Abstract
Comprehensive reviews of pre licensure rotavirus strain prevalence data indicated the global importance of six rotavirus genotypes, G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and G12P[8]. Since 2006, two vaccines, the monovalent Rotarix (RV1) and the pentavalent RotaTeq (RV5) have been available in over 100 countries worldwide. Of these, 60 countries have already introduced either RV1 or RV5 in their national immunization programs. Post licensure vaccine effectiveness is closely monitored worldwide. This review aimed at describing the global changes in rotavirus strain prevalence over time. The genotype distribution of the nearly 47,000 strains that were characterized during 2007–2012 showed similar picture to that seen in the preceding period. An intriguing finding was the transient predominance of heterotypic strains, mainly in countries using RV1. Unusual and novel antigen combinations continue to emerge, including some causing local outbreaks, even in vaccinated populations. In addition, vaccine strains have been found in both vaccinated infants and their contacts and there is evidence for genetic interaction between vaccine and wild-type strains. In conclusion, the post-vaccine introduction strain prevalence data do not show any consistent pattern indicative of selection pressure resulting from vaccine use, although the increased detection rate of heterotypic G2P[4] strains in some countries following RV1 vaccination is unusual and this issue requires further monitoring.
Keywords: Surveillance, Rotarix, RotaTeq, Genotype, Rotavirus
1. Introduction
From 2006 onward, two rotavirus vaccines, the monovalent Rotarix (RV1) and the pentavalent Rotateq (RV5) have been licensed in >100 countries worldwide and have been recommended by the World Health Organization (WHO) for routine immunization of all children worldwide (Dennehy, 2008; WHO, 2009a). As of May 2014, 60 countries worldwide have introduced either RV1 and/or RV5 into their national childhood immunization programs (PATH, 2014). RV1 is a monovalent vaccine composed of a single human-derived rotavirus strain of G1P[8] specificity, whereas RV5 is a pentavalent vaccine containing bovine-human reassortant rotaviruses expressing human surface antigens of G1–G4 and P[8] (Parashar et al., 2006; Cortese et al., 2009). Whereas the composition of RV1 and RV5 is different, the multiple vaccine doses administered a few weeks apart imitate the role of sequential natural rotavirus infections in infants and young children, which stimulate the development of both type specific (i.e. homotypic) and heterotypic protective immunity against a variety of group A rotavirus (RVA) strains (Velazquez et al., 1996).
Efficacy, safety and strain-specific effectiveness of RVA vaccines are being closely monitored in post licensure surveillance. Rotavirus strain surveillance targets the characterization of both neutralization antigens, VP7 or G and VP4 or P, of RVAs. Previous reviews on rotavirus strain prevalence (using G and P type data) focused on the pre vaccine licensure period. Three major reviews reported global prevalence data and several regional reviews summarized relevant information from a continent or a WHO region (Gentsch et al., 2005; Santos and Hoshino, 2005; Bányai et al., 2012; Usonis et al., 2012; Ogilvie et al., 2011; Khoury et al., 2011; Todd et al., 2010; Castello et al., 2004; Sanchez-Padilla et al., 2009). The largest data set analyzed so far included over 100,000 strains between 1996 and 2007 to provide baseline data for evaluation of any possible changes in strain distribution following vaccine introduction (Bányai et al., 2012). That study listed >70 individual G–P antigen combinations from 281 studies and 100 countries worldwide, and, together with other major reviews, highlighted the spatiotemporal fluctuation of genotype prevalence in various populations without vaccine pressure. During the 1990s and 2000s at least 2 novel strains, G9P[8] and G12P[8], emerged to become medically important globally in addition to the historically well known four endemic strains, G1P[8], G2P[4], G3P[8], and G4P[8]. In fact, the emerging variants of G9 and G12 strains were inferred to have spread worldwide over the course of a decade to become, respectively, the 5th and 6th most commonly reported RVA strains (Matthijnssens et al., 2010). In addition to these six globally important genotypes, some strains have been demonstrated to be locally or regionally important. Historical examples include G5P[8] strains in South America and G8P[6] strains in parts of Africa (Cunliffe et al., 2000, 2010; Alfieri et al., 1996; Gouvea et al., 1994).
With the availability of vaccines and the observation that newly emerging RVAs may reshape the global landscape of medically important strains, it has become even more important to monitor strain prevalence to assess vaccine effectiveness against various RVA strains. Strain surveillance will also allow studies of the evolution and epidemiology of field strains under vaccine immune selective pressure, to identify possible breakthrough events and genetic interplay between vaccine and field strains. The objective of this review is to gather information about spatial and temporal changes in strain prevalence and summarize reports about possible vaccination associated changes during the first six years of vaccine use. To obtain the required information, we reviewed studies reporting RVA prevalence data from 2007 onward and abstracted data on reported G–P combinations. In addition, strain characterization studies (such as those reporting whole genome sequences or sequencing and phylogenetic analyses of neutralization antigens) were also evaluated and combined with prevalence surveys if background information suggested that data from different sources could be merged.
2. Medically important RVA strains
From 2007 to 2012 the genotypes of 46,967 RVA strains were reported from a total of 81 countries (Table 1). Of the whole data set, 8224 strains were characterized in the WHO African region (AFR), 2341 in the Eastern Mediterranean region (EMR), 6756 in the Americas region (AMR), 14,438 in the European region (EUR), 5728 in the South East Asia region (SEAR), and 9480 in the Western pacific region (WPR) (Fig. 1). Among the 60 countries with a national RV immunization program, 15 were found to have reported post licensure prevalence data. Globally, the most commonly reported six strains were found in ~73% of the samples (G1P[8], 31.2%; G2P[4], 13.0%; G3P[8], 10.7%; G9P[8], 10.2%; G4P[8], 5.0%; G12P[8], 2.7%), or in ~87% of specimens (out of 39,403) when non-typeable strains and mixed infections were removed before the calculation.
Table 1.
Countries reporting RVA strain prevalence in humans, 2007–2012.
| Country | Year of sample collection | No. of strains | G1P[8] | G2P[4] | G3P[8] | G4P[8] | G9P[8] | G12P[8] | OTHERS | NT | Mix | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African region | ||||||||||||
| African Rotavirus Surveillance Network (Ghana, Kenya,Uganda, Zambia, Cameroon, Tanzania, Zimbabwe, Ethiopia) | JUN 2006–2012 | 4638 | 954 | 295 | 129 | 68 | 361 | 293 | 1330 | 609 | 599 | Mwenda et al. (2010, 2014) |
| Burkina Faso | DEC 2009–MAR 2011 | 156 | 16 | 7 | 66 | 47 | 9 | 11 | Nordgren et al. (2012a,b) | |||
| Cameroon | 2010–2011 | 135 | 8 | 1 | 1 | 73 | 40 | 7 | 5 | Ndze et al. (2013) | ||
| Ethiopia | AUG 2007–MAR 2012 | 215 | 44 | 23 | 9 | 37 | 67 | 11 | 24 | Abebe et al. (2014) | ||
| Gambia | 2008–2010 | 204 | 52 | 5 | 124 | 19 | 4 | Kwambana et al. (2014) | ||||
| Ghana | APR 2007–FEB 2011 | 1015 | 224 | 73 | 7 | 6 | 3 | 249 | 239 | 214 | Breiman et al. (2012), Enweronu-Laryea et al. (2013) | |
| Ivory Coast | DEC 2007–JUN 2010 | 90 | 22 | 9 | 8 | 16 | 0 | 19 | 16 | Karamoko and Dabonne (2013), Akoua-Koffi et al. (2014) | ||
| Kenya | APR 2007–AUG 2011 | 246 | 76 | 1 | 62 | 56 | 38 | 13 | Breiman et al. (2012), Kiulia et al. (2014) | |||
| Madagascar | FEB 2008–MAY 2009 | 104 | 15 | 51 | 13 | 25 | Razafindratsimandresy et al. (2013) | |||||
| Malawi | 2006–2007 | 131 | 25 | 2 | 11 | 6 | 76 | 9 | 2 | Cunliffe et al. (2010), Steele et al. (2012) | ||
| Mali | APR 2007–MAR 2009 | 370 | 201 | 16 | 4 | 127 | 22 | Breiman et al. (2012) | ||||
| Niger | APR 2010–MAR 2012 | 449 | 14 | 167 | 16 | 154 | 29 | 39 | 30 | Page et al. (2014) | ||
| Nigeria | JUN 2010–JAN 2011 | 19 | 1 | 6 | 1 | 11 | Japhet et al. (2012) | |||||
| Réunion Island | AUG 2012–NOV 2012 | 20 | 15 | 4 | 1 | Caillère et al. (2013) | ||||||
| South Africa | 2012 | 123 | 2 | 16 | 7 | 54 | 38 | 2 | 4 | Iyaloo et al. (2013) | ||
| Tanzania | JAN 2010–JUN 2012 | 309 | 161 | 2 | 1 | 107 | 38 | Hokororo et al. (in press), Moyo et al. (2014) | ||||
| Total | 8224 | 1806 | 625 | 160 | 84 | 595 | 630 | 2323 | 1040 | 961 | ||
| American region | ||||||||||||
| Argentina | JAN 2007–DEC 2011 | 912 | 67 | 168 | 232 | 23 | 188 | 143 | 9 | 57 | 25 | Esteban et al. (2010), Mandile et al. (2014), Stupka et al. (2009, 2012) |
| Bolivia | JAN 2007–JUN 2011 | 740 | 16 | 134 | 52 | 253 | 167 | 55 | 63 | Patel et al. (2013), Rivera et al. (2013) | ||
| Brazil | MAR 2006–MAR 2012 | 980 | 87 | 521 | 11 | 64 | 117 | 111 | 69 | Assis et al. (2013), Borges et al. (2011), Carvalho-Costa et al. (2009), Cilli et al. (2011), Nakagomi et al. (2008), Dulgheroff et al. (2012), Gómez et al. (2013), Gurgel et al. (2009), Luchs et al. (2012, 2013), Luchs and Timenetsky (2014), Nozawa et al. (2010), Sáfadi et al. (2010), Soares et al. (2012, 2014) | ||
| Canada | 2007–2011 | 323 | 192 | 26 | 45 | 5 | 32 | 6 | 5 | 12 | Chetrit et al. (2013), McDermid et al. (2012), Ward et al. (2013) | |
| Chile | JUL 2006–MAR 2010 | 238 | 12 | 14 | 177 | 15 | 20 | Lucero et al. (2012), O’Ryan et al. (2009) | ||||
| Colombia | 2008–2012 | 467 | 1 | 191 | 5 | 26 | 103 | 61 | 80 | Peláez-Carvajal et al. (2014) | ||
| Cuba | 2007–2008 | 29 | 14 | 13 | 1 | 1 | Ribas et al. (2011) | |||||
| Guatemala | 2007–2010 | 147 | 91 | 15 | 37 | 4 | Cortes et al. (2012), Quaye et al. (2013) | |||||
| Honduras | 2009–2010 | 50 | 25 | 25 | Quaye et al. (2013) | |||||||
| Mexico | MAR 2010–MAY 2010 | 16 | 16 | Yen et al. (2011) | ||||||||
| Nicaragua | FEB 2007–OCT 2009 | 1095 | 336 | 341 | 39 | 42 | 68 | 266 | 3 | Bányai et al. (2009a,b), Becker-Dreps et al. (2011), Khawaja et al. (2013) | ||
| Paraguay | 2006–2007 | 143 | 69 | 37 | 6 | 23 | 8 | Martínez et al. (2010) | ||||
| Peru | JAN 2010–DEC 2012 | 42 | 2 | 5 | 4 | 19 | 12 | Espejo et al. (2014) | ||||
| USA | 2007–2011 | 1574 | 342 | 230 | 489 | 35 | 157 | 153 | 97 | 29 | 42 | Abdel-Haq et al. (2011), Boom et al. (2010), Cardemil et al. (2012), Clark et al. (2011), Hull et al. (2011), McDonald et al. (2012), Payne et al. (2009, 2013), Staat et al. (2011), Weinberg et al. (2012, 2013) |
| Total | 6756 | 1183 | 1694 | 873 | 107 | 967 | 300 | 686 | 639 | 307 | ||
| Western pacific region | ||||||||||||
| Australia | JUL 2006–SEP 2011 | 1715 | 543 | 635 | 167 | 37 | 106 | 46 | 149 | 32 | Cowley et al. (2013), Kirkwood et al. (2007, 2008, 2009, 2010, 2011) | |
| China | 2006–2011 | 2516 | 534 | 78 | 1245 | 6 | 103 | 137 | 299 | 114 | Chen et al. (2013), Dong et al. (2013), Li et al. (2009), Shen et al. (2013), Wang et al. (2009, 2011, 2013), Zeng et al. (2010), Zhang et al. (2012) | |
| Fiji | JAN 2006–DEC 2007 | 116 | 1 | 98 | 1 | 2 | 14 | Jenney et al. (2009) | ||||
| Japan | JUL 2006–AUG 2012 | 682 | 268 | 23 | 295 | 1 | 80 | 15 | Chan-it et al. (2011), Dey et al. (2009), Kamiya et al. (2011), Komoto et al. (2013), Kuzuya et al. (2014), Thongprachum et al. (2013), Yamamoto et al. (2011); | |||
| Malaysia | SEP 2007–DEC 2007 | 12 | 4 | 5 | 3 | Zuridah et al. (2010) | ||||||
| Papua New Guinea | APR 2008–NOV 2010 | 244 | 122 | 20 | 56 | 2 | 6 | 2 | 8 | 28 | Horwood et al. (2012) | |
| Singapore | SEP 2005–APR 2008 | 320 | 125 | 49 | 61 | 1 | 68 | 16 | Phua et al. (2013) | |||
| South Korea | 2006–2012 | 1398 | 507 | 38 | 125 | 18 | 77 | 1 | 364 | 172 | 96 | Han et al. (2010), Jeong et al. (2011), Park et al. (2011), Shim et al. (2010, 2011), Than et al., 2013 |
| Taiwan | JAN 2005–DEC 2010 | 1831 | 891 | 111 | 347 | 214 | 18 | 250 | Hwang et al. (2012), Wu et al. (2011a,b, 2012) | |||
