Abstract
Summary: Following trauma, imaging of brain stem lesions is often inconclusive. In a man who suffered a lethal accident, postmortem MR diffusion tensor (DT) imaging of the brain and neuropathologic examination were performed. DT imaging showed a disorganization of fibers in the brain stem that was not found in 2 controls and corresponded to changes on neuropathologic correlation. Diffusion tensor imaging provides an insight into the organization of myelinated structures of the CNS, potentially allowing diagnosis of traumatic fiber tract rupture.
Diffusion-weighted MR imaging (DWI) is very sensitive to changes in tissular motion and has been successfully used to investigate acute human stroke, in both the brain and spine.1 As a modification of DWI, diffusion tensor (DT) imaging enables exploration of the anatomy of myelinated pathways by demonstrating water movement along these structures.2,3 Recent studies highlight the role of DT imaging in the examination of brain tumor localization and spreading, alterations in neonate brains, and chronic alcoholism and other neurologic or psychiatric disorders. Furthermore, DT imaging is frequently used for neuroanatomic studies.4,5 The examination of mechanically traumatized brain by using DT imaging is rarely reported in the literature,6–8 and recent publications are focused on patient recovery following brain injury. An attempt to display mechanical ruptures of brain fibers by using DT imaging techniques has, to our best knowledge, not yet been made. We performed diffusion tensor imaging with a line-scan technique that provides line-by-line spin-echo sampling of each section. Compared with echo-planar techniques, this sequence is less sensitive to susceptibility-related distortions and is therefore better suited for imaging the brain stem and structures close to the base of the skull. Because inclusions of air that easily produce artifacts and distortions in the more ubiquitous echo-planar diffusion sequences are often present intracranially in posttraumatic patients with skull fractures, the line-scan technique is the method of choice where increased scan time is less of an issue.
In the field of forensic medicine, radiologic studies are presently being proposed as an adjunct or a replacement of traditional autopsy methods.9,10 Within one study that aims to evaluate MR imaging and CT imaging for the benefit of forensic medicine,9 postmortem examinations have been performed by using these techniques. Among them, a patient with suspected brain stem injury who died in a hang-glider crash was investigated by using DT imaging. The goal of this investigation was to evaluate whether DT imaging was principally suited for the detection of mechanical brain stem fiber tract ruptures.
Description of Technique
Case Presentation
A 49-year-old man was hang-gliding with friends when he suddenly crashed to the ground and his neck hit a tree. A bystander observed a horizontal hematoma on the right side of his neck when the man was found lying dead on the ground immediately after the incident. No resuscitation attempts were performed. An investigation into the cause of the crash concluded that the victim’s unfamiliarity with a new hang-glider was to blame.
Imaging
MR imaging was performed on a 1.5T clinical scanner equipped with a head coil (GE Signa; General Electric Systems, Milwaukee, Wisc) 26 hours postmortem. The brain was scanned in situ and without prior fixation. For DT imaging, a multisection line-scan sequence was used with the following parameters: for each section 6 images with high b values (1000 seconds/mm2) in 6 different noncollinear directions (relative gradient amplitudes: [Gx, Gy, Gz] = {1,1,0, 0,1,1, 1,0,1 [−1,1,0], [0,−1,1][1,0,–1]}) and 2 images with low b value (5 seconds/mm2); field of view, 16 × 12 cm; 3-mm thick sagittal sections with a 0.5-mm spacing; TR, 3808 milliseconds; TE, 96 milliseconds; matrix, 128 × 128 with NEX, 1. Four sagittal images were obtained covering the whole brain stem. The images were exported to an external workstation, and maps of the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) were calculated on a pixel-by-pixel basis from these images after interpolation to a matrix size of 256 × 256; the diffusion tensors were reconstructed by using the homemade image analysis software Xphase (S. Maier, Boston, Mass., unpublished software).
Sixteen fast spin-echo (FSE) T2-weighted images in the sagittal plane were acquired (TR, 4000 milliseconds; TE, 96 milliseconds; FOV, 24 × 24 cm; 2-mm-thick with 0.2-mm spacing; matrix, 512 × 256; NEX, 6) in addition to 27 axial FSE T2-weighted images (TR, 4000 ms; TE, 96 milliseconds; FOV, 24 × 24 cm; 5-mm-thick; 1.0-mm spacing; matrix, 512 × 256; NEX, 4) and 27 axial FSE inversion recovery images (TR, 3000 ms; TE, 14; FOV, 24 × 24 cm; 5-mm-thick; 1.0-mm spacing; matrix, 256 × 256; NEX, 3).
