Abstract
Summary: Intravenous immunoglobulin therapy is useful against various immune system disorders and viral infections. It is generally safe, and serious adverse reactions are uncommon. We report a rare case of acute encephalopathy following intravenous immunoglobulin therapy for human herpes virus 6 infection in a child. MR imaging findings suggest that the dominant causative mechanism of acute encephalopathy is cytotoxic edema, and the findings indicate 2 primary mechanisms. Reversibility of the restriction of water diffusion (low apparent diffusion coefficient value) on diffusion-weighted MR imaging suggests intramyelinic edema in the myelin sheath, and an increase of glutamate and glutamine complex peak on MR spectroscopy suggests excitotoxic injury to the neurons and astrocytes.
Intravenous immunoglobulin therapy is useful against various immune system disorders and viral infections. Serious adverse reactions following this therapy are uncommon; aseptic meningitis is one such reaction. Our patient developed aseptic meningitis following intravenous immunoglobulin therapy, and MR imaging findings revealed that the adverse effects extended to the brain parenchyma as well as to the meninges. We report a case of acute encephalopathy associated with intravenous immunoglobulin therapy and suggest its pathological cause on the basis of diffusion-weighted MR imaging and MR spectroscopy findings.
Case Report
A 2-year-old boy was admitted with acute-onset generalized tonic convulsion. On physical examination, he was drowsy and had a fever of 39°C. Initial hematology data showed leukocytosis (22,800/mm3), and cerebrospinal fluid analysis showed normal cell count and protein content. CT of the brain revealed neither hemorrhage nor infarction. The convulsive seizure was well controlled with anticonvulsant (phenytoin) and sedative drugs (midazolam). After 3 days, his temperature returned to normal, and the anticonvulsant and sedative drug administration was stopped. Although the patient had no further signs of convulsive seizure after termination of medication, he remained drowsy.
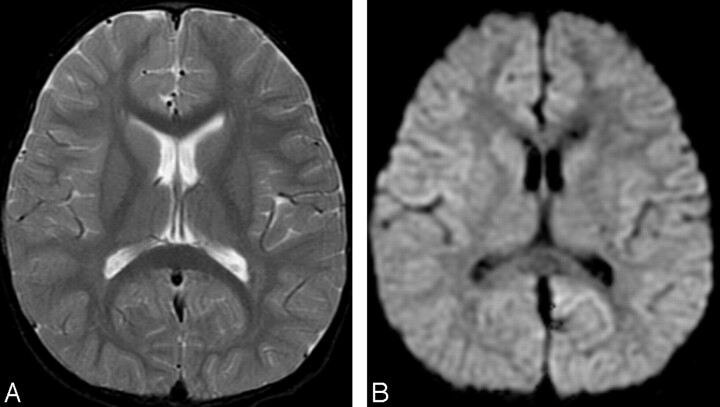
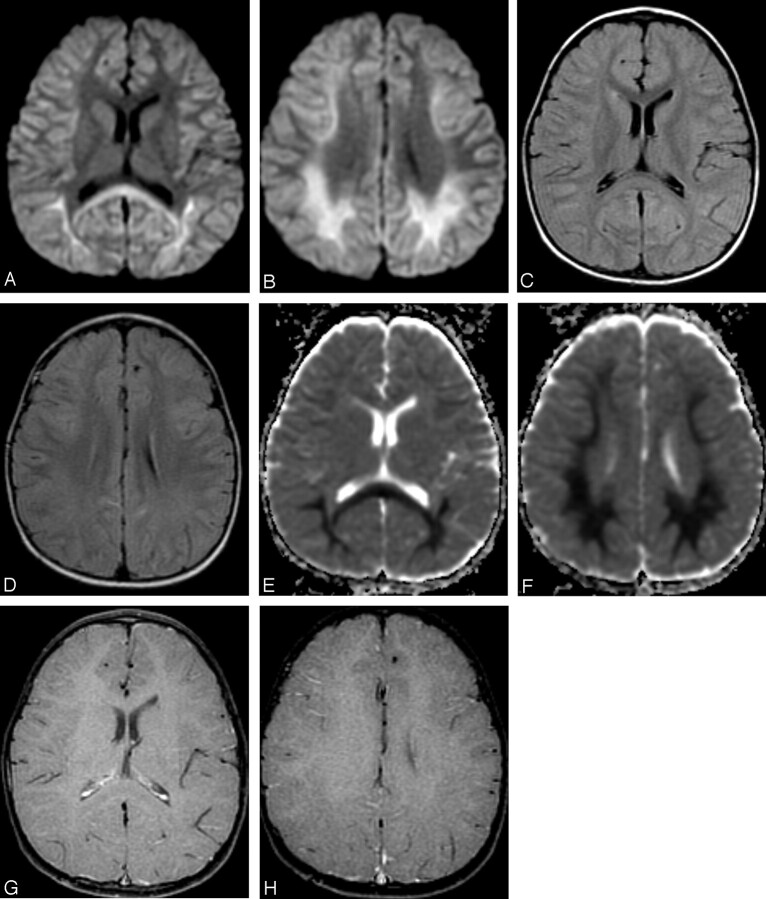
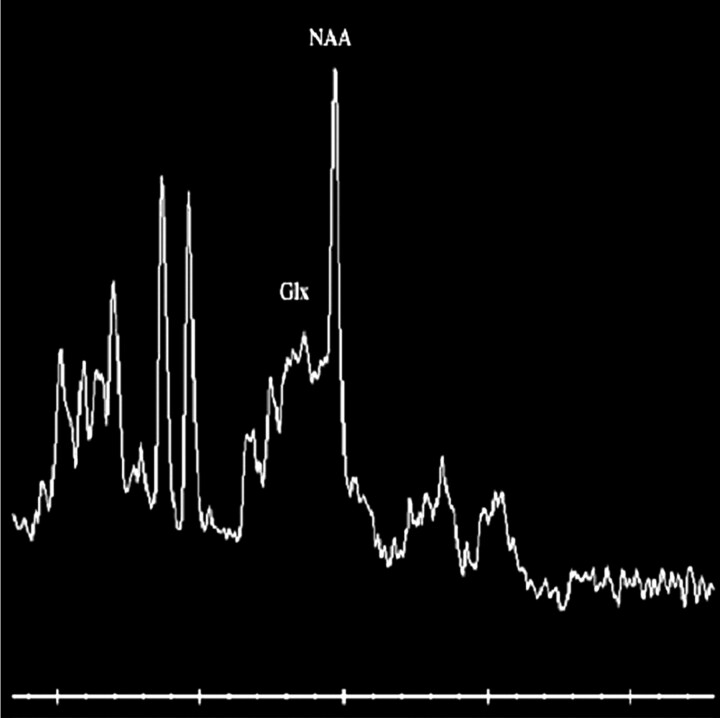