| Vietnam | SEP 2006–NOV 2008 | 646 | 106 | 11 | 450 | 1 | 38 | 12 | 28 | Breiman et al. (2012), Matsushima et al. (2012), Ngo et al. (2009), Tra My et al. (2011) | ||
| Total | 9480 | 3101 | 965 | 2844 | 64 | 656 | 7 | 638 | 907 | 298 | ||
| South East Asian region | ||||||||||||
| Bangladesh | 2007–2009 | 439 | 120 | 85 | 112 | 19 | 54 | 24 | 25 | Afrad et al. (2013), Breiman et al. (2012), Rahman et al. (2011) | ||
| India | 2007–2012 | 3424 | 788 | 540 | 296 | 97 | 346 | 1090 | 267 | Kang et al. (2013), Mangayarkarasi et al. (2012), Mathew et al. (2014), Mukherjee et al. (2009, 2012, 2013), Mullick et al. (2013), Reesu et al. (2013) | ||
| Nepal | NOV 2006–SEP 2011 | 849 | 40 | 91 | 36 | 23 | 498 | 66 | 95 | Ansari et al. (2013), Gauchan et al. (2013), Sherchand et al. (2009, 2012) | ||
| Thailand | 2007–MAY 2013 | 1016 | 454 | 71 | 431 | 36 | 11 | 13 | Chaimongkol et al. (2012), Chieochansin et al. (2014), Khananurak et al. (2010), Maiklang et al. (2012) | |||
| Total | 5728 | 1402 | 787 | 431 | 0 | 480 | 150 | 911 | 1180 | 387 | ||
| E. mediterranean region | ||||||||||||
| Bahrain | APR 2006–APR 2007 | 16 | 10 | 2 | 2 | 2 | Musawi et al. (2013) | |||||
| Iran | OCT 2006–SEP 2008 | 69 | 34 | 2 | 4 | 28 | 1 | Modaress and Rahbarimanesh (2011) | ||||
| Iraq | JAN 2008–DEC 2008 | 98 | 6 | 5 | 3 | 3 | 49 | 29 | 3 | Ahmed et al. (2013) | ||
| Jordan | JAN 2006–APR 2008 | 563 | 299 | 55 | 13 | 8 | 48 | 34 | 85 | 21 | Kaplan et al. (2011), Kareman and Alkafajei (2012), Salem et al. (2011) | |
| Libya | OCT 2007–SEP 2008 | 176 | 49 | 3 | 5 | 116 | 1 | 1 | 1 | Abugalia et al., 2011 | ||
| Morocco | JUN 2006–MAY 2009 | 547 | 301 | 50 | 2 | 5 | 62 | 35 | 13 | 79 | Benhafid et al., 2013 | |
| Oman | JUN 2006–DEC 2007 | 234 | 7 | 60 | 1 | 21 | 123 | 22 | Al Awaidy et al. (2009) | |||
| Pakistan | JAN 2008–SEP 2010 | 290 | 67 | 39 | 3 | 15 | 101 | 52 | 13 | Alam et al. (2013), Iftikhar et al. (2012), Tamim et al. (2013) | ||
| Tunisia | 2007–2010 | 270 | 74 | 61 | 55 | 27 | 3 | 22 | 3 | 25 | Fredj et al. (2012), Hassine-Zaafrane et al. (2011) | |
| Yemen | NOV 2007–MAR 2009 | 78 | 43 | 9 | 16 | 1 | 7 | 2 | Kirby et al. (2011) | |||
| Total | 2341 | 890 | 284 | 73 | 50 | 263 | 4 | 276 | 336 | 165 | ||
| European region | ||||||||||||
| Austria | 2010–2011 | 44 | 10 | 23 | 3 | 3 | 3 | 2 | Paulke-Korinek et al. (2013) | |||
| Belgium | 2007–2010 | 334 | 86 | 149 | 24 | 26 | 22 | 2 | 19 | 6 | Braeckman et al. (2012), Heylen et al. (2013), Zeller et al. (2010, 2012) | |
| Bulgaria | 2007–2008 | 352 | 139 | 88 | 7 | 45 | 1 | 14 | 22 | 36 | Mladenova et al. (2010, 2012) | |
| Estonia | JAN 2007–DEC 2008 | 124 | 5 | 43 | 2 | 16 | 5 | 6 | 42 | 5 | Soeorg et al. (2012) | |
| Finland | 2007–2008 | 292 | 214 | 9 | 13 | 38 | 2 | 8 | 8 | Räsänen et al. (2011) | ||
| France | 2007–2009 | 1380 | 853 | 45 | 46 | 37 | 345 | 8 | 10 | 36 | De Rougemont et al. (2011) | |
| Germany | JAN 2008–DEC 2010 | 342 | 71 | 13 | 136 | 54 | 59 | 3 | 6 | Pietsch et al. (2009, 2011) | ||
| Greece | JAN 2007–AUG 2010 | 207 | 67 | 41 | 76 | 13 | 2 | 8 | Koukou et al. (2011), Trimis et al. (2011) | |||
| Hungary | JAN 2007–DEC 2011 | 2380 | 1068 | 351 | 22 | 557 | 162 | 15 | 83 | 83 | 39 | László et al. (2012) |
| Ireland | 2007–2011 | 148 | 106 | 5 | 16 | 11 | 1 | 9 | Cashman et al. (2012), Gunn et al. (2012) | |||
| Israel | NOV 2007–DEC 2009 | 187 | 61 | 50 | 20 | 10 | 10 | 5 | 31 | Muhsen et al. (2010) | ||
| Italy | 2007–2011 | 3042 | 1308 | 287 | 88 | 286 | 640 | 1 | 90 | 107 | 235 | Finamore et al. (2011), Grassi et al. (2012), Ianiro et al. (2013, 2014), Medici et al. (2011), Ruggeri et al. (2011), Zuccotti et al. (2010) |
| Kazakhstan | JAN 2007–DEC 2009 | 446 | 113 | 106 | 65 | 27 | 11 | 3 | 24 | 71 | 26 | Vainio et al. (2013) |
| Kyrgyzstan | JAN 2007–DEC 2009 | 409 | 205 | 52 | 51 | 6 | 6 | 15 | 16 | 25 | 33 | Vainio et al. (2013) |
| Netherlands | MAY 2008–NOV 2009 | 54 | 19 | 2 | 8 | 13 | 2 | 4 | 6 | Friesema et al. (2012) | ||
| Portugal | JAN 2007–MAR 2007 | 207 | 10 | 142 | 29 | 7 | 8 | 11 | Antunes et al. (2009) | |||
| Russia | JAN 2007–MAY 2011 | 1416 | 311 | 90 | 58 | 655 | 7 | 10 | 257 | 28 | Zhirakovskaya et al. (2012) | |
| Slovenia | JAN 2007–OCT 2008 | 823 | 528 | 81 | 19 | 110 | 28 | 15 | 23 | 19 | Steyer et al. (2009) | |
| Spain | OCT 2006–DEC 2011 | 1111 | 443 | 113 | 13 | 6 | 288 | 133 | 14 | 64 | 37 | Téllez Castillo et al. (2010), Cilla et al. (2010, 2013), Gutiérrez-Gimeno et al. (2010), Sánchez-Fauquier et al. (2013) |
| Sweden | OCT 2007–OCT 2008 | 604 | 467 | 17 | 24 | 36 | 52 | 4 | 4 | Rinder et al. (2014) | ||
| Switzerland | DEC 2006–JUN 2007 | 113 | 59 | 4 | 4 | 4 | 5 | 15 | 22 | Lacroix et al. (2010) | ||
| Turkey | JAN 2008–JAN 2009 | 100 | 7 | 6 | 1 | 19 | 31 | 10 | 26 | Tapisiz et al. (2011) | ||
| Ukraine, Tajikistan, Georgia | 2007 | 323 | 102 | 59 | 12 | 59 | 63 | 2 | 8 | 18 | Mirzayeva et al. (2009) | |
| Total | 14438 | 6252 | 1770 | 630 | 2027 | 1827 | 185 | 403 | 760 | 584 |
Fig. 1.

Geographical distribution of medically important RVA strains between 2007 and 2012. WHO regions are highlighted by various colors; dark shade shows countries providing data from any given region. In addition to the six globally common G–P combinations, regionally common, unusual and rare strains are also shown and referred to as ‘Other’. Mixed infection with multiple G and/or P types and non-typeable strains were not included in the calculations.
At the level of WHO regions, the combined prevalence of the six common genotypes ranged from 62.7% (AFR) to 96.9% (EUR). G1P[8] strains predominated in all but one WHO region; the only exception was AMR, where G2P[4] strains were relatively more commonly reported. On the contrary, among the globally common strains, the prevalence of G4P[8] and G12P[8] strains was negligible in several regions (e.g., WPR: G4P[8], 0.8%; G12P[8], 0.1%; EMR: G12P[8], 0.2%; SEAR: G4P[8], not detected). All other common genotypes had >1% prevalence (regional ranges in the whole study period: G1P[8], 20.4–47.7%; G2P[4], 10.0–29.2%; G3P[8], 2.6–34.4%; G4P[8], up to 15.5%; G9P[8], 7.9–16.6%; G12P[8], 0.1–10.1%). Furthermore, in the AFR an additional seven genotypes were found at a prevalence greater than 1%, including G2P[6] (8.2%), G1P[6] (6.0%), G3P[6] (5.5%), G12P[6] (3.5%), G8P[6] (1.7%), G8P[4] (1.8%), and G1P[4] (1.1%). These data demonstrate that genotype P[6] RVA strains continue to circulate at marked prevalence in the AFR. Somewhat less genotype diversity among medically important strains was seen in the AMR (G1P[6], 2.1%; G9P[6], 2.2%; G9P[4], 1.7%), in the EMR (G2P[6], 3.9%; G1P[6], 3.8%; G1P[4], 1.8%; G9P[6], 1%), in the SEAR (G12P[6], 14.9%; G9P[4], 1.6%; G1P[6], 1.2%; G9P[6], 1.1%), and in the WPR (G4P[6], 2.4%; G3P[4], 1%). No additional prevalent strains were found in EUR. Of note is that several of the regionally common strains were reported from single countries that contributed a disproportionately large number of strains to the regional total. Thus, the regional prevalence data may be somewhat biased by the relative significance of unusual strains reported from countries with greater sample size analyzed.
In order to study annual fluctuation of RVA strains within and between countries, data were summarized at the level of study sites of reporting countries whenever possible. With this approach temporal and spatial changes were observed both in countries with and without a rotavirus vaccination program (Fig. 2). Predominance and sustained circulation of G1P[8] strains was observed in parts of North America, and many countries in the EMR and the EUR and even in selected countries within the AFR and the WPR, where G1P[8] strains were otherwise regionally not prevalent. Genotype G2P[4] was predominant in many countries utilizing RV1 in their childhood immunization program, including Latin American countries and Belgium and Austria in Europe (Braeckman et al., 2012; Gurgel et al., 2009; Nakagomi et al., 2008; Paulke-Korinek et al., 2013; Peláez-Carvajal et al., 2014). However, territories in Australia using RV5 as well as several countries worldwide with low vaccine usage also reported high G2P[4] prevalence that was sustained over consecutive years (Fig. 2). Among the historically important genotypes, G3P[8] was reported to prevail in several study sites in the United States and Australia using RV1 and RV5 vaccine, but this genotype was also prevalent in several countries without universal rotavirus vaccination programs including Thailand, Germany, Argentina and the Reunion Island, as well as some SEAR countries (e.g., China, Japan, and Vietnam), where it has been prevalent over consecutive years (Breiman et al., 2012; Caillère et al., 2013; Chen et al., 2013; Ngo et al., 2009; Hull et al., 2011; Kamiya et al., 2011; Kuzuya et al., 2014; Kirkwood et al., 2007, 2008, 2009, 2010, 2011; Li et al., 2009; Maiklang et al., 2012; Stupka et al., 2012; Pietsch et al., 2009, 2011; Thongprachum et al., 2013; Wang et al., 2009, 2011; Zeng et al., 2010). Genotype G4P[8] strains were reported to prevail in a few countries worldwide (e.g., Ivory Coast, Estonia, Greece, Hungary, and Russia) typically during a single epidemic season, and G4P[8] strains were also predominant at some Australian RV1 sites (Akoua-Koffi et al., 2014; Karamoko and Dabonne, 2013; Kirkwood et al., 2007, 2008, 2009, 2010, 2011; Koukou et al., 2011; László et al., 2012; Soeorg et al., 2012; Trimis et al., 2011; Zhirakovskaya et al., 2012). Finally, many countries worldwide reported transient increase in the frequency of genotypes G9P[8] and G12P[8] that emerged during the 1990s, including some high vaccine coverage areas within the USA (Fig. 2).
Fig. 2.

Spatiotemoral shift in predominance of the globally common six G–P combinations and some others. Data were broken down by study year whenever possible. Color codes refer to a particular G–P combination. Diagonal color patterns indicate two different G–P combinations circulated at similar great prevalence. The dates when universal RVA vaccination was implemented in a given country and the prefererred RVA vaccine are indicated where relevant.
3. Minority and novel wild-type RVA strains
No globally emerging new strains were identified in the past six years, but several locally or regionally important strains were recognized including some with partially or fully heterotypic serotypes compared to the two vaccines (Fig. 2). One example is G9P[4] strains identified in Guatemala and Bangladesh during 2009–2010 and 2011, respectively (Quaye et al., 2013; Afrad et al., 2013). G12P[6] strains predominated in Nepal between 2007 and 2011 and in Malawi in 2007, G1P[6] and/or G2P[6] prevailed in some study years in Columbia, Gambia and Iraq, and G4P[6]s were locally important in some regions of Hungary and South Korea during 2008 (Ahmed et al., 2013; Ansari et al., 2013; Gauchan et al., 2013; Jeong et al., 2011; Kwambana et al., 2014; László et al., 2012; Peláez-Carvajal et al., 2014; Sherchand et al., 2009, 2012; Steele et al., 2012). Another example of a regionally emerging genotype is G6P[6], recently reported in several African countries among genotyped strains (prevalence range 3.4–17.6%) (Nordgren et al., 2012a,b; Page et al., 2014). In addition, phylogenetic analyses identified a possible epidemiological linkage between African and European G6P[6] strains (Nordgren et al., 2012b; Ianiro et al., 2013). A bovine RVA related human strain, G10P[14], was described in several individuals of an aboriginal population in Australia vaccinated with RV1 (Cowley et al., 2013).
Two VP4 genotypes, P[6] and P[11], were originally associated with asymptomatic infections on neonatal wards (Das et al., 1994; Gentsch et al., 1993; Dunn et al., 1993; Sukumaran et al., 1992; Gorziglia et al., 1986). Subsequent studies, however, found P[6] to be very common among pathogenic strains. The most salient example is the high prevalence of genotype P[6] among RV patients in many sub-Saharan countries of Africa (Todd et al., 2010). RVA strains bearing genotype P[11], have been also detected in children with diarrhea in several countries worldwide, including India, Pakistan, Estonia, Slovenia, and Italy (Table 2).
Table 2.
Detection of rare RVA strains in humans, 2007–2012.