DT imaging was also performed with the same parameters in 2 controls: one patient who died of heart failure (the brain was also imaged in situ without fixation) and therefore suffered no mechanical brain stem trauma (DT imaging scan 22 hours postmortem) and one living volunteer. This healthy volunteer had no prior history of head or spinal trauma.
Neuropathologic Examination
Autopsy was performed 40 hours postmortem in the trauma patient (38 hours postmortem in the control). Analogically to the imaging, the brain stem was cut in sagittal planes. Brain stem tissue was examined histologically by using hematoxylin-eosin (H&E) and amyloid precursor protein (APP) staining. In the injury case, in addition to brain stem trauma, autopsy revealed craniocerebral trauma including an open parietal skull fracture and orbital roof fracture on the right side, fracture of the skull base of the posterior fossa, subarachnoid and small frontal intracerebral white matter hemorrhage, and tiny hemorrhage of one cerebellar tonsil. The body of the second vertebra was fractured. Further traumatic hemorrhage was found in the right neck muscles and the right hand region, and the right arm was fractured. In addition to the cerebral and spinal findings, histologic examination showed a positive fat embolism of the lung vessels as a result of the traumatic impact. The cause of death was central respiratory insufficiency due to brain stem injury. No findings that could have served as a medical reason for the crash were observed at the autopsy.
Findings at Imaging and Neuropathologic Examination
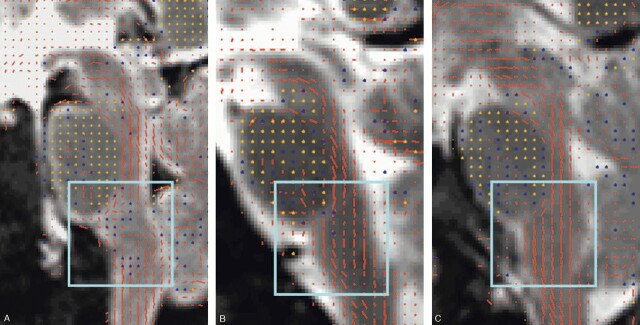
The trauma patient showed gross disorganization of brain stem fibers on diffusion tensor images of the cervical spinal cord substance in comparison to our control data (Fig 1).
Fig 1.
A, Diffusion tensors overlaid on T2 image in the patient with cervical trauma. Disorganization of the distal brain stem white matter tracts is present.
B, Diffusion tensors overlaid on T2 image in the patient who died after cardiac arrest shows intact fibers with an overall slight wavy configuration.
C, Diffusion tensors overlaid on T2 image in a healthy living volunteer showing the homogenous character of the craniocaudal brain stem white matter tracts.
The principal eigenvector of the diffusion tensor scaled by the fractional anisotropy (FA) is represented by its projection onto the sagittal plane by using red dashes and by its through-plane component by using dots (yellow dot for strong component, blue dot for intermediate component, and missing dot for a weak component). For clarity, tensors were visualized in this figure at a lower resolution than measured, displaying a single vector for every 9 pixels of the interpolated image (ie, 1.5-times lower resolution in both dimensions than measured).
In the living healthy volunteer, the normal anatomy of the vertical white matter tracts in the upper cervical spine and brain stem were observed (Fig 1C), which were only slightly disorganized in the patient who had died of cardiac arrest (Fig 1B). This contrasted greatly with our postmortem examination of the trauma patient where we found a disruption of the normally longitudinally oriented fibers in the lower brain stem below the pons, which was reflected by a gross disorganization of the fiber tracts in the DT images (Fig 1A). On the FA maps, we found an area of hyperintensity to be present at the level of the transition between the pons and medulla (Fig 2B), corresponding to the presence of hemorrhage. ADC values at the level of the lesion: 0.235 × 10−5 cm2/s for the trauma patient, 0.230 × 10−5 cm2/s for the cardiac arrest patient, and 0.650 × 10−5 cm2/s for the volunteer; FA values were: 0.425 for the trauma patient, 0.265 for the cardiac arrest patient, and 0.342 for the volunteer.
Fig 2.
