Abstract
SUMMARY: A 20-year-old woman recently diagnosed with acute posterior multifocal placoid pigment epitheliopathy developed headaches, weakness, and paresthesias. MR imaging of the brain revealed an acute infarct (demonstrated by diffusion-weighted images) in the head of the right caudate nucleus, a chronic infarct with encephalomalacia in the body of the corpus callosum, and multiple foci of abnormal signal intensity in the white matter of the centrum semiovale.
Acute posterior multifocal placoid pigment epitheliopathy (APMPPE) is a chorioretinal disease that has been associated with multiple neurologic complications, including headaches, aseptic meningitis, transient ischemic attacks, and stroke.1 The case presented in this article is unique not only because APMPPE is a rare entity, but also because it is unusual for these patients to develop ischemic brain infarcts and even more unusual to depict them on the acute stage on MR imaging of the brain, especially when the infarcts are clinically silent, as in our case. Moreover, to the best of our knowledge, the neuroimaging findings of APMPPE have not been reported in the radiologic literature.
Case Report
A 20-year-old woman presented for neurology consultation complaining of multiple acute episodes of right-sided paresthesia and right-sided weakness affecting the face, arm, and leg, as well as language disturbance, photophobia, phonophobia, and intense headaches. She had been diagnosed with APMPPE 1.5 months earlier and was treated by her ophthalmologist with a short course of steroids. The patient had been having headaches for several months. On presentation, physical examination revealed visual acuity of 20/25 of the left eye and 20/70 of the right eye. Sensory and motor examination was unremarkable at that time. No CSF or laboratory studies were performed.
MR imaging of the brain was performed 40 days after the initial episode of neurologic deficit and 3 months after diagnosis of APMPPE, on a 3T magnet (Signa Excite; GE Medical Systems, Milwaukee, Wisc). The head of the right caudate nucleus demonstrated restricted diffusion, normal T1-weighted signal intensity, and slightly increased T2-weighted and fluid-attenuated inversion recovery (FLAIR) signal intensity (Fig 1A–D). No mass or mass effect was seen. There was no abnormal enhancement after the intravenous administration of gadolinium-based contrast material. These findings were consistent with an acute stroke of the head of the right caudate nucleus. There was a focal lesion in the posterior aspect of the body of the corpus callosum, slightly to the right of the midline, which was isointense to CSF in the center and slight hyperintense to gray matter on the T2-weighted and FLAIR sequences in the periphery, without restricted diffusion or abnormal enhancement (Figs 1E–G). This lesion was consistent with a chronic ischemic stroke with central cystic encephalomalacia. In addition, there were multiple foci of increased signal intensity on the T2-weighted and FLAIR sequences in the periventricular white matter and subcortical white matter of the centrum semiovale bilaterally and symmetrically, without abnormal enhancement or restricted diffusion (Fig 1H). These lesions most likely represented microvascular ischemic demyelination, because of central nervous system (CNS) vasculitis, though other demyelinating processes, such as multiple sclerosis, could also demonstrate similar findings in a young patient. There was no evidence of abnormal meningeal enhancement.
Fig 1.