| Strain | Country | No./freq. | Comment | Refs. | Strain | Country | No./freq. | Comment | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| G1P[5] | Bangladesh | 1/0.6% | Detected during RV5 trial | Breiman et al. (2012) | G6P[1] | Pakistan | 2/2.7% | Bovine like | Tamim et al. (2013) |
| G1P[9] | Pakistan | 6/3.5% | Iftikhar et al. (2012) | G6P[4] | Belgium | 1/0.9% | Zeller et al. (2010) | ||
| Italy | 1/1% | Grassi et al. (2012) | G6P[6] | Burkina Faso | 13/23% | Nordgren et al. (2012a) | |||
| Taiwan | 1/<0.1% | Hwang et al. (2012) | Burkina Faso | 11/11% | Putative bovine-human reassortant G6-P[6]-I2-A2-N2-T2-E2-H2 | Nordgren et al. (2012b) | |||
| South Korea | 1/0.3% | Shim et al. (2011) | Niger | 13/2.9% | Page et al. (2014) | ||||
| South Korea | 1/0.4% | Park et al. (2011) | Italy | 1/N.R. | G6-P[6]-I2-A2-N2-T2-E2-H2 | Ianiro et al. (2013) | |||
| G1P[10] | Gambia | 21/10.2% | Kwambana et al. (2014) | G6P[8] | Belgium | 1/0.9% | Zeller et al. (2010) | ||
| Oman | 3/1.2% | Al Awaidy et al. (2009) | Bangladesh | 1/0.6% | Detected during RV5 vaccine trial | Breiman et al. (2012) | |||
| South Korea | 2/0.7% | Park et al. (2011) | G6P[9] | Tunisia | 1/0.7% | Feline like | Fredj et al. (2012) | ||
| G1P[11] | Estonia | 1/0.8% | Soeorg et al. (2012) | France | 1/0.1% | de Rougemont et al. (2011) | |||
| G2P[3] | Turkey | 1/1% | Tapisiz et al. (2011) | Italy | 1/N.R. | G6-P[9]-I2-A3-N2-T3-E3-H3 | Ianiro et al. (2013) | ||
| G2P[5] | Mali | 1/0.2% | Detected during RV5 trial | Breiman et al. (2012) | Hungary | 7/0.3% | László et al. (2012) | ||
| Australia | 1/0.2% | Detected in an RV5 territory (Western Australia) | Kirkwood et al. (2011) | Slovenia | 2/0.2% | Steyer et al. (2009) | |||
| G2P[9] | South Korea | 1/0.2% | Jeong et al. (2011) | Japan | 2/N.R. | Possible humanbovine-feline reassortant, G6-P[9]-I2-R2-C2-M2-A3-N2-T3-E3-H3 | Yamamoto et al. (2011) | ||
| South Korea | 1/0.4% | Park et al. (2011) | G6P[11] | Slovenia | 1/0.1% | Bovine like G6-P[11]-I2-R2-C2-M2-A13-N2-T6-E2-H3 | Steyer et al. (2009, 2013) | ||
| G2P[10] | Oman | 3/1.2% | Al Awaidy et al. (2009) | G6P[14] | Spain | 1/0.3% | Cilla et al. (2010) | ||
| Italy | 2/N.R. | Finamore et al. (2011) | France | 1/0.1% | de Rougemont et al. (2011) | ||||
| Italy | 1/1% | Grassi et al. (2012) | Slovenia | 1/0.1% | Steyer et al. (2009) | ||||
| Turkey | 2/2% | Tapisiz et al. (2011) | India | 1/N.R. | Bovine like G6-P[14]-I2-R2-C2-M2-A11-N2-T6-E2-H3 | Mullick et al. (2013) | |||
| China | 1/0.1% | Li et al. (2009) | G8P[1] | Ghana | 1/0.7% | Detected during RV5 trial | Breiman et al. (2012) | ||
| South Korea | 1/0.4% | Park et al. (2011) | G8P[9] | Australia | 2/0.4% | Kirkwood et al. (2011) | |||
| G2P[14] | Gambia | 1/0.5% | Kwambana et al. (2014) | G8P[14] | Spain | 1/0.3% | Cilla et al. (2010) | ||
| G3P[3] | Brazil | 1/N.R. | Canine like | Luchs et al. (2012) | France | 1/0.1% | Bovine like | de Rougemont et al. (2011) | |
| G3P[9] | Madagascar | 1/0.9% | Feline like | Razafindratsimandresy et al. (2013) | Greece | 1/0.8% | Koukou et al. (2011) | ||
| USA | 1/0.4% | Abdel-Haq et al. (2011) | Spain | 1/0.8% | Sánchez-Fauquier et al. (2013) | ||||
| USA | 1/1.2% | Cardemil et al. (2012) | Taiwan | 1/<0.1% | Bovine like | Hwang et al. (2012), Wu et al. (2012) | |||
| USA | 1/0.5% | Hull et al. (2011) | Australia | 1/0.8% | Kirkwood et al. (2009) | ||||
| Canada | 1/7.6% | Feline like | Ward et al. (2013) | Australia | 1/33% | Bovine like | Swiatek et al. (2010) | ||
| Libya | 1/0.6% | Abugalia et al. (2011) | G9P[9] | Tunisia | 1/0.8% | Hassine-Zaafrane et al. (2011) | |||
| Hungary | 9/0.4% | László et al. (2012) | Portugal | 2/0.9% | Antunes et al. (2009) | ||||
| Taiwan | 1/<0.1% | Feline like | Hwang et al. (2011, 2012) | Bulgaria | 1/0.3% | Mladenova et al. (2010) | |||
| China | 1/0.1% | Li et al. (2009) | Slovenia | 1/0.1% | Steyer et al. (2009) | ||||
| South Korea | 1/0.3% | Shim et al. (2011) | Kazakhstan | 1/0.2% | Vainio et al. (2013) | ||||
| China | 1/N.R. | G3-P[9]-I3-R3-C3-M3-A3-N3-T3-E3-H6; Feline/Canine like | Wang et al. (2013) | South Korea | 1/0.4% | Park et al. (2011) | |||
| China | 2/0.5% | Feline like | Wang et al. (2009) | G9P[10] | Oman | 1/0.4% | Al Awaidy et al. (2009) | ||
| G3P[10] | India | 1/N.R. | G3-P[10]-I2-R2-C2-M5-A2-N5-T5-E2-H3; Simian and caprine like | Mukherjee et al. (2012) | Pakistan | 8/4.6% | Iftikhar et al. (2012) | ||
| G3P[14] | South Africa | 1/0.8% | Enweronu-Laryea et al. (2013) | Turkey | 4/4% | Tapisiz et al. (2011) | |||
| G3P[19] | Taiwan | 3/0.2% | Porcine like | Wu et al. (2011b) | G9P[11] | Pakistan | 2/1.1% | Diarrheic children | Iftikhar et al. (2012) |
| G3P[24] | USA | Staat et al. (2011) | Italy | 1/N.R. | Diarrheic child | Finamore et al. (2011) | |||
| G3P[25] | Taiwan | 1/<0.1% | Porcine like | Wu et al. (2011a) | G9P[19] | Taiwan | 1/<0.1% | Porcine like | Hwang et al. (2012) |
| G4P[9] | Portugal | 1/0.5% | Antunes et al. (2009) | G10P[14] | Slovenia | 6/0.7% | Bovine like | Steyer et al. (2009) | |
| G4P[10] | Gambia | 1/0.5% | Kwambana et al. (2014) | Australia | 6/N.R. | G10-P[14]-I2-R2-C2-M2-A11-N2-T6-E2-H3 Bovine/ovine like | Cowley et al. (2013) | ||
| Turkey | 1/1% | Tapisiz et al. (2011) | G10P[15] | China | 1/N.R. | Ovine like | Zhang et al. (2012) | ||
| G5P[6] | Bulgaria | 1/N.R. | G5-P[6]-I1-R1-C1-M1-A8-N1-T1-E1-H1; possible porcine-human reassortant | Mladenova et al. (2012) | G11P[25] | India | 1/N.R. | Porcine like G11-P[25]-I12-R1-C1-M1-A1-N1-T1-E1-H1 | Mullick et al. (2013) |
| Japan | 1/N.R. | Porcine like G5-P[6]-I5-R1-C1-M1-A8-N1-T1-E1-H1 | Komoto et al. (2013) | South Korea | 1/N.R. | Porcine-human reassortant G11-P[25]-I12-R1-C1-M1-A1-N1-T1-E1-H1 | Than et al. (2013) | ||
| Taiwan | 1/<0.1% | Porcine like | Hwang et al. (2012) | G12P[9] | Paraguay | 1/1.6% | Martínez et al. (2010) | ||
| G5P[8] | Turkey | 2/2% | Tapisiz et al. (2011) | Slovenia | 2/0.2% | Steyer et al. (2009) | |||
| G5P[19] | Taiwan | 1/<0.1% | Porcine like | Wu et al. (2011b) | G14P[24] | USA | 1/N.R. | Possible equinehuman-bovine reassortant G14-P24-I9-R2-C3-M3-A9-N3-T3-E3-H6 | Weinberg et al. (2013) |
In previous comprehensive reviews which collected and published data from the pre licensure period, at least 74 G–P combinations were identified. In studies of published data for the 2007–2012 period, an additional 11 new antigen combinations were identified, each from sporadic cases. These included a G14P[24] strain from the USA that contained simian-, equine- and bovine RVA related genes in the whole genomic configuration, a G3P[25] strain and a G5P[19] from Taiwan both carrying porcine RVA related genes in their genomes, two G6P[1] strains from Pakistan closely related to bovine RVA strains in their VP4 and VP7 genes, a G10P[15] rotavirus identified in China that shared over 99% VP4 and VP7 gene sequence identities with ovine rotaviruses, two G8P[9] detected in Australia, a G2P[3] strain from Turkey, a G2P[14] strain from Gambia, a G3P[24] from the USA, a G1P[5] strain from Bangladesh, and a G2P[5] strain from Mali (Breiman et al., 2012; Hwang et al., 2012; Kirkwood et al., 2011; Kwambana et al., 2014; Staat et al., 2011; Tamim et al., 2013; Tapisiz et al., 2011; Weinberg et al., 2013; Zhang et al., 2012). Some of the rare strains were detected from diarrheic patients during vaccine trials and the shared genotypes with vaccine strains indicate a possible vaccine strain origin (Table 2).
4. Detection and evolution of vaccine strains in patients with diarrhea
The mechanisms of attenuation and potential reversion to regain the virulent phenotype of rotavirus vaccine strains are unknown. A temporal association between vaccination and diarrhea in a low proportion of vaccinees has been documented in large vaccine trials as well as during strain surveillance in the post licensure period (Payne et al., 2010; Bowen and Payne, 2012; Boom et al., 2012; Donato et al., 2012). In addition, vaccine derived strains have been described in contacts of vaccinees and even in patients without history of vaccination or apparent contact with a vaccinated infant. For some of these cases only RV1/RV5 derived strains but no other common enteropathogens were detected, suggesting that the vaccine strains could have (re)gained a pathogenic phenotype.
Concerning putative RV5 derived vaccine strains, several G6 and/or P[5] RVAs were identified in RV5 vaccine trials suggestive of vaccine origin of the identified strains in infants. The G6 VP7 and P[5] VP4 genotypes are components of the RV5 vaccine and are co-expressed with the human-derived P[8] VP4 and G1-G4 VP7 antigens, respectively, as individual reassortants (Fig. 3A). Of note is that in these vaccine trials no further analysis was conducted to provide evidence for the possible bovine parental RVA strain (i.e. WC3) origin of the G6 VP7 or the P[5] VP4 antigens (Breiman et al., 2012). Following the introduction of RV5 in routine vaccination programs, only few authors have reported detailed molecular analysis of vaccine derived strains. For example, Payne et al. (2010) used a genome sequencing approach to demonstrate that the identified G1P[8] strain detected in a 30 month old US child suffering from diarrhea was fully RV5 vaccine origin. Because both G1 VP7 and P[8] VP4 specificities are expressed on separate reassortants of the pentavalent vaccine, identification of a single G1P[8] strain is consistent with reassortment between two RV5 vaccine strains. Boom et al. (2012) described four RV5 derived vaccine strains by partial genome sequencing (including only VP3, VP4, VP6 and VP7 genes). Donato et al. (2012) described 13 RV5 derived strains from Australian patients by targeting only four genes (VP3, VP4, VP6 and VP7). Of these, four patients were found to shed vaccine derived (vd) G1P[8] reassortants. Hemming and Vesikari (2012, 2014) reported several vdG1P[8] strains in diarrheic patients from Finland by sequencing of selected genes; one report from Finland raised the possibility that vaccine-derived strains may be acquired from the community. Bucardo et al. (2012) demonstrated for the first time the possible rescue of a RV5 origin NSP2 gene in wild-type G1P[8] strains detected in two Nicaraguan children. Altogether, some data indicate that vdG1P[8] reassortants of the RV5 strains might be more transmissible and more virulent in humans than the original vaccine component strains individually.
Fig. 3.
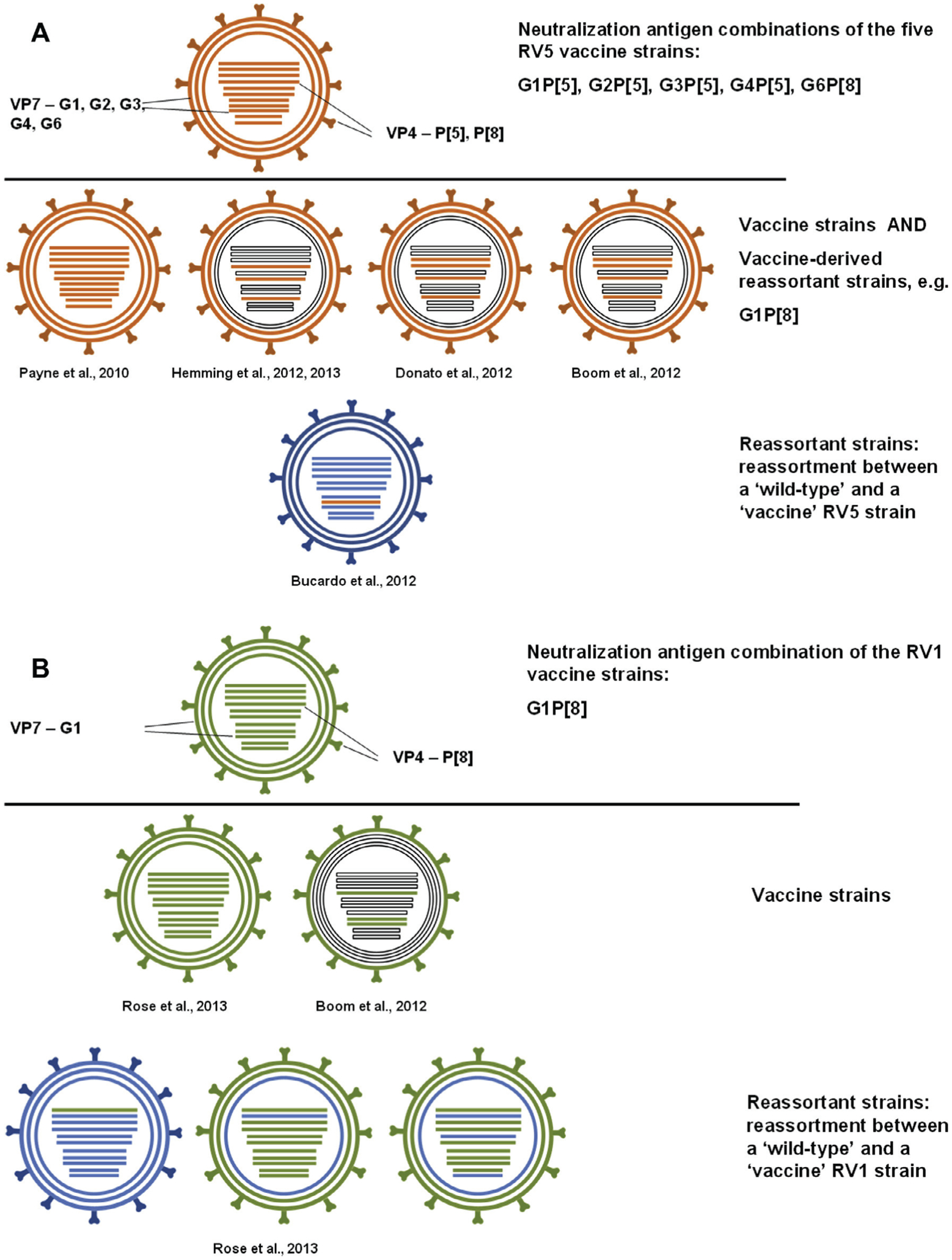
Evolution of RV vaccines. The virions and the parental genomic constellations of RV1 and RV5 are shown in orange and green, respectively. Wild type strains are represented by blue color. Missing sequence information is shown as empty bars.
With regard to RV1 origin strains, Rose et al. (2013) reported several cases from Brazil demonstrating natural reassortment events between the RV1 vaccine strain and wild-type strains (Fig. 3B). Among the reassortants identified were two mono-reassortant strains; one strain carried an RV1 derived VP1 gene on the configuration of a wild-type strain and another strain carried a wild-type derived VP2 gene on the configuration of the RV1 vaccine strain. Another reassortant strain carried the VP2, NSP1 and NSP5 gene from a wild-type strain, whereas the remaining genes were of RV1 vaccine strain origin. In addition, Boom et al. (2012), in their aforementioned paper, reported a RV1 associated case by sequencing of the VP7, VP4 and NSP2 genes, where the affected child was non-vaccinated and had no contact history with a vaccinated child.