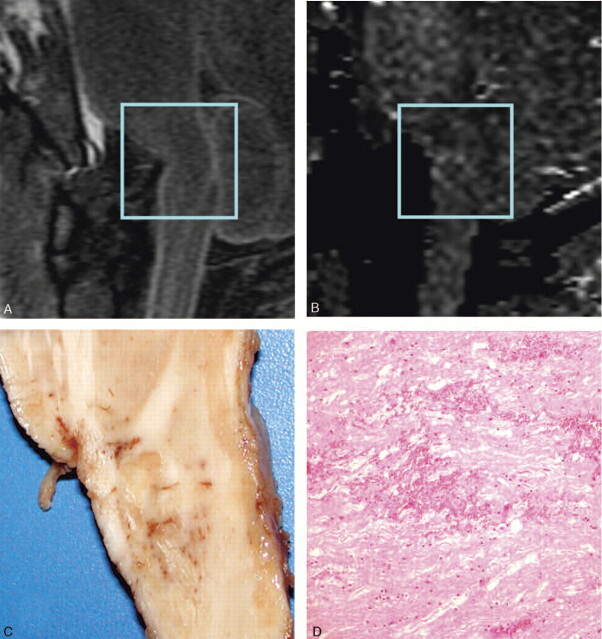
A, Sagittal T2-weighted image showing no visible changes in the brain stem tissue. The brain stem is partially dislocated following traumatic impact because of compression by the fractured clivus.
B, Sagittal FA map showing an area of higher anisotropy at the level of the transition between the pons and medulla, likely due to hemorrhage at the pontomedullary junction, but noise contributions cannot be ruled out.
C, Macroscopy: sagittal autopsy specimen showing punctate hemorrhagic changes and partially disrupted brain stem tissue at the level of the transition between pons and medulla
D, Microscopy: histologic section of brain stem fiber tract demonstrating acute hemorrhage and edema (paraffin; H&E; scale bar = 60 μm). Axonal swellings indicating a prolonged interval between the traumatic lesion and death are absent due to the immediate death of the patient.
All these findings were well correlated with the autopsy findings both macroscopically and microscopically (Fig 2C, D). These findings also contrasted with the conventional MR imaging, which showed no changes in the brain stem tissue at the level of the DT imaging alterations (Fig 2A); however, the brain stem showed partial dislocation following traumatic impact, and a small bone fragment was visible near the injury site.
Neuropathologic examination revealed macroscopically the presence of a small hemorrhagic white matter disruption at the level of the transition between pons and medulla, which was also reflected in the histologic specimens (Fig 2C, -D). The control case showed no micro- and macropathologic alterations of the brain stem tissue.
Discussion
DT imaging has been applied to track axonal anatomy in the human brain stem in vivo.2,3 Postmortem, it has been used to evaluate changes in the white matter in dementia11 and for serial evaluation of axonal function in patients with brain death.12 Concerning traumatic brain injury, Huisman et al and Schaefer et al reported their observations by using DT imaging in diffuse axonal injury.6,7
Disruption of the white matter tracts below the pons and in the medulla oblongata was observed in the DT imaging examination of the presented case of mechanical brain stem trauma, but it was present in neither the healthy living volunteer nor a patient who died of cardiac arrest. The changes seen on the diffusion tensor tractograms corresponded perfectly with the neuropathologic findings of hemorrhagic fiber ruptures both macro- and microscopically (Figs 1 and 2). Changes on the FA images corresponding to those seen in the tractograms were also observed (Fig 2B). The hyperintensities that were present on the FA images in the injured brain stem region were in accordance with the histologic observation of hemorrhage and edema in the traumatized region. The FA values were higher than in the controls, but an effect of noise cannot be ruled out in this single case.
In contrast to diffusion imaging, T2-weighted images or inversion-recovery images did not reveal any post-traumatic changes of the spinal cord and brain stem (eg, edema, hemorrhage; Fig 2A, -C) other than the partial posttraumatic dislocation of the brain stem.
In the neuropathologic correlation material for the DT imaging findings, no pathologic changes were represented in the control cadaver case.
