occlusive vascular events.19 Recently, combination therapy of aspirin and a thienopyridine drug (ticlopidine or clopidogrel) has come to be used for the patients after vascular stent placement to prevent embolic stroke, restenosis, and acute obstruction. This is because their additive effects through different pathways provide more substantial benefit than single drug therapy, even allowing for the increased risk of bleeding.19–22 Therefore, a low-dose combination of aspirin (81 mg) and ticlopidine (200 mg) is usually adopted at our institute for patients at high risk of ischemic stroke and for patients after CAS. In this study, our choice of antiplatelet therapy is considered to be an effective measure to prevent thromboembolic strokes and restenosis even for patients with peristent ulceration.
Conclusion
We found that peristent ulceration after CAS improves spontaneously and does not cause embolic strokes. Any concerted effort to prevent peristent ulceration such as high-pressure postdilation is not necessary. Antiplatelet therapy can be used in any follow-up therapy after CAS.
Fig 2.
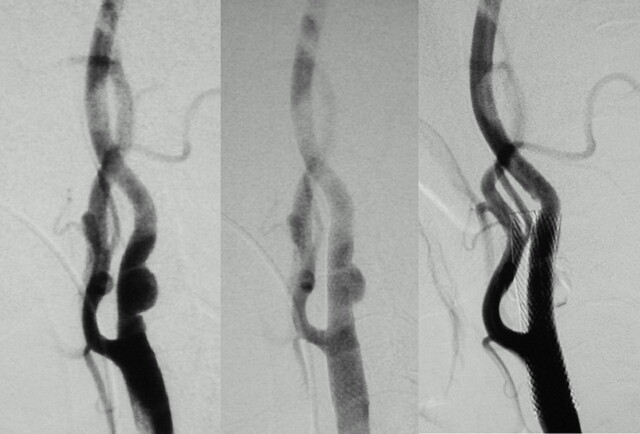
Patient 26, a 77-year-old woman with multiple infarction in the right cerebral hemisphere. Right carotid angiogram (lateral view) revealing mild stenosis with irregular carotid wall, which is considered as a plaque ulceration (left). Diffuse shallow peristent ulceration in the internal carotid artery (arrows) just after CAS (center) improved after 4 months (right).
Fig 3.
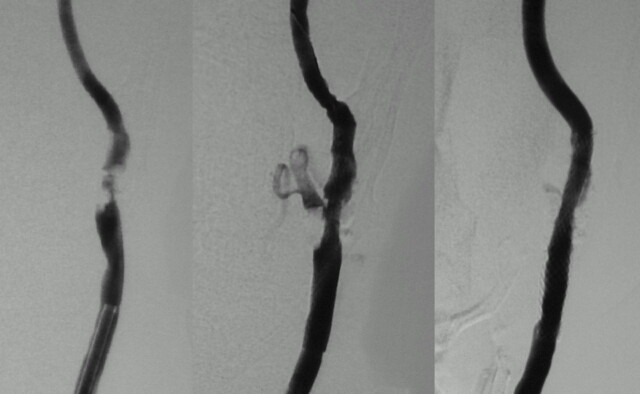
Patient 21, a 70-year-old man with bilateral hemispheric TIAs. Right carotid angiogram (lateral view) showing mild stenosis with plaque ulceration (left). Large peristent ulceration just after CAS (center) disappeared after 1 month (right). (Left carotid stenosis was also treated with CAS.)
Fig 4.
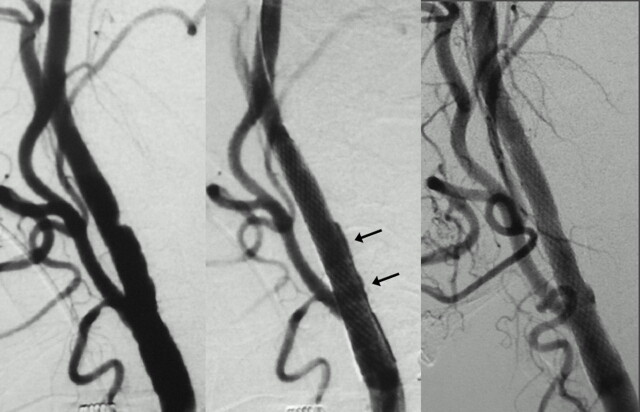
Patient 25, an 81-year-old man with crescendo TIAs. Right carotid angiogram (lateral view) revealing mild-to-moderate stenosis with large plaque ulceration (left). Large peristent ulceration just after CAS (center) disappeared after 4 months (right).
Acknowledgments
We thank Mr. Tom Greensmith for assistance in preparing this article.