5. Concluding remarks
In a previous review, we described the decline of the historically dominant G1P[8] strains over time between 1996 and 2007, and the simultaneous global emergence of G9P[8] and G12P[8] strains (Bányai et al., 2012). Analysis of new data in the vaccine post licensure period indicates the resurgence of G1P[8] strains to become the most prevalent genotype in many countries worldwide although not to levels described in earlier reviews. The sustained predominance of G2P[4] strains reported in the Americas and parts of Europe and Australia, where RV1 vaccine is used in national immunization programs, raised concerns whether this strain that is fully heterotypic to the G1P[8] strain in RV1 was being selected because of vaccine pressure (Grimwood and Kirkwood, 2008; Matthijnssens et al., 2009, 2012). However, contemporary data from other countries in the same regions with low rates of RV1 use as well as from countries using RV5 indicates sustained prevalence of G2P[4] strains in these countries as well, diminishing the initial concern that a fully heterotypic strain may overwhelm a highly vaccinated population. Nevertheless, the very high prevalence of G2P[4] strains reported in some countries after implementation of RV1 vaccination is highly unusual, and this issue requires further monitoring.
Although the emergence and transient predominance of fully heterotypic strains in countries using either RV1 or RV5 were observed, in several instances fully or partially homotypic strains replaced these fully heterotypic strains over several years post-vaccine implementation. Furthermore, many pre-licensure and post-licensure studies have shown that both RV1 and RV5 have high effectiveness against non-vaccine strains (Justino et al., 2011; Yen et al., 2011; Braeckman et al., 2012; Cortese et al., 2013; Patel et al., 2013). Despite these reassuring findings, it is important to note that among the 60 countries which introduced RV1 and/or RV5 in national immunization programs (PATH, 2014), only a few have so far reported detailed, longitudinal strain prevalence data, and thus it is not possible to exclude the possibility of some strain selection related to vaccine pressure over several years after vaccine implementation.
Regardless, an important question that we need to ask is what the implication of a putative vaccine use associated strain selection would be? Currently there is no conclusive evidence associating RVA genotype and disease severity. Furthermore, although from a historical perspective it may be very unusual for genotype G2P[4] to predominate for several years, one needs to remember that a single genotype, G1P[8], predominated over several consecutive years in many countries during the 1980s to late 1990s (Hull et al., 2011; Bányai et al., 2009a,b; Arista et al., 2006). In some studies the long term prevalence of G1P[8] strains was linked to antigenic drift and intra-typic shift leading to new combinations of different co-circulating G1 VP7 and P[8] VP4 variants (lineages) (Bányai et al., 2009a,b; Arista et al., 2006). Natural fluctuation of the common RVA strains during consecutive seasons may attest to their fitness; however, vaccine breakthrough events may result from lineage changes in VP4, VP7 and other rotavirus genes. Recent sequencing studies have identified marked intra-typic heterogeneity even within G2P[4] strains, thus their unusual epidemiology in high vaccine use countries need to be reconsidered in relation to the emergence of the novel genetic and antigenic variants of G2P[4] strains (Do et al., 2014; Dennis et al., 2014; Donato et al., 2014; Giammanco et al., 2014).
With continued enhanced RVA surveillance in the post-licensure period additional new genotypes were identified in humans, most typically from sporadic cases. Sequencing of several genes or the full genomes of many of these strains often uncovered relationships with animal RVAs. Animal origin RVAs identified, for example, were the bovine/ovine origin G6, G8, and P[14] specificities (de Rougemont et al., 2011;Wu et al., 2012; Swiatek et al., 2010; Steyer et al., 2009; Cowley et al., 2013; Mullick et al., 2013), the porcine origin G5, P[6], and P[19] genotypes (Mladenova et al., 2012; Komoto et al., 2013; Hwang et al., 2012; Wu et al., 2011b), the feline/canine origin G3, P[3] and P[9] specificities (Luchs et al., 2012; Hwang et al., 2011), and more interestingly the putative equine origin G14 specificity (Weinberg et al., 2012, 2013). Phylogenetic analyses of sporadic rare strains in the respective studies were not consistent with regard to the epidemiological relationships among the spatially isolated cases. For example, human RVA strains carrying the P[3] and P[9] VP4 genotype specificities detected in different locations appear to be more similar to each other and often to the putative canine and feline hosts (Luchs et al., 2012; Hwang et al., 2011), whereas bovine/ovine-like P[14] or porcine-like P[6], P[19] and P[25] strains detected at various geographic locations appear to be more diverse genetically (Wu et al., 2011a,b; Mladenova et al., 2012). So far, very few strains have been characterized in sufficient detail; therefore one cannot make conclusions about possible differences in the driving forces of these specificities in their respective host species. Nonetheless, all these findings raise the possibility that rare RVA genotypes detected in humans originate from local gene pools harbored by one or multiple animal host species with humans merely a heterologous dead end host for these rare strains, whereas other strains might have some limited potential for human-to-human transmissibility and thus, are able more readily to disperse geographically.
Whereas the mechanisms and the potential implications of possible selection of heterotypic strains by vaccine pressure, including those that are currently considered uncommon remain to be understood, evidence has been published that vaccine strains may exchange genetic material with wild type strains (Bucardo et al., 2012; Rose et al., 2013). Such mechanisms were identified for both vaccines indicating that either single-gene or multi-gene reassortment could lead to new strains potentially pathogenic to infants. Although mechanisms of attenuation and reversion still await exploration, the detection of vaccine strain derived genes in virulent reassortant wild-type strains during the post licensure surveillance period has implications for routine surveillance methods. The laboratory protocol recommended for strain monitoring relies on amplified fragment length polymorphism driven by genotype specific primers (WHO, 2009b). However, currently used primer cocktails do not contain some of the genotype specificities expressed by RV5. Furthermore, this approach cannot be used to differentiate vaccine and wild-type strains. Alternative methods may be needed, particularly because typing primers may cross react with heterotypic strains and emergence of new sequence variants may lead to reduced reactivity with the homotypic primer. Furthermore, the number of individual typing primers in a cocktail is another limiting factor. One possible alternative to address these issues is to use unbiased massively parallel sequencing of whole genomes to obtain new insight into mechanisms of evolution within and between field RVA strains and vaccine strains.
In conclusion, the post-vaccine introduction strain prevalence data in countries using either RV1 or RV5 do not show any consistent pattern indicative of selection pressure resulting from vaccine use. The six genotype combinations responsible for a majority of infections in most of the world remained medically important during 2007–2012. Only a few putative novel candidates for possible new pandemics were identified, including some rapidly spreading genotypes, such as G6P[6] and G9P[4], in Africa and Latin America, respectively, whose genetically closely related counterparts have been identified in several continents. The question of whether strain evolution over the long term will result in declining vaccine effectiveness to particular types or whether escape mutants due to completely novel gene constellations will emerge is still open. The observed dominance of the fully heterotypic G2P[4] strains in some countries in some years following RV1 vaccination is unusual, however, and this issue requires further monitoring. As more post-vaccine implementation data on strain diversity become available from many low-income countries in Africa that have recently introduced either RV1 or RV5 in routine childhood immunization programs and from high and middle income countries that have been using for vaccines for several years, further evidence of vaccine selection pressure on circulating rotavirus strains should be examined.
Acknowledgments
K.B. was supported by the Hungarian Scientific Research Fund (OTKA, T100727) and the Momentum program awarded by the Hungarian Academy of Sciences. B.L. was supported by the ‘Erdős Pál’ scholarship.
References
- Abdel-Haq N, Amjad M, McGrath E, Chearskul P, Amer A, Salimnia H, Asmar BI, 2011. Emergence of human rotavirus genotype G9 in metropolitan Detroit between 2007 and 2009. J. Med. Microbiol 60, 761–767. [DOI] [PubMed] [Google Scholar]
- Abebe A, Teka T, Kassa T, Seheri M, Beyene B, Teshome B, Kebede F, Habtamu A, Maake L, Kassahun A, Getahun M, Mitiku K, Mwenda JM, 2014. Hospital-based surveillance for rotavirus gastroenteritis in children younger than 5 years of age in Ethiopia: 2007–2012. Pediatr. Infect. Dis. J 33 (Suppl. 1), S28–S33. [DOI] [PubMed] [Google Scholar]
- Abugalia M, Cuevas L, Kirby A, Dove W, Nakagomi O, Nakagomi T, Kara M, Gweder R, Smeo M, Cunliffe N, 2011. Clinical features and molecular epidemiology of rotavirus and norovirus infections in Libyan children. J. Med. Virol 83, 1849–1856. [DOI] [PubMed] [Google Scholar]
- Afrad MH, Hassan Z, Farjana S, Moni S, Barua S, Das SK, Faruque ASG, Azim T, Rahman M, 2013. Changing profile of rotavirus genotypes in Bangladesh, 2006–2012. BMC Infect. Dis 13, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Klena J, Albana A, Alhamdani F, Oskoff J, Soliman M, Heylen E, Teleb N, Husain T, Matthijnssens J, 2013. Characterization of human rotaviruses circulating in Iraq in 2008: atypical G8 and high prevalence of P[6] strains. Infect. Genet. Evol 16, 212–217. [DOI] [PubMed] [Google Scholar]
- Akoua-Koffi C, Asse Kouadio V, Yao Atteby JJ, 2014. Hospital-based surveillance of rotavirus gastroenteritis among children under 5 years of age in the Republic of Ivory Coast: a cross-sectional study. BMJ Open 4, e003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Awaidy SA, Bawikar S, Al Busaidy S, Baqiani S, Al Abedani I, Varghese R, Abdoan HS, Al Abdoon H, Bhatnagar S, Al Hasini KS, Mohan P, Shah S, Elamir E, Klena J, Ahmed SF, Teleb N, Parashar U, Patel MM, 2009. Considerations for introduction of a rotavirus vaccine in Oman: rotavirus disease and economic burden. J. Infect. Dis 200 (Suppl. 1), S248–S253. [DOI] [PubMed] [Google Scholar]
- Alam MM, Khurshid A, Shaukat S, Suleman RM, Sharif S, Angez M, Malik SA, Ahmed TM, Aamir UB, Naeem M, Zaidi SSZ, 2013. Epidemiology and genetic diversity of rotavirus strains in children with acute gastroenteritis in Lahore, Pakistan. PLoS One 8, e67998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri AA, Leite JP, Nakagomi O, Kaga E, Woods PA, Glass RI, Gentsch JR, 1996. Characterization of human rotavirus genotype P[8]G5 from Brazil by probe-hybridization and sequence. Arch. Virol 141, 2353–2364. [DOI] [PubMed] [Google Scholar]
- Ansari S, Sherchand JB, Rijal BP, Parajuli K, Mishra SK, Dahal RK, Shrestha S, Tandukar S, Chaudhary R, Kattel HP, Basnet A, Pokhrel BM, 2013. Characterization of rotavirus causing acute diarrhoea in children in Kathmandu, Nepal, showing the dominance of serotype G12. J. Med. Microbiol 62, 114–120. [DOI] [PubMed] [Google Scholar]
- Antunes H, Afonso A, Iturriza M, Martinho I, Ribeiro C, Rocha S, Magalhães C, Carvalho L, Branca F, Gray J, 2009. G2P[4] the most prevalent rotavirus genotype in 2007 winter season in an European non-vaccinated population. J. Clin. Virol 45, 76–78. [DOI] [PubMed] [Google Scholar]
- Arista S, Giammanco GM, De Grazia S, Ramirez S, Lo Biundo C, Colomba C,Cascio A, Martella V, 2006. Heterogeneity and temporal dynamics of evolution of G1 human rotaviruses in a settled population. J. Virol 80, 10724–10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis AS, Valle DA, Antunes GR, Tibiriça SH, Assis RM, Leite JP, Carvalho IP, Rosa e Silva ML, 2013. Rotavirus epidemiology before and after vaccine introduction. J. Pediatr 89, 470–476. [DOI] [PubMed] [Google Scholar]
- Bányai K, Gentsch JR, Martella V, Bogdán Á, Havasi V, Kisfali P, Szabó A, Mihály I, Molnár P, Melegh B, Szqcs G, 2009a. Trends in the epidemiology of human G1P[8] rotaviruses: a Hungarian study. J. Infect. Dis 200, S222–S227. [DOI] [PubMed] [Google Scholar]
- Bányai K, Esona MD, Mijatovic S, Kerin TK, Pedreira C, Mercado J, Balmaseda A, Perez MC, Patel MM, Gentsch JR, 2009b. Zoonotic bovine rotavirus strain in a diarrheic child, Nicaragua. J. Clin. Virol 46, 391–393. [DOI] [PubMed] [Google Scholar]
- Bányai K, László B, Duque J, Steele AD, Nelson EA, Gentsch JR, Parashar UD,2012. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine 30 (Suppl. 1), A122–A130. [DOI] [PubMed] [Google Scholar]
- Becker-Dreps S, Paniagua M, Zambrana LE, Bucardo F, Hudgens MG, Weber DJ, Morgan DR, Espinoza F, 2011. Rotavirus prevalence in the primary care setting in Nicaragua after universal infant rotavirus immunization. Am. J. Trop. Med. Hyg 85, 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hadj Fredj M, Zeller M, Fodha I, Heylen E, Chouikha A, Van Ranst M, Matthijnssens J, Trabelsi A, 2012. Molecular characterization of the NSP4 gene of human group A rotavirus strains circulating in Tunisia from 2006 to 2008. Infect. Genet. Evol 12, 997–1004. [DOI] [PubMed] [Google Scholar]