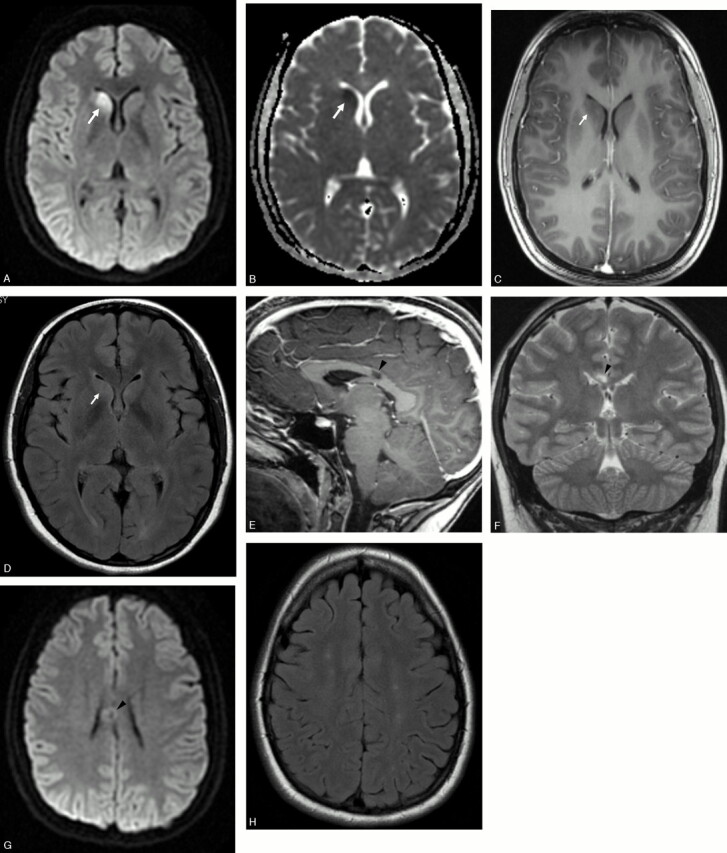
A, Axial diffusion-weighted image (b = 1000) demonstrates increased signal intensity in the head of the right caudate nucleus (arrow).
B, Axial apparent diffusion coefficient map demonstrates decreased apparent diffusion in the head of the right caudate nucleus (arrow).
C, Axial contrast-enhanced 3D fast-spoiled gradient (FSPGR) T1-weighted image (flip angle/TR/TE, 13/5/1.2) after intravenous gadolinium demonstrates normal signal intensity and no enhancement in the head of the right caudate nucleus (arrow).
D, Axial FLAIR image (TR/TE/TI, 8200/123/2000) demonstrates mild increased signal intensity in the head of the right caudate nucleus (arrow).
E, Sagittal contrast-enhanced 3D-FSPGR T1-weighted image (FL/TR/TE, 13/5/1.2) after intravenous gadolinium demonstrates a nonenhancing lesion isointense to CSF in the body of the corpus callosum (arrowhead).
F, Coronal fast spin-echo T2-weighted (TR/TE, 4000/103) image demonstrates the corpus callosum lesion with signal intensity isointense to CSF (arrowhead).
G, Axial diffusion-weighted (b = 1000) image at the level of the lesion in the corpus callosum demonstrates no restricted diffusion (arrowhead).
H, Axial FLAIR image (TR/TE/TI, 8200/123/2000) at the level of the centrum semiovale demonstrates multiple small foci of high signal intensity in the white matter.
Follow-up neurology visits 3 months after the onset of symptoms disclosed that her headaches had resolved spontaneously. Her paresthesias also resolved 2.5 months after her symptoms appeared. She still had subjective eye complaints regarding her visual field deficits, and was scheduled for follow-up with her neuro-ophthalmologist.
Discussion
APMPPE typically affects young adult patients without sex predilection and presents with bilateral acute loss of visual acuity, usually after a flulike syndrome.2 On fundoscopic examination, multifocal yellow-white placoid lesions at the level of the pigment epithelium and choroid of the retina are seen.2 It is a vasculitis of the choroid that secondarily affects the retina and pigment epithelium3 and has been associated with other systemic manifestations of vasculitis.4 The etiology of the vasculitis causing APMPPE is unknown, though various conjectures have associated it with viral infections, Lyme disease, postvaccination autoimmune response, collagen vascular diseases, such as lupus, juvenile rheumatoid arthritis, and other systemic processes, such as sarcoidosis.1
Cerebral ischemia in patients with APMPPE is unusual and manifests clinically as strokes, or less frequently as transient ischemic attacks, with cerebral vasculitis the presumed mechanism of injury.5–9 These patients usually have evidence of inflammation of the CSF or aseptic meningitis.1 Infarcts usually affect the basal ganglia or the posterior circulation.1,7 Large ischemic strokes can occur and have been reported to result in patient death.10,11 The onset of stroke usually occurs within 4–5 months of the onset of ocular disease, though is typically concomitant.1 Strokes can also occur or recur during the tapering of immunosuppressive therapy.1
Caudate nucleus infarcts are uncommon, because of the rich blood supply from the anterior and lateral lenticulostriate arteries and the recurrent artery of Heubner. Caudate nucleus infarctions are more frequently caused by small vessel disease than emboli11 and have been reported in patients with APMPPE.1 The patient we are presenting had an ischemic infarction of the head of the right caudate nucleus, which is presumed to be due to small vessel vasculitis, on the basis of the underlying diagnosis of APMPPE.