References
- 1.Fox DJ Jr, Moran CJ, Cross DT 3rd, et al. Long-term outcome after angioplasty for symptomatic extracranial carotid stenosis in poor surgical candidates. Stroke 2002;33:2877–80 [DOI] [PubMed] [Google Scholar]
- 2.Shawl F, Kadro W, Domanski MJ, et al. Safety and efficacy of elective carotid artery stenting in high-risk patients. J Am Coll Cardiol 2000;35:1721–28 [DOI] [PubMed] [Google Scholar]
- 3.Mathur A, Roubin GS, Iyer SS, et al. Predictors of stroke complicating carotid artery stenting. Circulation 1998;97:1239–45 [DOI] [PubMed] [Google Scholar]
- 4.Troyer A, Saloner D, Pan XM, et al. Major carotid plaque surface irregularities correlate with neurologic symptoms. J Vasc Surg 2002;35:741–47 [DOI] [PubMed] [Google Scholar]
- 5.Park AE, McCarthy WJ, Pearce WH, et al. Carotid plaque morphology correlates with presenting symptomatology. J Vasc Surg 1998;27:872–78; discussion 878–79 [DOI] [PubMed] [Google Scholar]
- 6.Schminke U, Motsch L, Hilker L, et al. Three-dimensional ultrasound observation of carotid artery plaque ulceration. Stroke 2000;31:1651–55 [DOI] [PubMed] [Google Scholar]
- 7.Molloy J, Markus HS. Asymptomatic embolization predicts stroke and TIA risk in patients with carotid artery stenosis. Stroke 1999;30:1440–43 [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 2000;31:622–30 [DOI] [PubMed] [Google Scholar]
- 9.AbuRahma AF, Kyer PD 3rd, Robinson PA, et al. The correlation of ultrasonic carotid plaque morphology and carotid plaque hemorrhage: clinical implications. Surgery 1998;124:721–26;discussion 726–28 [DOI] [PubMed] [Google Scholar]
- 10.Leisch F, Kerschner K, Hofmann R, et al. Carotid sinus reactions during carotid artery stenting: predictors, incidence, and influence on clinical outcome. Catheter Cardiovasc Interv 2003;58:516–23 [DOI] [PubMed] [Google Scholar]
- 11.Harrop JS, Sharan AD, Benitez RP, et al. Prevention of carotid angioplasty-induced bradycardia and hypotension with temporary venous pacemakers. Neurosurgery 2001;49:814–820;discussion 820–12 [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis: on behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 2000;31:615–21 [DOI] [PubMed] [Google Scholar]
- 13.Ohyama M, Mizushige K, Ohyama H, et al. Carotid turbulent flow observed by convergent color Doppler flowmetry in silent cerebral infarction. Int J Cardiovasc Imaging 2002;18:119–24 [DOI] [PubMed] [Google Scholar]
- 14.Orlandi G, Fanucchi S, Fioretti C, et al. Characteristics of cerebral microembolism during carotid stenting and angioplasty alone. Arch Neurol 2001;58:1410–13 [DOI] [PubMed] [Google Scholar]
- 15.Muller-Hulsbeck S, Grimm J, Jahnke T, et al. Flow patterns from metallic vascular endoprostheses: in vitro results. Eur Radiol 2001;11:893–901 [DOI] [PubMed] [Google Scholar]
- 16.Lovett JK, Gallagher PJ, Hands LJ, et al. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation 2004;110:2190–97 [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann R, Mintz GS, Mehran R, et al. Tissue proliferation within and surrounding Palmaz-Schatz stents is dependent on the aggressiveness of stent implantation technique. Am J Cardiol 1999;83:1170–74 [DOI] [PubMed] [Google Scholar]
- 18.Phatouros CC, Higashida RT, Malek AM, et al. Endovascular stenting for carotid artery stenosis: preliminary experience using the shape-memory- alloy-recoverable-technology (SMART) stent. AJNR Am J Neuroradiol 2000;21:732–38 [PMC free article] [PubMed] [Google Scholar]
- 19.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller C, Roskamm H, Neumann FJ, et al. A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary artery stents. J Am Coll Cardiol 2003;41:969–73 [DOI] [PubMed] [Google Scholar]
- 21.Herbert JM, Bernat A, Samama M, et al. The antiaggregating and antithrombotic activity of ticlopidine is potentiated by aspirin in the rat. Thromb Haemost 1996;76:94–98 [PubMed] [Google Scholar]
- 22.Bhatt DL, Bertrand ME, Berger PB, et al. Meta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stenting. J Am Coll Cardiol 2002;39:9–14 [DOI] [PubMed] [Google Scholar]