Although difficult, diffusion imaging of the brain stem and spinal cord is not impossible to perform, as has been shown in smaller series, by using among others more standard echo-planar techniques. The technique of line-scan diffusion imaging was used in our examinations because it has one main advantage over echo-planar diffusion imaging: the absence of the important susceptibility artifacts and image distortions present when imaging the contents of the posterior fossa. One main drawback of the line-scan technique is the length of the scanning, which can be problematic in the acute setting of stroke but obviously is of no importance in this case. Because of its inherent insensitivity to susceptibility-induced artifacts, line scanning also facilitates the acquisition of sagittal sections that allow imaging of the white matter tracts in the cervical spine in the longer dimension, which is why the technique has been used by others with success as well.
Although our findings have been produced postmortem, we believe that, with improvements in scanning technology, it will be possible to perform these studies in the clinical setting on patients who have severe neurologic deficits attributable to disruption of tracts following severe trauma but who cannot be imaged conventionally. In the forensic context, the technique of DWI and DT imaging might also become a valuable tool because the examination of the dorsal neck region is technically difficult and time-consuming at autopsy. With the current trend of developing minimally invasive autopsy techniques analogous to “keyhole surgery,” the postmortem use of imaging techniques as an adjunct to autopsy is of increasing interest in forensic casework, offering new possibilities in the evaluation and displaying of traumatologic findings.
Our investigation demonstrates that DT imaging can provide detailed information concerning white matter tract integrity and/or disruption in cases of cervical trauma, allowing the diagnosis of mechanical fiber tract ruptures. The finding of ruptured axonal structures is of great importance not only in forensic assessment, but also in the clinical context where no possibility of histologic sampling exists. DT imaging provides unique information about traumatic changes of the brain tissue that cannot be obtained with other imaging techniques.
Acknowledgments
Drs. Lövblad and Kreis are supported by grants from the Swiss National Science Foundation regarding diffusion tensor imaging (grants 3100-066348 and 3100-59082). We express our thanks to Therese Perinat, Urs Koenigsdorfer, and Roland Dorn, from the Institute of Forensic Medicine, Bern, for their excellent technical support, and to Karin Zwygart and Verena Beutler, from the Department of Clinical Research, University of Bern, for proficient help with data acquisition.
References
- 1.Roberts TP, Rowley HA. Diffusion weighted magnetic resonance imaging in stroke. Eur J Radiol 2003;45:185–94 [DOI] [PubMed] [Google Scholar]
- 2.Mamata H, Mamata Y, Westin CF, et al. High-resolution line scan diffusion tensor MR imaging of white matter fiber tract anatomy. AJNR Am J Neuroradiol 2002;23:67–75 [PMC free article] [PubMed] [Google Scholar]
- 3.Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med 2000;44:625–32 [DOI] [PubMed] [Google Scholar]
- 4.Stieltjes B, Kaufmann WE, van Zijl PC, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage 2001;14:723–35 [DOI] [PubMed] [Google Scholar]
- 5.Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol 2004;25:356–69 [PMC free article] [PubMed] [Google Scholar]
- 6.Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 2004;25:370–76 [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer PW, Huisman TA, Sorensen AG, et al. Diffusion-weighted MR imaging in closed head injury: high correlation with initial Glasgow coma scale score and score on modified Rankin scale at discharge radiology.2004;233:58–66 [DOI] [PubMed] [Google Scholar]
- 8.Levin HS. Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj 2003;17:665–74 [DOI] [PubMed] [Google Scholar]
- 9.Thali MJ, Yen K, Schweitzer W, et al. Virtopsy, a new imaging horizon in forensic pathology: virtual autopsy by postmortem multislice computed tomography (MSCT) and magnetic resonance imaging (MRI)—a feasibility study. J Forensic Sci 2003;48:386–403 [PubMed] [Google Scholar]
- 10.Yen K, Sonnenschein M, Thali MJ, et al. Postmortem multislice computed tomography and magnetic resonance imaging of odontoid fractures, atlantoaxial distractions and ascending medullary edema. Int J Legal Med 2005;119:129–36 [DOI] [PubMed] [Google Scholar]
- 11.Larsson EM, Englund E, Sjobeck M, et al. MRI with diffusion tensor imaging post-mortem at 3.0 T in a patient with frontotemporal dementia. Dement Geriatr Cogn Disord 2004;17:316–19 [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Honda Y, Fujii Y, et al. Serial evaluation of axonal function in patients with brain death by using anisotropic diffusion-weighted magnetic resonance imaging. J Neurosurg 2004;100:56–60 [DOI] [PubMed] [Google Scholar]