- Benhafid M, Elomari N, Elqazoui M, Meryem AI, Rguig A, Filali-maltouf A, Elaouad R, 2013. Diversity of rotavirus strains circulating in children under 5 years of age admitted to hospital for acute gastroenteritis in Morocco, June 2006 to May 2009. J. Med. Virol, 354–362. [DOI] [PubMed] [Google Scholar]
- Boom JA, Sahni LC, Payne DC, Gautam R, Lyde F, Mijatovic-Rustempasic S, Bowen MD, Tate JE, Rench MA, Gentsch JR, Parashar UD, Baker CJ, 2012. Symptomatic infection and detection of vaccine and vaccine-reassortant rotavirus strains in 5 children: a case series. J. Infect. Dis 206, 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR, Patel MM, Baker CJ, Parashar UD, 2010. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 125, e199–e207. [DOI] [PubMed] [Google Scholar]
- Borges AMT, Dias e Souza M, Fiaccadori FS, Cardoso D, 2011. Monitoring the circulation of rotavirus among children after the introduction of the RotarixTM vaccine in Goiânia, Brazil. Mem. Inst. Oswaldo Cruz 106, 499–501. [DOI] [PubMed] [Google Scholar]
- Bowen MD, Payne DC, 2012. Rotavirus vaccine-derived shedding and viral reassortants. Exp. Rev. Vacc 11, 1311–1314. [DOI] [PubMed] [Google Scholar]
- Braeckman T, Van Herck K, Meyer N, Pirçon JY, Soriano-Gabarró M, Heylen E, Zeller M, Azou M, Capiau H, De Koster J, Maernoudt AS, Raes M, Verdonck L, Verghote M, Vergison A, Matthijnssens J, Van Ranst M, Van Damme P, RotaBel Study Group, 2012. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ 345, e4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, Hille D, Ciarlet M, Neuzil KM, 2012. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine 30 (Suppl. 1), A24–A29. [DOI] [PubMed] [Google Scholar]
- Bucardo F, Rippinger CM, Svensson L, Patton JT, 2012. Vaccine-derived NSP2 segment in rotaviruses from vaccinated children with gastroenteritis in Nicaragua. Infect. Genet. Evol 12, 1282–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillère N, Vilain P, Brottet E, Kaplon J, Ambert-Balay K, Polycarpe D, Filleul L,2013. A major outbreak of gastroenteritis in Réunion Island in 2012: first identification of G12 rotavirus on the Island. Euro. Surveill 18, 20476. [PubMed] [Google Scholar]
- Cardemil CV, Cortese MM, Medina-Marino A, Jasuja S, Desai R, Leung J, Rodriguez-Hart C, Villarruel G, Howland J, Quaye O, Tam KI, Bowen MD, Parashar UD, Gerber SI, Rotavirus Investigation Team, 2012. Two rotavirus outbreaks caused by genotype G2P[4] at large retirement communities: cohort studies. Ann. Intern. Med 157, 621–631. [DOI] [PubMed] [Google Scholar]
- Carvalho-Costa FA, Araújo IT, Santos de Assis RM, Fialho AM, de Assis Martins CM, Bóia MN, Leite JP, 2009. Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerg. Infect. Dis 15, 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman O, Collins PJ, Lennon G, Cryan B, Martella V, Fanning S, Staines A, O’Shea H, 2012. Molecular characterization of group A rotaviruses detected in children with gastroenteritis in Ireland in 2006–2009. Epidemiol. Infect 140, 247–259. [DOI] [PubMed] [Google Scholar]
- Castello AA, Arvay ML, Glass RI, Gentsch J, 2004. Rotavirus strain surveillance in Latin America: a review of the last nine years. Pediatr. Infect. Dis. J 23 (Suppl. 10), S168–S172. [DOI] [PubMed] [Google Scholar]
- Chaimongkol N, Khamrin P, Malasao R, Thongprachum A, Ushijima H, Maneekarn N, 2012. Genotypic linkages of gene segments of rotaviruses circulating in pediatric patients with acute gastroenteritis in Thailand. Infect. Genet. Evol 12, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Chan-it W, Thongprachum A, Dey SK, Phan TG, Khamrin P, Okitsu S, Nishimura S, Kobayashi M, Kikuta H, Baba T, Yamamoto A, Sugita K, Hashira S, Tajima T, Ishida S, Mizuguchi M, Ushijima H, 2011. Detection and genetic characterization of rotavirus infections in non-hospitalized children with acute gastroenteritis in Japan, 2007–2009. Infect. Genet. Evol 11, 415–422. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li Z, Han D, Cui D, Chen X, Zheng S, Yu F, Liu J, Lai S, Yan Y, Lin Z, Shi Z, Wu T, Li L, Yang W, 2013. Viral agents associated with acute diarrhea among outpatient children in southeastern China. Pediatr. Infect. Dis. J 32, e285–e290. [DOI] [PubMed] [Google Scholar]
- Chetrit E, L’homme Y, Sohal JS, Quach C, 2013. Group A rotaviruses in children with gastroenteritis in a Canadian pediatric hospital: the prevaccine era. Can. J. Infect. Dis. Med. Microbiol 24, e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieochansin T, Vutithanachot V, Theamboonlers A, Poovorawan Y, 2014. Evaluation of the rapid test for human rotavirus A in Thai children with acute gastroenteritis. Clin. Lab 60, 511–514. [DOI] [PubMed] [Google Scholar]
- Cilla G, Montes M, Gomariz M, Piñeiro L, Pérez-Trallero E, 2010. Rotavirus genotypes in children in the Basque Country (northern Spain) over a 13-year period (July 1996-June 2009). Eur. J. Clin. Microbiol. Infect. Dis 29, 955–960. [DOI] [PubMed] [Google Scholar]
- Cilla G, Montes M, Gomariz M, Alkorta M, Iturzaeta A, Perez-Yarza EG, Perez-Trallero E, 2013. Rotavirus genotypes in children in the Basque Country (North of Spain): rapid and intense emergence of the G12[P8] genotype. Epidemiol. Infect 141, 868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilli A, Luchs A, Morillo SG, Costa FF, Carmona RC, Timenetsky MC, 2011. Characterization of rotavirus and norovirus strains: a 6-year study (2004–2009). J. Pediatr 87, 445–449. [DOI] [PubMed] [Google Scholar]
- Clark HF, Lawley D, DiStefano D, Matthijnssens J, Dinubile MJ, 2011. Distribution of rotavirus genotypes causing nosocomial and community-acquired acute gastroenteritis at the Children’s Hospital of Philadelphia in the new rotavirus vaccine era. Hum. Vacc 7, 1118–1123. [DOI] [PubMed] [Google Scholar]
- Cortes J, Arvelo W, Lopez B, Reyes L, Kerin T, Gautam R, Patel M, Parashar U, Lindblade KA, 2012. Rotavirus disease burden among children <5 years of age – Santa Rosa, Guatemala, 2007–2009. Trop. Med. Int. Health 17, 254–259. [DOI] [PubMed] [Google Scholar]
- Cortese MM, Parashar UD, Centers for Disease Control and Prevention, 2009. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep 58(RR-2), 1–25. [PubMed] [Google Scholar]
- Cortese MM, Immergluck LC, Held M, Jain S, Chan T, Grizas AP, Khizer S, Barrett C, Quaye O, Mijatovic-Rustempasic S, Gautam R, Bowen MD, Moore J, Tate JE, Parashar UD, Vázquez M, 2013. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics 132, e25–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley D, Donato CM, Roczo-Farkas S, Kirkwood CD, 2013. Novel G10P[14] rotavirus strain, northern territory, Australia. Emerg. Infect. Dis 219, 1324–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe NA, Gentsch JR, Kirkwood CD, Gondwe JS, Dove W, Nakagomi O, Nakagomi T, Hoshino Y, Bresee JS, Glass RI, Molyneux ME, Hart CA, 2000. Molecular and serologic characterization of novel serotype G8 human rotavirus strains detected in Blantyre, Malawi. Virology 274, 309–320. [DOI] [PubMed] [Google Scholar]
- Cunliffe NA, Ngwira BM, Dove W, Thindwa BD, Turner AM, Broadhead RL, Molyneux ME, Hart CA, 2010. Epidemiology of rotavirus infection in children in Blantyre, Malawi, 1997–2007. J. Infect. Dis 202(Suppl.), S168–S174. [DOI] [PubMed] [Google Scholar]
- Das BK, Gentsch JR, Cicirello HG, Woods PA, Gupta A, Ramachandran M, Kumar R, Bhan MK, Glass RI, 1994. Characterization of rotavirus strains from newborns in New Delhi. Ind. J. Clin. Microbiol 32, 1820–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rougemont A, Kaplon J, Pillet S, Mory O, Gagneur A, Minoui-Tran A, Meritet JF, Mollat C, Lorrot M, Foulongne V, Gillet Y, Nguyen-Bourgain C, Alain S, Agius G, Lazrek M, Colimon R, Fontana C, Gendrel D, Pothier P, 2011. Molecular and clinical characterization of rotavirus from diarrheal infants admitted to pediatric emergency units in France. Pediatr. Infect. Dis. J 30, 118–124. [DOI] [PubMed] [Google Scholar]
- Dennehy PH, 2008. Rotavirus vaccines: an overview. Clin. Microbiol. Rev 21, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis AF, McDonald SM, Payne DC, Mijatovic-Rustempasic S, Esona MD, Edwards KM, Chappell JD, Patton JT, 2014. Molecular epidemiology of contemporary G2P[4] human rotaviruses cocirculating in a single U.S. community: footprints of a globally transitioning genotype. J. Virol 88, 3789–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey SK, Thongprachum A, Ota Y, Phan TG, Nishimura S, Mizuguchi M, Okitsu S, Ushijima H, 2009. Molecular and epidemiological trend of rotavirus infection among infants and children in Japan. Infect. Genet. Evol 9, 955–961. [DOI] [PubMed] [Google Scholar]
- Do LP, Nakagomi T, Doan YH, Kitahori Y, Nakagomi O, 2014. Molecular evolution of the VP7 gene of Japanese G2 rotaviruses before vaccine introduction. Arch. Virol 159, 315–319. [DOI] [PubMed] [Google Scholar]
- Donato CM, Ch’ng LS, Boniface KF, Crawford NW, Buttery JP, Lyon M, Bishop RF, Kirkwood CD, 2012. Identification of strains of RotaTeq rotavirus vaccine in infants with gastroenteritis following routine vaccination. J. Infect. Dis 206, 377–383. [DOI] [PubMed] [Google Scholar]
- Donato CM, Zhang ZA, Donker NC, Kirkwood CD, 2014. Characterization of G2P[4] rotavirus strains associated with increased detection in Australian states using the RotaTeq® vaccine during the 2010–2011 surveillance period. Infect. Genet. Evol doi: 0.1016/j.meegid.2014.05.020. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Dong HJ, Qian Y, Huang T, Zhu RN, Zhao LQ, Zhang Y, Li RC, Li YP, 2013. Identification of circulating porcine-human reassortant G4P[6] rotavirus from children with acute diarrhea in China by whole genome analyses. Infect. Genet. Evol 20, 155–162. [DOI] [PubMed] [Google Scholar]
- Dulgheroff AC, Figueiredo EF, Moreira LP, Moreira KC, Moura LMS, Gouvêa VS, Domingues AL, 2012. Distribution of rotavirus genotypes after vaccine introduction in the Triângulo Mineiro region of Brazil: 4-Year follow-up study. J. Clin. Virol 55, 67–71. [DOI] [PubMed] [Google Scholar]
- Dunn SJ, Greenberg HB, Ward RL, Nakagomi O, Burns JW, Vo PT, Pax KA, Das M, Gowda K, Rao CD, 1993. Serotypic and genotypic characterization of human serotype 10 rotaviruses from asymptomatic neonates. J. Clin. Microbiol 31, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enweronu-Laryea CC, Sagoe KW, Damanka S, Lartey B, Armah GE, 2013. Rotavirus genotypes associated with childhood severe acute diarrhoea in southern Ghana: a cross-sectional study. Virol. J 10, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo PW, Peralta FO, Pacheres HC, Del Valle LJ, Tapia AC, Mayra JB, Ruiz J, Del Valle Mendoza J, 2014. Diarrhoea caused by rotavirus in a regional Peruvian hospital: determination of circulating genotypes. Trans. R. Soc. Trop. Med. Hyg 108, 425–430. [DOI] [PubMed] [Google Scholar]
- Esteban LE, Rota RP, Gentsch JR, Jiang B, Esona M, Glass RI, Glikmann G, Castello AA, 2010. Molecular epidemiology of group A rotavirus in Buenos Aires, Argentina 2004–2007: reemergence of G2P[4] and emergence of G9P[8] strains. J. Med. Virol 82, 1083–1093. [DOI] [PubMed] [Google Scholar]
- Finamore E, Vitiello M, Kampanaraki A, Rao M, Galdiero M, Galdiero E, Bevilacqua P, Gallo MA, Galdiero M, 2011. G2 as an emerging rotavirus strain in pediatric gastroenteritis in southern Italy. Infection 39, 113–119. [DOI] [PubMed] [Google Scholar]
- Friesema IH, de Boer RF, Duizer E, Kortbeek LM, Notermans DW, Norbruis OF, Bezemer DD, van Heerbeek H, van Andel RN, van Enk JG, Fraaij PL, Koopmans MP, Kooistra-Smid AM, van Duynhoven YT, 2012. Etiology of acute gastroenteritis in children requiring hospitalization in the Netherlands. Eur. J. Clin. Microbiol. Infect. Dis 31, 405–415. [DOI] [PubMed] [Google Scholar]
- Gauchan P, Nakagomi T, Sherchand JB, Yokoo M, Pandey BD, Cunliffe NA, Nakagomi O, 2013. Continued circulation of G12P[6] rotaviruses over 28 months in Nepal: successive replacement of predominant strains. Trop. Med. Health 41, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch JR, Das BK, Jiang B, Bhan MK, Glass RI, 1993. Similarity of the VP4 protein of human rotavirus strain 116E to that of the bovine B223 strain. Virology 194, 424–430. [DOI] [PubMed] [Google Scholar]
- Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, Jain V, Cunliffe NA, Nakagomi O, Kirkwood CD, Fischer TK, Parashar UD, Bresee JS, Jiang B, Glass RI, 2005. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J. Infect. Dis 192 (Suppl. 1), S146–S159. [DOI] [PubMed] [Google Scholar]
- Giammanco GM, Bonura F, Zeller M, Heylen E, Van Ranst M, Martella V, Bányai K, Matthijnssens J, De Grazia S, 2014. Evolution of DS-1-like human G2P[4] rotaviruses assessed by complete genome analyses. J. Gen. Virol 95, 91–109. [DOI] [PubMed] [Google Scholar]
- Gómez MM, da Silva MF, Zeller M, Heylen E, Matthijnssens J, Ichihara MY, Rose TL, de Mello Volotão E, Leite JP, 2013. Phylogenetic analysis of G1P[6] group A rotavirus strains detected in Northeast Brazilian children fully vaccinated with RotarixTM. Infect. Genet. Evol 19, 395–402. [DOI] [PubMed] [Google Scholar]
- Gorziglia M, Hoshino Y, Buckler-White A, Blumentals I, Glass R, Flores J, Kapikian AZ, Chanock RM, 1986. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc. Natl. Acad. Sci. U.S.A 83, 7039–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V, de Castro L, Timenetsky MC, Greenberg H, Santos N, 1994. Rotavirus serotype G5 associated with diarrhea in Brazilian children. J. Clin. Microbiol 32, 1408–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi T, Bagordo F, Cavallaro A, Guido M, Malaventura C, Gabutti G, De Donno A, 2012. Sequence analysis of human rotavirus strains: comparison of clinical isolates from Northern and Southern Italy. Eur. J. Clin. Microbiol. Infect. Dis 31, 575–582. [DOI] [PubMed] [Google Scholar]
- Grimwood K, Kirkwood CD, 2008. Human rotavirus vaccines: too early for the strain to tell. Lancet 371, 1144–1145. [DOI] [PubMed] [Google Scholar]