Corpus callosum infarctions are also very rare, because of the rich blood supply from the paired pericallosal and posterior pericallosal arteries, and are also most frequently related to small vessel disease.12 We assume the lesion in the corpus callosum in this case also represents an old infarct with central cystic encephalomalacia due to vasculitis secondary to the underlying diagnosis of APMPPE.
In light of the clinical and imaging context, the periventricular and subcortical white matter lesions seen in our case are most likely due to microvascular ischemic demyelination due to CNS vasculitis. This pattern can also be seen in patients with demyelination related to other vasculitides, multiple sclerosis, or chronic small vessel disease (usually in older patients).
The differential diagnosis in a young patient with multiple ischemic infarcts in different stages, small-vessel supratentorial white matter disease, and loss of visual acuity includes recurrent emboli, vasculitis (systemic lupus erythematosus, Takayasu arteritis, Susac syndrome, Behcet syndrome, neurosyphilis, and APMPPE) and thrombophilias (antiphospholipid antibodies, sickle cell disease). Multiple or recurrent emboli in young patients are usually related to congenital heart disease. Vasculitis from systemic lupus erythematosus can result in multiple brain infracts and in optic neuropathy, which may account for visual symptoms.13 Takayasu arteritis is a large vessel vasculitis that occurs most frequently in adolescents and young adults; patients usually have visual symptoms and can complicate with strokes believed to be secondary to microemboli.14 Patients typically present with extremity claudication, asymmetric or diminished pulses, and renovascular hypertension.14 Susac syndrome (encephalopathy, branch retinal artery occlusions, and hearing loss) has constant multifocal supratentorial white matter lesions and corpus callosum involvement (usually central), and frequent deep gray nuclei involvement. Sensorineural hearing loss (due to choclear involvement) is constant.15 Behcet syndrome is a disease of young adults that may involve the brain (neuro-Behcet), frequently affecting the basal ganglia and/or brain stem.16 Recurrent aphtous oral ulcers are constant and necessary to establish the diagnosis; genital ulcers and eye lesions (uveitis or retinal vasculitis) are frequent.16 Syphilis may result in retinal vasculitis17 as well as brain vasculitis leading to stroke.18 Antiphospholipid antibodies and sickle cell disease are recognized risk factors for recurrent brain infarcts that have also been related to eye infarcts and visual symptoms.19,20 Giant cell arteritis is another vasculitis that can also produce visual loss and ischemic CNS infarcts, but it typically occurs in patients older than 50 years of age.21
In conclusion, APMPPE-related CNS vasculitis should be considered in young patients with stroke (especially when multiple, in the basal ganglia, posterior circulation, or when they do not follow a particular vascular distribution), particularly when there is associated acute loss of visual acuity or history of APMPPE.
References
- 1.Comu S, Verstraeten T, Rinkoff JS, et al. Neurological manifestations of acute posterior multifocal placoid pigment epitheliopathy. Stroke 1996;27:996–1001 [DOI] [PubMed] [Google Scholar]
- 2.Gass JDM. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol 1968;80:177–85 [DOI] [PubMed] [Google Scholar]
- 3.Holt WS, Regan CD, Trempe C. Acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol 1976;81:403–12 [DOI] [PubMed] [Google Scholar]
- 4.Hsu CT, Harlan JB, Goldberg MF, et al. Acute posterior multifocal placoid pigment epitheliopathy associated with a systemic necrotizing vasculitis. Retina 2003;23:64–68 [DOI] [PubMed] [Google Scholar]
- 5.Smith CH, Savino PJ, Beck RW, et al. Acute posterior multifocal placoid pigment epitheliopathy and cerebral vasculitis. Arch Neurol 1983;40:48–50 [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JM, Bresnick GH, Bell CL, et al. Acute posterior multifocal placoid pigment epitheliopathy with cerebral vasculitis. J Clin Neuroophthalmol 1988;8:195–201 [PubMed] [Google Scholar]
- 7.Bewermeyer H, Nelles G, Huber M, et al. Pontine infarction in acute posterior multifocal placoid epitheliopathy. J Neurol 1993;241:22–26 [DOI] [PubMed] [Google Scholar]
- 8.Al Kawi A, Wang DZ, Kishore K, et al. A case of ischemic cerebral infarction associated with acute posterior multifocal placoid pigment epitheliopathy, CNS vasculitis, vitamin B(12) deficiency and homocysteinemia. Cerebrovasc Dis 2004. 21;18:338–39 [DOI] [PubMed] [Google Scholar]
- 9.Wilson CA, Choromokos EA, Sheppard R. Acute posterior multifocal placoid pigment epitheliopathy and cerebral vasculitis. Arch Ophthalmol 1988;106:796–800 [DOI] [PubMed] [Google Scholar]
- 10.Hammer ME, Grizzard WS, Travies D. Death associated with acute, multifocal, placoid pigment epitheliopathy. Arch Ophthalmol 1989;107:170–71 [DOI] [PubMed] [Google Scholar]
- 11.Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke 1999;30:100–108 [DOI] [PubMed] [Google Scholar]
- 12.Kasow DL, Destian S, Braun C, et al. Corpus callosum infarcts with atypical clinical and radiologic presentations. AJNR Am J Neuroradiol 2000;21:1876–80 [PMC free article] [PubMed] [Google Scholar]
- 13.Sklar EM, Schatz NJ, Glaser JS, et al. MR of vasculitis-induced optic neuropathy. AJNR Am J Neuroradiol 1996;17:121–28 [PMC free article] [PubMed] [Google Scholar]
- 14.Kumral E, Evyapan D, Aksu K, et al. Microembolus in patients with Takayasu’s arteritis. Stroke 2002;33:712–16 [DOI] [PubMed] [Google Scholar]
- 15.Susac JO, Murtagh FR, Egan RA, et al. MRI findings in Susac’s syndrome. Neurology 2003;61:1783–87 [DOI] [PubMed] [Google Scholar]
- 16.Ackman-Demor G, Serdaroglu P, Tasci B. Clinical patterns of neurological involvement in Behcet’s disease: evaluation of 200 patients: the Neuro-Behcet Study Group. Brain 1999;122:2171–82 [DOI] [PubMed] [Google Scholar]
- 17.Yokoi M, Kase M. Retinal vasculitis due to secondary syphilis. Jpn J Ophthalmol 2004;48:65–67 [DOI] [PubMed] [Google Scholar]
- 18.Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry 2004;75:1727–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine SR, Deegan MJ, Futrell N, et al. Cerebrovascular and neurologic disease associated with antiphospholipid antibodies: 48 cases. Neurology 1990;40:1181–89 [DOI] [PubMed] [Google Scholar]
- 20.Fine LC, Petrovic V, Irvine AR, et al. Spontaneous central retinal artery occlusion in hemoglobin sickle cell disease. Am J Ophthalmol 2000;129:680–81 [DOI] [PubMed] [Google Scholar]
- 21.Mohan N, Kerr G. Spectrum of giant cell vasculitis. Curr Rheumatol Rep 2000;2:390–95 [DOI] [PubMed] [Google Scholar]