- Gunn L, Feeney SA, Cashman O, Collins PJ, Coyle PV, Shea HO, 2012. Molecular characterization of group A rotavirus found in elderly patients in Ireland; predominance of G1P[8], continued presence of G9P[8], and emergence of G2P[4]. J. Med. Virol 84, 2008–2017. [DOI] [PubMed] [Google Scholar]
- Gurgel RG, Bohland AK, Vieira SC, Oliveira DM, Fontes PB, Barros VF, Ramos MF, Dove W, Nakagomi T, Nakagomi O, Correia JB, Cunliffe N, Cuevas LE, 2009. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology 137, 1970–1975. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Gimeno MV, Martin-Moreno JM, Díez-Domingo J, Asensi-Botet F, Hernández-Marco R, Correcher-Medina P, Sánchez-Fauquier A, 2010. Nosocomial rotavirus gastroenteritis in Spain: a multicenter prospective study. Pediatr. Infect. Dis. J 29, 23–27. [DOI] [PubMed] [Google Scholar]
- Han TH, Kim CH, Chung JY, Park SH, Hwang ES, 2010. Genetic characterization of rotavirus in children in South Korea from 2007 to 2009. Arch. Virol 155, 1663–1673. [DOI] [PubMed] [Google Scholar]
- Hassine-Zaafrane M, Sdiri-Loulizi K, Ben Salem I, Kaplon J, Ayouni S, Ambert-Balay K, Sakly N, Pothier P, Aouni M, 2011. The molecular epidemiology of circulating rotaviruses: three-year surveillance in the region of Monastir, Tunisia. BMC Infect. Dis 11, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming M, Vesikari T, 2012. Vaccine-derived human-bovine double reassortant rotavirus in infants with acute gastroenteritis. Pediatr. Infect. Dis. J 31, 992–994. [DOI] [PubMed] [Google Scholar]
- Hemming M, Vesikari T, 2014. Detection of rotateq vaccine-derived, double- reassortant rotavirus in a 7-year-old child with acute gastroenteritis. Pediatr. Infect. Dis. J 33, 655–656. [DOI] [PubMed] [Google Scholar]
- Heylen E, Zeller M, Ciarlet M, De Coster S, Van Ranst M, Matthijnssens J, 2013. Complete genetic characterization of human G2P[6] and G3P[6] rotavirus strains. Infect. Genet. Evol 13, 27–35. [DOI] [PubMed] [Google Scholar]
- Horwood PF, Luang-Suarkia D, Bebes S, Boniface K, Datta SS, Siba PM, Kirkwood CD, 2012. Surveillance and molecular characterization of group A rotaviruses in Goroka, Papua New Guinea. Am. J. Trop. Med. Hyg 87, 1145–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokororo A, Kidenya BR, Seni J, Mapaseka S, Mphahlele J, Mshana SE, in press. Predominance of rotavirus G1[P8] genotype among under-five children with gastroenteritis in Mwanza, Tanzania. J. Trop. Pediatr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, Cortese MM, Parashar UD, Glass RI, Bowen MD, 2011. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr. Infect. Dis. J 30 (Suppl. 1), S42–S47. [DOI] [PubMed] [Google Scholar]
- Hwang KP, Huang YC, Bányai K, Wu HS, Chang FY, Yang DC, Hsiung CA, Lin JS, Jiang B, Gentsch JR, Wu FT, 2011. Severe gastroenteritis associated with G3P[9] rotavirus in Taiwan. Infection 39, 271–275. [DOI] [PubMed] [Google Scholar]
- Hwang KP, Wu FT, Bányai K, Wu HS, Yang DC, Huang YC, Lin JS, Hsiung CA, Huang JC, Jiang B, Gentsch JR, 2012. Identification of porcine rotavirus-like genotype P[6] strains in Taiwanese children. J. Med. Microbiol 61, 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiro G, Delogu R, Camilloni B, Lorini C, Ruggeri FM, Fiore L, 2013. Detection of unusual G6 rotavirus strains in Italian children with diarrhoea during the 2011 surveillance season. J. Med. Virol 85, 1860–1869. [DOI] [PubMed] [Google Scholar]
- Ianiro G, Delogu R, Bonomo P, Castiglia P, Ruggeri FM, Fiore L, 2014. Molecular characterization of human G8P[4] rotavirus strains in Italy: proposal of a more complete subclassification of the G8 genotype in three major lineages. Infect. Genet. Evol 21, 129–133. [DOI] [PubMed] [Google Scholar]
- Iftikhar T, Butt A, Nawaz K, Sarwar Y, Ali A, Mustafa T, Haque A, 2012. Genotyping of rotaviruses detected in children admitted to hospital from Faisalabad region, Pakistan. J. Med. Virol 84, 2003–2007. [DOI] [PubMed] [Google Scholar]
- Iyaloo S, Mapuroma F, Seheri M, Peenze I, Kruger T, Walaza S, Cohen C, Page N, 2013. Rotavirus surveillance in South Africa, 2012. Comm. Dis. Surv. Bull 11, 37–41. [Google Scholar]
- Japhet MO, Adesina OA, Famurewa O, Svensson L, Nordgren J, 2012. Molecular epidemiology of rotavirus and norovirus in Ile-Ife, Nigeria: high prevalence of G12P[8] rotavirus strains and detection of a rare norovirus genotype. J. Med. Virol 84, 1489–1496. [DOI] [PubMed] [Google Scholar]
- Jenney A, Tikoduadua L, Buadromo E, Barnes G, Kirkwood CD, Boniface K, Bines J, Mulholland K, Russell F, 2009. The burden of hospitalised rotavirus infections in Fiji. Vaccine 27 (Suppl. 5), F108–F111. [DOI] [PubMed] [Google Scholar]
- Jeong HS, Lee KB, Jeong AY, Jo MY, Jung SY, Ahn JH, Jee Y, Kim J, Cheon DS, 2011. Genotypes of the circulating rotavirus strains in the seven prevaccine seasons from September 2000 to August 2007 in South Korea. Clin. Microbiol. Infect 17, 232–235. [DOI] [PubMed] [Google Scholar]
- Justino MC, Linhares AC, Lanzieri TM, Miranda Y, Mascarenhas JD, Abreu E, Guerra SF, Oliveira AS, da Silva VB, Sanchez N, Meyer N, Shafi F, Ortega-Barria E, Soriano-Gabarró M, Colindres RE, 2011. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belem, Brazil. Pediatr. Infect. Dis. J 30, 396–401. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Nakano T, Kamiya H, Yui A, Taniguchi K, Parashar U, Rotavirus Epidemiology Study Group, 2011. Rotavirus-associated acute gastroenteritis hospitalizations among Japanese children aged <5 years: active rotavirus surveillance in Mie Prefecture, Japan. Jpn. J. Infect. Dis 64, 482–487. [PubMed] [Google Scholar]
- Kang G, Desai R, Arora R, Chitamabar S, Naik TN, Krishnan T, Deshpande J, Gupte MD, Venkatasubramaniam S, Gentsch JR, Parashar UD, Indian Rotavirus Strain Surveillance Network, Mathew A, Anita Sr., Ramani S, Sowmynarayanan TV, Moses PD, Agarwal I, Simon A, Bose A, Arora R, Chhabra P, Fadnis P, Bhatt J, Shetty SJ, Saxena VK, Mathur M, Jadhav A, Roy S, Mukherjee A, Singh NB, 2013. Diversity of circulating rotavirus strains in children hospitalized with diarrhea in India, 2005–2009. Vaccine 31, 2879–2883. [DOI] [PubMed] [Google Scholar]
- Kaplan NM, Kirby A, Abd-Eldayem SA, Dove W, Nakagomi T, Nakagomi O, Cunliffe NA, 2011. Detection and molecular characterisation of rotavirus and norovirus infections in Jordanian children with acute gastroenteritis. Arch. Virol 156, 1477–1480. [DOI] [PubMed] [Google Scholar]
- Karamoko Y, Dabonne S, 2013. Rotavirus VP7 and VP 4 genotyping in stool samples from children with acute diarrhea in Williamsville area (Abidjan, Côte d’Ivoire). Int. J. Sci. Adv. Tech 3, 21–24. [Google Scholar]
- Kareman J, Alkafajei A, 2012. Epidemiological, clinical and laboratory features of rotavirus gastroenteritis among hospitalized children less than five years old in selected hospitals in Jordan, 2007–2008. Med. J. Basrah Univ 30, 30–39. [Google Scholar]
- Khananurak K, Vutithanachot V, Simakachorn N, Theamboonlers A, Chongsrisawat V, Poovorawan Y, 2010. Prevalence and phylogenetic analysis of rotavirus genotypes in Thailand between 2007 and 2009. Infect. Genet. Evol 10, 537–745. [DOI] [PubMed] [Google Scholar]
- Khawaja S, Cardellino A, Mast TC, 2013. Hospital-based surveillance and analysis of genotype variation in Nicaragua after the introduction of the pentavalent rotavirus vaccine. Pediatr. Infect. Dis. J 33, e25–e28. [DOI] [PubMed] [Google Scholar]
- Khoury H, Ogilvie I, El Khoury AC, Duan Y, Goetghebeur MM, 2011. Burden of rotavirus gastroenteritis in the Middle Eastern and North African pediatric population. BMC Infect. Dis 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A, Al-Eryani A, Al-Sonboli N, Hafiz T, Beyer M, Al-Aghbari N, Al-Moheri N, Dove W, Cunliffe NA, Cuevas LE, 2011. Rotavirus and norovirus infections in children in Sana’a, Yemen. Trop. Med. Int. Health 16, 680–684. [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, Cannan D, Bogdanovic-Sakran N, Bishop RF, Barnes GL, National Rotavirus Surveillance Group, 2007. Australian rotavirus surveillance program: annual report, 2006–07. Commun. Dis. Intell. Q. Rep 31, 375–379. [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, Cannan D, Boniface K, Bishop RF, Barnes GL, Australian Rotavirus Surveillance Group, 2008. Australian rotavirus surveillance program annual report, 2007/08. Commun. Dis. Intell. Q. Rep 32, 425–429. [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, Boniface K, Bishop RF, Barnes GL, Australian Rotavirus Surveillance Group, 2009. Australian rotavirus surveillance program annual report, 2008/2009. Commun. Dis. Intell. Q. Rep 33, 382–388. [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, Boniface K, Bishop RF, Barnes GL, 2010. Australian rotavirus surveillance program: annual report, 2009/2010. Commun. Dis. Intell. Q. Rep 34, 427–434. [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, Roczo S, Boniface K, Barne GL, Bishop RF, Australian Rotavirus Surveillance Group, 2011. Australian rotavirus surveillance program annual report, 2010/11. Commun. Dis. Intell. Q. Rep 35, 281–287. [DOI] [PubMed] [Google Scholar]
- Kiulia NM, Nyaga MM, Seheri ML, Wolfaardt M, van Zyl WB, Esona MD, Irimu G, Inoti M, Gatinu BW, Njenga PK, Taylor MB, Nyachieo A, 2014. Rotavirus G and P types circulating in the eastern region of Kenya: predominance of G9 and emergence of G12 genotypes. Pediatr. Infect. Dis. J 33 (Suppl. 1), S85–S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoto S, Maeno Y, Tomita M, Matsuoka T, Ohfu M, Yodoshi T, Akeda H, Taniguchi K, 2013. Whole genomic analysis of a porcine-like human G5P[6] rotavirus strain isolated from a child with diarrhoea and encephalopathy in Japan. J. Gen. Virol 94, 1568–1575. [DOI] [PubMed] [Google Scholar]
- Koukou D, Grivea I, Roma E, Tsioni H, Trimis G, Galanakis E, Farmaki E, Iosifidis E, Michos A, Siamopoulou-mavridou A, Kalmanti M, Papadopoulou H, Roilides E, Theodoridou M, Syrogiannopoulos GA, Syriopoulou V, Greek Rotascore Extension Study Group, 2011. Frequency, clinical characteristics, and genotype distribution of rotavirus gastroenteritis in Greece (2007–2008). J. Med. Virol 83, 165–169. [DOI] [PubMed] [Google Scholar]
- Kuzuya M, Fujii R, Hamano M, Kida K, Mizoguchi Y, Kanadani T, Nishimura K, Kishimoto T, 2014. Prevalence and molecular characterization of G1P[8] human rotaviruses possessing DS-1-like VP6, NSP4, and NSP5/6 in Japan. J. Med. Virol 86, 1056–1064. [DOI] [PubMed] [Google Scholar]
- Kwambana BA, Ikumapayi UN, Sallah N, Dione M, Jarju S, Panchalingham S, Jafali J, Lamin M, Betts M, Adeyemi M, Akinsola A, Bittaye O, Jasseh M, Kotloff KL, Levine MM, Nataro JP, Corrah T, Hossain MJ, Saha D, Antonio M, 2014. High genotypic diversity among rotavirus strains infecting Gambian children. Pediatr. Infect. Dis. J 33 (Suppl. 1), S69–S75. [DOI] [PubMed] [Google Scholar]
- Lacroix L, Galetto-Lacour A, Altwegg M, Egli K, Schmidt M, Gervaix A, 2010. Disease burden of rotavirus gastroenteritis in children up to 5 years of age in two Swiss cantons: paediatrician- and hospital-based surveillance. Eur. J. Pediatr 169, 319–325. [DOI] [PubMed] [Google Scholar]
- László B, Kónya J, Dandár E, Deák J, Farkas Á, Gray J, Grósz G, Iturriza-Gomara M, Jakab F, Juhász Á, Kisfali P, Kovács J, Lengyel G, Martella V, Melegh B, Mészáros J, Molnár P, Nyúl Z, Papp H, Pátri L, Puskás E, Sántha I, Schneider F, Szomor K, Tóth A, Tóth E, Szqcs G, Bányai K, 2012. Surveillance of human rotaviruses in 2007–2011, Hungary: exploring the genetic relatedness between vaccine and field strains. J. Clin. Virol 55, 140–146. [DOI] [PubMed] [Google Scholar]
- Li D, Liu N, Yu J, Zhang Q, Cui S, Zhang D, Yang S-H, Cao D, Xu Z, Duan Z, 2009. Molecular epidemiology of G9 rotavirus strains in children with diarrhoea hospitalized in Mainland China from January 2006 to December 2007. Vaccine 27 (Suppl. 5), F40–F45. [DOI] [PubMed] [Google Scholar]
- Lucero Y, Mamani N, Cortés H, Peña A, Vergara R, O’Ryan M, 2012. Genotipos de rotavirus aislados de niños chilenos con gastroenteritis atendidos en dos hospitales públicos: variantes virales circulantes en un país con uso limitado de vacunas anti-rotavirus [Rotavirus genotypes in children with gastroenteritis assisted in two public hospitals from Chile: viral strains circulating in a country without a universal vaccination against rotavirus]. Rev. Chil. Infectol 29, 142–148. [DOI] [PubMed] [Google Scholar]
- Luchs A, Cilli A, Morillo SG, Carmona RC, Timenetsky MC, 2012. Rare G3P[3] rotavirus strain detected in Brazil: possible human-canine interspecies transmission. J. Clin. Virol 54, 89–92. [DOI] [PubMed] [Google Scholar]
- Luchs A, Morillo SG, Ribeiro CD, Cilli A, Calux SJ, Carmona RC, Timenetsky MC, 2013. Rotavirus G2P[4] and G2P[4]+[6] infections during norovirus gastroenteritis outbreak: summer season 2010, Brazil. Rev. Soc. Bras. Med. Trop 46, 227–230. [DOI] [PubMed] [Google Scholar]
- Luchs A, Timenetsky MC, 2014. G8P[6] rotaviruses isolated from Amerindian children in Mato Grosso do Sul, Brazil, during 2009: close relationship of the G and P genes with those of bovine and bat strains. J. Gen. Virol 95, 627–641. [DOI] [PubMed] [Google Scholar]
- Maiklang O, Vutithanachot V, Vutithanachot C, Hacharoen P, Chieochansin T, Poovorawan Y, 2012. Prevalence of group A genotype human rotavirus among children with dirarrhea in Thailand, 2009–2011. Southeast Asian J. Trop. Med. Public Health 43, 904–916. [PubMed] [Google Scholar]
- Mandile MG, Esteban LE, Argüelles MH, Mistchenko A, Glikmann G, Castello AA, 2014. Surveillance of group A Rotavirus in Buenos Aires 2008–2011, long lasting circulation of G2P[4] strains possibly linked to massive monovalent vaccination in the region. J. Clin. Virol 60, 282–289. [DOI] [PubMed] [Google Scholar]
- Mangayarkarasi V, Prema A, Gunasekaran P, Babu BV, Kaveri K, 2012. A unique human rotavirus (non vaccine) G9P[4] genotype infection in a child with gastroenteritis. Ind. Pediatr 49, 569–571. [DOI] [PubMed] [Google Scholar]
- Martínez M, Amarilla AA, Galeano ME, Aquino VH, Fariña N, Russomando G, Parra GI, 2010. Predominance of rotavirus G2P[4] and emergence of G12P[9] strains in Asunción, Paraguay, 2006–2007. Arch. Virol 155, 525–533. [DOI] [PubMed] [Google Scholar]
- Mathew MA, Paulose A, Chitralekha S, Nair MK, Kang G, Kilgore P, 2014. Prevalence of rotavirus diarrhea among hospitalized under-five children. Ind. Pediatr 51, 27–31. [DOI] [PubMed] [Google Scholar]
- Matsushima Y, Nakajima E, Nguyen TA, Shimizu H, Kano A, Ishimaru Y, Phan TG, Ushijima H, 2012. Genome sequence of an unusual human G10P[8] rotavirus detected in Vietnam. J. Virol 86, 10236–10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Bányai K, Rahman M, Zeller M, Beutels P, Van Damme P, Van Ranst M, 2009. Rotavirus disease and vaccination: impact on genotype diversity. Fut. Microbiol 4, 1303–1316. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Heylen E, Zeller M, Rahman M, Lemey P, Van Ranst M, 2010. Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Mol. Biol. Evol 27, 2431–2436. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Nakagomi O, Kirkwood CD, Ciarlet M, Desselberger U, Van Ranst M, 2012. Group A rotavirus universal mass vaccination: how and to what extent will selective pressure influence prevalence of rotavirus genotypes? Exp. Rev Vacc 11, 1347–1354. [DOI] [PubMed] [Google Scholar]
- McDermid A, Le Saux N, Grudeski E, Bettinger JA, Manguiat K, Halperin SA, Macdonald L, Déry P, Embree J, Vaudry W, Booth TF, Members of the Canadian Immunization Monitoring Program, Active, 2012. Molecular characterization of rotavirus isolates from select Canadian pediatric hospitals. BMC Infect. Dis 12, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, McKell AO, Rippinger CM, McAllen JK, Akopov A, Kirkness EF, Payne DC, Edwards KM, Chappell JD, Patton JT, 2012. Diversity and relationships of cocirculating modern human rotaviruses revealed using large-scale comparative genomics. J. Virol 86, 9148–9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici MC, Abelli LA, Guerra P, Dodi I, Dettori G, Chezzi C, 2011. Case report: detection of rotavirus RNA in the cerebrospinal fluid of a child with rotavirus gastroenteritis and meningism. J. Med. Virol 83, 1637–1640. [DOI] [PubMed] [Google Scholar]
- Mirzayeva R, Cortese MM, Mosina L, Biellik R, Lobanov A, Chernyshova L, Lashkarashvili M, Turkov S, Iturriza-Gomara M, Gray J, Parashar UD, Steele D, Emiroglu N, Rotavirus Surveillance Network, 2009. Rotavirus burden among children in the newly independent states of the former union of soviet socialist republics: literature review and first-year results from the rotavirus surveillance network. J. Infect. Dis 200 (Suppl. 1), S203–S214. [DOI] [PubMed] [Google Scholar]
- Mladenova Z, Korsun N, Geonova T, Iturriza-Gómara M, 2010. Molecular epidemiology of rotaviruses in Bulgaria: annual shift of the predominant genotype. Eur. J. Clin. Microbiol. Infect. Dis 29, 555–562. [DOI] [PubMed] [Google Scholar]
- Mladenova Z, Papp H, Lengyel G, Kisfali P, Steyer A, Steyer AF, Esona MD, Iturriza-Gómara M, Bányai K, 2012. Detection of rare reassortant G5P[6] rotavirus, Bulgaria. Infect. Genet. Evol 12, 1676–1684. [DOI] [PubMed] [Google Scholar]
- Modaress S, Rahbarimanesh A, 2011. Human rotavirus genotypes detection among hospitalized children, a study in Tehran, Iran. Arch. Iran. Med 14, 39–45. [PubMed] [Google Scholar]
- Moyo SJ, Blomberg B, Hanevik K, Kommedal O, Vainio K, Maselle SY, Langeland N, 2014. Genetic diversity of circulating rotavirus strains in Tanzania prior to the introduction of vaccination. PLoS One 9, e97562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, Cohen D, 2010. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis-associated hospitalizations in Israel: a case-control study. Hum. Vacc 6, 450–454. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Dutta D, Ghosh S, Bagchi P, Chattopadhyay S, Nagashima S, Kobayashi N, Dutta P, Krishnan T, Naik TN, Chawla-Sarkar M, 2009. Full genomic analysis of a human group A rotavirus G9P[6] strain from Eastern India provides evidence for porcine-to-human interspecies transmission. Arch. Virol 154, 733–746. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Mullick S, Kobayashi N, Chawla-Sarkar M, 2012. The first identification of rare human group A rotavirus strain G3P[10] with severe infantile diarrhea in eastern India. Infect. Genet. Evol 12, 1933–1937. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Mullick S, Deb AK, Panda S, Chawla-Sarkar M, 2013. First report of human rotavirus G8P[4] gastroenteritis in India: evidence of ruminants-to-human zoonotic transmission. J. Med. Virol 85, 537–545. [DOI] [PubMed] [Google Scholar]
- Mullick S, Mukherjee A, Ghosh S, Pazhani GP, Sur D, Manna B, Nataro JP, Levine MM, Ramamurthy T, Chawla-Sarkar M, 2013. Genomic analysis of human rotavirus strains G6P[14] and G11P[25] isolated from Kolkata in 2009 reveals interspecies transmission and complex reassortment events. Infect. Genet. Evol 14, 15–21. [DOI] [PubMed] [Google Scholar]
- Musawi MA, Zainaldeen H, Shafi F, Anis S, Deantonio R, 2013. Rotavirus gastroenteritis in children under 5 years in the Kingdom of Bahrain: hospital-based surveillance. Clin. Epidemiol 5, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwenda JM, Ntoto KM, Abebe A, Enweronu-Laryea C, Amina I, Mchomvu J, Kisakye A, Mpabalwani EM, Pazvakavambwa I, Armah GE, Seheri LM, Kiulia NM, Page N, Widdowson MA, Steele AD, 2010. Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African Rotavirus Surveillance Network. J. Infect. Dis 202(Suppl.), S5–S11. [DOI] [PubMed] [Google Scholar]
- Mwenda JM, Tate JE, Parashar UD, Mihigo R, Agócs M, Serhan F, Nshimirimana D, 2014. African rotavirus surveillance network: a brief overview. Pediatr. Infect. Dis. J 33 (Suppl. 1), S6–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T, Cuevas LE, Gurgel RG, Elrokhsi SH, Belkhir YA, Abugalia M, Dove W, Montenegro FM, Correia JB, Nakagomi O, Cunliffe NA, Hart CA, 2008. Apparent extinction of non-G2 rotavirus strains from circulation in Recife, Brazil, after the introduction of rotavirus vaccine. Arch. Virol 153, 591–593. [DOI] [PubMed] [Google Scholar]
- Ndze VN, Papp H, Achidi EA, Gonsu KH, László B, Farkas S, Kisfali P, Melegh B, Esona MD, Bowen MD, Bányai K, Gentsch JR, Odama AM, 2013. One year survey of human rotavirus strains suggests the emergence of genotype G12 in Cameroon. J. Med. Virol 85, 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo TC, Nguyen BM, Dang DA, Nguyen HT, Nguyen TT, Tran VN, Vu TT, Ogino M, Alam MM, Nakagomi T, Nakagomi O, Yamashiro T, 2009. Molecular epidemiology of rotavirus diarrhoea among children in Haiphong, Vietnam: the emergence of G3 rotavirus. Vaccine 27 (Suppl. 5), F75–F80. [DOI] [PubMed] [Google Scholar]
- Nordgren J, Bonkoungou IJ, Nitiema LW, Sharma S, Ouermi D, Simpore J, Barro N, Svensson L, 2012a. Rotavirus in diarrheal children in rural Burkina Faso: high prevalence of genotype G6P[6]. Infect. Genet. Evol 12, 1892–1898. [DOI] [PubMed] [Google Scholar]
- Nordgren J, Nitiema LW, Sharma S, Ouermi D, Traore AS, Simpore J, Svensson L, 2012b. Emergence of unusual G6P[6] rotaviruses in children, Burkina Faso, 2009–2010. Emerg. Infect. Dis 18, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa CM, Kerntopf GF, Czernisz Eda S, Albuquerque D, Romanin P, Freitas JF, Santos N, Benati FJ, Pietruchinski E, Linhares RE, 2010. Detection and characterization of human rotavirus in hospitalized patients in the cities of Ponta Grossa, Londrina and Assai – PR, Brazil. Braz. J. Infect. Dis 14, 553–557. [DOI] [PubMed] [Google Scholar]
- Ogilvie I, Khoury H, El Khoury AC, Goetghebeur MM, 2011. Burden of rotavirus gastroenteritis in the pediatric population in Central and Eastern Europe: serotype distribution and burden of illness. Hum. Vacc 7, 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Ryan ML, Lucero Y, Prado V, Santolaya ME, Rabello M, Solis Y, Berríos D, O’Ryan-Soriano MA, Cortés H, Mamani N, 2009. Symptomatic and asymptomatic rotavirus and norovirus infections during infancy in a Chilean birth cohort. Pediatr. Infect. Dis. J 28, 879–884. [DOI] [PubMed] [Google Scholar]
- Page AL, Jusot V, Mamaty AA, Adamou L, Kaplon J, Pothier P, Djibo A, Manzo ML, Toure B, Langendorf C, Collard JM, Grais RF, 2014. Rotavirus surveillance in urban and rural areas of Niger, April 2010–March 2012. Emerg. Infect. Dis 20, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar UD, Alexander JP, Glass RI, Advisory Committee on Immunization Practices, 2006. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep 55(RR-12), 1–13. [PubMed] [Google Scholar]
- Park S, Oh S, Lee J, Park G, Choi S, Chae Y, Kim H, 2011. Genotypes of rotavirus associated with acute gastroenteritis in Seoul, Korea. Microbiol. Immunol 55, 641–644. [DOI] [PubMed] [Google Scholar]
- Patel MM, Patzi M, Pastor D, Nina A, Roca Y, Alvarez L, Iniguez V, Rivera R, Tam KI, Quaye O, Bowen M, Parashar U, De Oliveira LH, 2013. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ 346, f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATH, 2014. Rotavirus vaccine introductions: worldwide, PATH Available from: http://sites.path.org/rotavirusvaccine/rotavirus-advocacy-and-communications-toolkit/country-introduction-maps-and-list.
- Paulke-Korinek M, Kollaritsch H, Aberle SW, Zwazl I, Schmidle-Loss B, Vécsei A, Kundi M, 2013. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine 31, 2686–2691. [DOI] [PubMed] [Google Scholar]
- Payne DC, Szilagyi PG, Staat MA, Edwards KM, Gentsch JR, Weinberg GA, Hall CB, Curns AT, Clayton H, Griffin MR, Fairbrother G, Parashar UD, 2009. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr. Infect. Dis. J 28, 948–953. [DOI] [PubMed] [Google Scholar]
- Payne DC, Edwards KM, Bowen MD, Keckley E, Peters J, Esona MD, Teel EN, Kent D, Parashar UD, Gentsch JR, 2010. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. Pediatrics 125, e438–441. [DOI] [PubMed] [Google Scholar]
- Payne DC, Boom JA, Staat MA, Edwards KM, Szilagyi PG, Klein EJ, Selvarangan R, Azimi PH, Harrison C, Moffatt M, Johnston SH, Sahni LC, Baker CJ, Rench MA, Donauer S, McNeal M, Chappell J, Weinberg GA, Tasslimi A, Tate JE, Wikswo M, Curns AT, Sulemana I, Mijatovic-Rustempasic S, Esona MD, Bowen MD, Gentsch JR, Parashar UD, 2013. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin. Infect. Dis 57, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peláez-Carvajal D, Cotes-Cantillo K, Paternina-Caicedo A, Gentsch J, de la Hoz-Restrepo F, Patel M, 2014. Characterization of rotavirus genotypes before and after the introduction of a monovalent rotavirus vaccine in Colombia. J. Med. Virol 86, 1083–1086. [DOI] [PubMed] [Google Scholar]
- Phua KB, Tee N, Tan N, Ramakrishnan G, Teoh YL, Bock H, Liu YF, 2013. A hospital-based surveillance of rotavirus gastroenteritis in children <5 years of age in Singapore. Pediatr. Infect. Dis. J 32, e426–e431. [DOI] [PubMed] [Google Scholar]
- Pietsch C, Petersen L, Patzer L, Liebert UG, 2009. Molecular characteristics of German G8P[4] rotavirus strain GER1H-09 suggest that a genotyping and subclassification update is required for G8. J. Clin. Microbiol 47, 3569–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch C, Schuster V, Liebert UG, 2011. A hospital based study on inter- and intragenotypic diversity of human rotavirus A VP4 and VP7 gene segments, Germany. J. Clin. Virol 50, 136–141. [DOI] [PubMed] [Google Scholar]
- Quaye O, McDonald S, Esona MD, Lyde FC, Mijatovic-Rustempasic S, Roy S, Banegas DJ, Quiñonez YM, Chinchilla BL, Santiago FG, Lozano HG, Rey-Benito G, de Oliveira LH, Gentsch JR, Bowen MD, 2013. Rotavirus G9P[4] in 3 countries in Latin America, 2009–2010. Emerg. Infect. Dis 19, 1332–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Alamgir AS, Saiada F, Hassan Z, Faruque AS, Cravioto A, Azim T, Rahman M, 2011. Co-circulation of G1, G2 and G9 rotaviruses in hospitalized patients in Bangladesh during 2006–2009. Hum. Vacc 7, 929–933. [DOI] [PubMed] [Google Scholar]
- Räsänen S, Lappalainen S, Halkosalo A, Salminen M, Vesikari T, 2011. Rotavirus gastroenteritis in Finnish children in 2006–2008, at the introduction of rotavirus vaccination. Scand. J. Infect. Dis 43, 58–63. [DOI] [PubMed] [Google Scholar]
- Razafindratsimandresy R, Heraud J, Ramarokoto CE, Rabemanantsoa S, Randremanana R, Andriamamonjy NS, Richard V, Reynes JM, 2013. Rotavirus genotypes in children in the community with diarrhea in Madagascar. J. Med. Virol 85, 1652–1660. [DOI] [PubMed] [Google Scholar]
- Reesu R, Bhattacharya D, Chaaithanya IK, Muruganandam N, Bharadwaj AP, Singhania M, Sugunan AP, Vijayachari P, 2013. Emergence of an unusual genotype of rotavirus in Andaman and Nicobar islands, India. Intervirology 56, 134–139. [DOI] [PubMed] [Google Scholar]
- Ribas ML, Nagashima S, Calzado A, Acosta G, Tejero Y, Cordero Y, Piedra D, Kobayashi N, 2011. Emergence of G9 as a predominant genotype of human rotaviruses in Cuba. J. Med. Virol 83, 738–744. [DOI] [PubMed] [Google Scholar]
- Rinder M, Tran AN, Bennet R, Brytting M, Cassel T, Eriksson M, Frithiof D, Gothefors L, Storsaeter J, Trollfors B, Valdimarsson S, Wennerström M, Johansen K, 2014. Burden of severe rotavirus disease leading to hospitalization assessed in a prospective cohort study in Sweden. Scand. J. Infect. Dis 46, 294–302. [DOI] [PubMed] [Google Scholar]
- Rivera R, Forney K, Castro MR, Rebolledo PA, Mamani N, Patzi M, Halkyer P, Leon JS, Iñiguez V, 2013. Rotavirus genotype distribution during the prevaccine period in Bolivia: 2007–2008. Int. J. Infect. Dis 17, e762–e767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose TL, Marques da Silva MF, Goméz MM, Resque HR, Ichihara MY, Volotão Ede M, Leite JP, 2013. Evidence of vaccine-related reassortment of rotavirus, Brazil, 2008–2010. Emerg. Infect. Dis 19, 1843–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri FM, Delogu R, Petouchoff T, Tcheremenskaia O, Petris S, De Fiore L, Study R, 2011. Molecular characterization of rotavirus strains from children with diarrhea in Italy, 2007–2009. J. Med. Virol 83, 1657–1668. [DOI] [PubMed] [Google Scholar]
- Sáfadi MA, Berezin EN, Munford V, Almeida FJ, de Moraes JC, Pinheiro CF, Racz ML, 2010. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in São Paulo, Brazil. Pediatr. Infect. Dis. J 29, 1019–1022. [DOI] [PubMed] [Google Scholar]
- Salem K, Bdour S, Zeller M, Van Ranst M, Matthijnssens J, 2011. Genotypes of rotavirus strains circulating in Amman, Jordan, in 2006/07 and their significance for the potential effectiveness of future rotavirus vaccination. Arch. Virol 156, 1543–1550. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fauquier A, González-Galán V, Arroyo S, Cabornero A, Ruiz-Burruecos A, Wilhelmi-De Cal I, 2013. Monitoring of children with acute gastroenteritis in Madrid, Spain during 2010–2011: rotavirus genotype distribution after the vaccines introduction. Enferm. Infecc. Microbiol. Clin 32, 280–284. [DOI] [PubMed] [Google Scholar]
- Sanchez-Padilla E, Grais RF, Guerin PJ, Steele AD, Burny ME, Luquero FJ, 2009. Burden of disease and circulating serotypes of rotavirus infection in sub-Saharan Africa: systematic review and meta-analysis. Lancet Infect. Dis 9, 567–576. [DOI] [PubMed] [Google Scholar]
- Santos N, Hoshino Y, 2005. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol 15, 29–56. [DOI] [PubMed] [Google Scholar]
- Shen Z, Wang G, Zhang W, Qian F, Li Y, Zhang M, Gu S, Wang M, Lin F, Hu Y, Yuan Z, Zhang J, 2013. Rotavirus infection and its genetic characterization in non-hospitalized adults with acute gastroenteritis in Shanghai, China. Arch. Virol 158, 1671–1677. [DOI] [PubMed] [Google Scholar]
- Sherchand JB, Nakagomi O, Dove W, Nakagomi T, Yokoo M, Pandey BD, Cuevas LE, Hart CA, Cunliffe NA, 2009. Molecular epidemiology of rotavirus diarrhea among children aged <5 years in Nepal: predominance of emergent G12 strains during 2 years. J. Infect. Dis 200 (Suppl. 1), S182–S187. [DOI] [PubMed] [Google Scholar]
- Sherchand JB, Tandukar S, Sherchan JB, Rayamajhi A, Gurung B, Shrestha L, Rijal B, Pokhrel BM, 2012. Hospital-based study in children with rotavirus gastroenteritis and other enteropathogens. J. Nepal Health Res. Coun 10, 130–135. [PubMed] [Google Scholar]
- Shim S, Jung Y, Le VP, Son DW, Ryoo E, Shim JO, Lim I, Kim W, 2010. Genetic variation of G4P[6] rotaviruses: evidence for novel strains circulating between the hospital and community. J. Med. Virol 82, 700–706. [DOI] [PubMed] [Google Scholar]
- Shim JO, Baek IH, Le VP, Ko EM, Seok WS, Uh Y, Kim JK, Ahn SY, Lee HS, Ryoo E, Shim SY, Song W, Lim I, Kim W, 2011. Molecular characterization of rotavirus diarrhea among children in South Korea: detection of an unusual G11 strain. Arch. Virol 156, 887–892. [DOI] [PubMed] [Google Scholar]
- Soares LS, Lobo PS, Mascarenhas JD, Neri DL, Guerra SF, de Oliveira AS, Maestri RP, Oliveira DS, de Menezes EM, Linhares AC, 2012. Identification of lineage III of G12 rotavirus strains in diarrheic children in the Northern Region of Brazil between 2008 and 2010. Arch. Virol 157, 135–139. [DOI] [PubMed] [Google Scholar]
- Soares LS, de Fátima Dos Santos Guerra S, do Socorro Lima de Oliveira A, da Silva Dos Santos F, de Fátima Costa de Menezes EM, Mascarenhas J, Linhares AC, 2014. Diversity of rotavirus strains circulating in Northern Brazil after introduction of a rotavirus vaccine: high prevalence of G3P[6] genotype. J. Med. Virol 86, 1065–1072. [DOI] [PubMed] [Google Scholar]
- Soeorg H, Tamm E, Huik K, Pauskar M, Mägi D, Pruudel K, Vainomäe L, Moosar L, Kirss K, Torm S, Närska M, Pütsepp A, Nurm H, Pruunsild K, Jänes A, Zilmer K, Lutsar I, 2012. Group A rotavirus genotypes circulating prior to implementation of a National Immunization Program in Estonia. Hum. Vacc. Immunother 8, 465–469. [DOI] [PubMed] [Google Scholar]
- Staat MA, Payne DC, Donauer S, Weinberg GA, Edwards KM, Szilagyi PG, Griffin MR, Hall CB, Curns AT, Gentsch JR, Salisbury S, Fairbrother G, Parashar UD, New Vaccine Surveillance Network (NVSN), 2011. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 128, e267–e275. [DOI] [PubMed] [Google Scholar]
- Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, Witte D, Todd S, Louw C, Kirsten M, Aspinall S, Van Doorn LJ, Bouckenooghe A, Suryakiran PV, Han HH, 2012. Human rotavirus vaccine RotarixTM provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infect. Dis 12, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer A, Bajželj M,Žnuderl K, Berce I, Drinovec B, Harlander T, Orešič N, Ravnik M, Štorman A, Trkov M, 2009. Molecular epidemiology of rotaviruses during rotavirus vaccine introduction in Slovenia [Molekularna epidemiologija rotavirusov v obdobju uvajanja rotavirusnega cepiva v Sloveniji]. Zdravniški Vestnik 78, 381–386. [Google Scholar]
- Steyer A, Sagadin M, Kolenc M, Poljšak-Prijatelj M, 2013. Whole genome sequence analysis of bovine G6P[11] rotavirus strain found in a child with gastroenteritis. Infect. Genet. Evol 13, 89–95. [DOI] [PubMed] [Google Scholar]
- Stupka JA, Carvalho P, Amarilla AA, Massana M, Parra GI, Argentinean National Surveillance Network for Diarrheas, 2009. National Rotavirus Surveillance in Argentina: high incidence of G9P[8] strains and detection of G4P[6] strains with porcine characteristics. Infect. Genet. Evol 9, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Stupka JA, Degiuseppe JI, Parra GI, Argentinean National Rotavirus Surveillance Network, 2012. Increased frequency of rotavirus G3P[8] and G12P[8] in Argentina during 2008–2009: whole-genome characterization of emerging G12P[8] strains. J. Clin. Virol 54, 162–167. [DOI] [PubMed] [Google Scholar]
- Sukumaran M, Gowda K, Maiya PP, Srinivas TP, Kumar MS, Aijaz S, Reddy RR, Padilla L, Greenberg HB, Rao CD, 1992. Exclusive asymptomatic neonatal infections by human rotavirus strains having subgroup I specificity and “long” RNA electropherotype. Arch. Virol 126, 239–251. [DOI] [PubMed] [Google Scholar]
- Swiatek DL, Palombo EA, Lee A, Coventry MJ, Britz ML, Kirkwood CD, 2010. Characterisation of G8 human rotaviruses in Australian children with gastroenteritis. Virus Res 148, 1–7. [DOI] [PubMed] [Google Scholar]
- Tamim S, Hasan F, Matthijnssens J, Sharif S, Shaukat S, Alam MM, Angez M, Suleman Rana M, Khurshid a., Zaidi SSZ, 2013. Epidemiology and phylogenetic analysis of VP7 and VP4 genes of rotaviruses circulating in Rawalpindi, Pakistan during 2010. Infect. Genet. Evol 14, 161–168. [DOI] [PubMed] [Google Scholar]
- Tapisiz A, Karahan ZC, Çiftçi E, Ince E, Doğru Ü, 2011. Changing patterns of rotavirus genotypes in Turkey. Curr. Microbiol 63, 517–522. [DOI] [PubMed] [Google Scholar]
- Téllez Castillo CJ, Montava Vilaplana R, Fernández Jiménez M, Ribes Fernández JM, Buesa Gómez J, 2010. Predominance of G9 rotavirus in Valencia and Castellón between 2005 and 2007. An. Pediatr 72, 49–54. [DOI] [PubMed] [Google Scholar]
- Than VT, Park JH, Chung IS, Kim JB, Kim W, 2013. Whole-genome sequence analysis of a Korean G11P[25] rotavirus strain identifies several porcine-human reassortant events. Arch. Virol 158, 2385–2393. [DOI] [PubMed] [Google Scholar]
- Thongprachum A, Chan-it W, Khamrin P, Okitsu S, Nishimura S, Kikuta H, Yamamoto A, Sugita K, Baba T, Mizuguchi M, Maneekarn N, Hayakawa S, Ushijima H, 2013. Reemergence of new variant G3 rotavirus in Japanese pediatric patients, 2009–2011. Infect. Genet. Evol 13, 168–174. [DOI] [PubMed] [Google Scholar]
- Todd S, Page NA, Steele DA, Peenze I, Cunliffe NA, 2010. Rotavirus strain types circulating in Africa: review of studies published during 1997–2006. J. Infect. Dis 202 (Suppl.), S34–42. [DOI] [PubMed] [Google Scholar]
- Tra My PV, Rabaa MA, Vinh H, Holmes EC, Hoang NV, Vinh NT, Phuong le T, Tham NT, Bay PV, Campbell JI, Farrar J, Baker S, 2011. The emergence of rotavirus G12 and the prevalence of enteric viruses in hospitalized pediatric diarrheal patients in southern Vietnam. Am. J. Trop. Med. Hyg 85, 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimis G, Koutsoumbari I, Kottaridi C, Palaiologou N, Assimakopoulou E, Spathis A, Lebessi E, Konstantopoulos A, Kafetzis D, Karakitsos P, Papaevangelou V, 2011. Hospital-based surveillance of rotavirus gastroenteritis in the era of limited vaccine uptake through the private sector. Vaccine 29, 7292–7295. [DOI] [PubMed] [Google Scholar]
- Usonis V, Ivaskeviciene I, Desselberger U, Rodrigo C, Pediatric ROTavirus European CommiTtee (PROTECT), 2012. The unpredictable diversity of co-circulating rotavirus types in Europe and the possible impact of universal mass vaccination programmes on rotavirus genotype incidence. Vaccine 30, 4596–4605. [DOI] [PubMed] [Google Scholar]
- Vainio K, Latipov R, Utegenova E, Kasymbekova K, Juraev R, Asilova M, Flem E, 2013. Rotavirus genotype distribution in Kyrgyzstan and Kazakhstan, 2007–2009. APMIS 121, 447–455. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM, 1996. Rotavirus infections in infants as protection against subsequent infections. N. Engl. J. Med 335, 1022–1028. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kobayashi N, Zhou X, Nagashima S, Zhu Z, Peng J, Liu M, Hu Q, Zhou D, Watanabe S, Ishino M, 2009. Phylogenetic analysis of rotaviruses with predominant G3 and emerging G9 genotypes from adults and children in Wuhan, China. J. Med. Virol 81, 382–389. [DOI] [PubMed] [Google Scholar]
- Wang YH, Zhou X, Ghosh S, Zhou DJ, Pang BB, Peng JS, Hu Q, Kobayashi N, 2011. Prevalence of human rotavirus genotypes in Wuhan, China, during 2008–2011: changing trend of predominant genotypes and emergence of strains with the P[8]b subtype of the VP4 gene. Arch. Virol 156, 2221–2231. [DOI] [PubMed] [Google Scholar]
- Wang YH, Pang BB, Zhou X, Ghosh S, Tang WF, Peng JS, Hu Q, Zhou DJ, Kobayashi N, 2013. Complex evolutionary patterns of two rare human G3P[9] rotavirus strains possessing a feline/canine-like H6 genotype on an AU-1-like genotype constellation. Infect. Genet. Evol 16, 103–112. [DOI] [PubMed] [Google Scholar]
- Ward P, Poitras E, Leblanc D, Gagnon CA, Brassard J, Houde A, 2013. Comparison of different RT-qPCR assays for the detection of human and bovine group A rotaviruses and characterization by sequences analysis of genes encoding VP4 and VP7 capsid proteins. J. Appl. Microbiol 114, 1435–1448. [DOI] [PubMed] [Google Scholar]
- Weinberg GA, Payne DC, Teel EN, Mijatovic-Rustempasic S, Bowen MD, Wikswo M, Gentsch JR, Parashar UD, 2012. First reports of human rotavirus G8P[4] gastroenteritis in the United States. J. Clin. Microbiol 50, 1118–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg GA, Teel EN, Mijatovic-Rustempasic S, Payne DC, Roy S, Foytich K, Parashar UD, Gentsch JR, Bowen MD, 2013. Detection of novel rotavirus strain by vaccine postlicensure surveillance. Emerg. Infect. Dis 19, 1321–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2009a. Meeting of the immunization strategic advisory group of experts, April 2009—conclusions and recommendations. Wkly. Epidemiol. Rec 84, 220–236. [PubMed] [Google Scholar]
- WHO, 2009. Manual of Rotavirus Detection and Characterization Methods, pp. 1–146. http://whqlibdoc.who.int/hq/2008/who_ivb_08.17_eng.pdf.
- Wu FT, Bányai K, Huang JC, Wu HS, Chang FY, Hsiung CA, Huang YC, Lin JS, Hwang KP, Jiang B, Gentsch JR, 2011a. Human infection with novel G3P[25] rotavirus strain in Taiwan. Clin. Microbiol. Infect 17, 1570–1573. [DOI] [PubMed] [Google Scholar]
- Wu FT, Bányai K, Huang JC, Wu HS, Chang FY, Yang JY, Hsiung CA, Huang YC, Lin JS, Hwang KP, Jiang B, Gentsch JR, 2011b. Diverse origin of P[19] rotaviruses in children with acute diarrhea in Taiwan: detection of novel lineages of the G3, G5, and G9 VP7 genes. J. Med. Virol 83, 1279–1287. [DOI] [PubMed] [Google Scholar]
- Wu FT, Bányai K, Wu HS, Yang DC, Lin JS, Hsiung CA, Huang YC, Hwang KP, Jiang B, Gentsch JR, 2012. Identification of a G8P[14] rotavirus isolate obtained from a Taiwanese child: evidence for a relationship with bovine rotaviruses. Jpn. J. Infect. Dis 65, 455–457. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D, Kawaguchiya M, Ghosh S, Ichikawa M, Numazaki K, Kobayashi N, 2011. Detection and full genomic analysis of G6P[9] human rotavirus in Japan. Virus Genes 43, 215–223. [DOI] [PubMed] [Google Scholar]
- Yen C, Figueroa JR, Uribe ES, Carmen-Hernández LD, Tate JE, Parashar UD, Patel MM, Richardson López-Collado V, 2011. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J. Infect. Dis 204, 783–786. [DOI] [PubMed] [Google Scholar]
- Zeller M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, Novo L, Verstappen N, Van Ranst M, Matthijnssens J, 2010. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 28, 7507–7513. [DOI] [PubMed] [Google Scholar]
- Zeller M, Heylen E, De Coster S, Van Ranst M, Matthijnssens J, 2012. Full genome characterization of a porcine-like human G9P[6] rotavirus strain isolated from an infant in Belgium. Infect. Genet. Evol 12, 1492–1500. [DOI] [PubMed] [Google Scholar]
- Zeng M, Zhang Y, Zhu Q, Wang X, Yu H, 2010. Clinical and molecular epidemiology of rotavirus in children with community-acquired and hospital-acquired diarrhea in Shanghai, China. Pediatr. Infect. Dis. J 29, 177–180. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tian L, Zhang L, Li B, Yang J, Liu Z, 2012. First report of a P[15] rotavirus isolated from human. J. Clin. Virol 55, 177–180. [DOI] [PubMed] [Google Scholar]
- Zhirakovskaya EV, Aksanova RK, Gorbunova MG, Tikunov A, Kurilshchikov AM, Sokolov SN, Netesov SV, Tikunova NV, 2012. Genetic diversity of group A rotavirus isolates found in Western Siberia in 2007–2011. Mol. Gen. Mikrobiol. Virusol 4, 22–41. [PubMed] [Google Scholar]
- Zuccotti G, Meneghin F, Dilillo D, Romanò L, Bottone R, Mantegazza C, Giacchino R, Besana R, Ricciardi G, Sterpa A, Altamura N, Andreotti M, Montrasio G, Macchi L, Pavan A, Paladini S, Zanetti A, Radaelli G, 2010. Epidemiological and clinical features of rotavirus among children younger than 5 years of age hospitalized with acute gastroenteritis in Northern Italy. BMC Infect. Dis 10, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuridah H, Kirkwood CD, Bishop RF, Yap KL, 2010. Circulating human group A rotavirus genotypes in Malaysia. J. Med. Virol 82, 707–711. [DOI] [PubMed] [Google Scholar]