T2-weighted and diffusion-weighted MR images showed no abnormal signal intensity in the brain (Fig 1A,-B). Serum examination showed high immunoglobulin G (IgG) titer for human herpes virus 6, and electroencephalography revealed generalized slow waves in both cerebral hemispheres. Prolongation of the infectious encephalopathy condition was suspected, and high-dose (1000 mg/kg) intravenous immunoglobulin therapy was administered in the hope of improving the neurologic symptoms. The patient showed no clinical symptoms during and after infusion, but disturbed consciousness and weakness of limbs appeared later that evening. Diffusion-weighted MR images showed remarkable high signal intensity in the white matter of the bilateral parietooccipital lobes and splenium of the corpus callosum (Fig 2A,-B). Apparent diffusion coefficient (ADC) maps showed a distinct decrease in ADC value in these lesions (Fig 2C,-D). FLAIR images showed only slight high signal intensity (Fig 2E,-F), and gadolinium-enhanced T1-weighted images showed no abnormal contrast enhancement in the meninges or brain (Fig 2G, -H). MR spectroscopy focused on the right parietal white matter showed a mild decrease of the peak of n-acetylaspartate and elevation of the peak of glutamate and glutamine complex (Fig 3). The cerebrospinal fluid analysis showed pleocytosis (112/mm3) with a predominance of mononuclear cells.
Fig 1.
Initial MR images obtained 3 days after onset (before intravenous immunoglobulin therapy).
A, Axial T2-weighted image (TR/TE, 4700/102) shows no abnormal signal intensity in the brain.
B, Axial diffusion-weighted images (echo-planar imaging; TR/TE, 7000/77; b = 1000) show no apparent restriction of diffusion.
Fig 2.
MR images obtained 2 days after intravenous immunoglobulin therapy (7 days after onset).
A, -B, Axial diffusion-weighted MR images (echo-planar imaging; TR/TE, 7000/80; b 1000) show remarkable high signal intensity in the bilateral parietooccipital white matter and splenium of the corpus callosum.
C, -D, Axial FLAIR images (TR/TE/TI, 8002/127/2000) show slight high signal intensity in the bilateral deep white matter.
E, -F, Apparent diffusion coefficient (ADC) maps show distinct decrease of ADC value in the deep white matter.
G, -H, Contrast-enhanced T1-weighted images (gradient-refocused echo; TR/TE, 950/14; flip angle, 50) show no abnormal contrast enhancement in the meninges and white matter.
Fig 3.
MR spectroscopic image (point-resolved spectroscopy sequence; TR/TE. 2000/30) focused on right parietal deep white matter shows a mild decrease of the peak of n-acetylaspartate (NAA) and elevation of the peak of glutamate and glutamine complex (Glx).
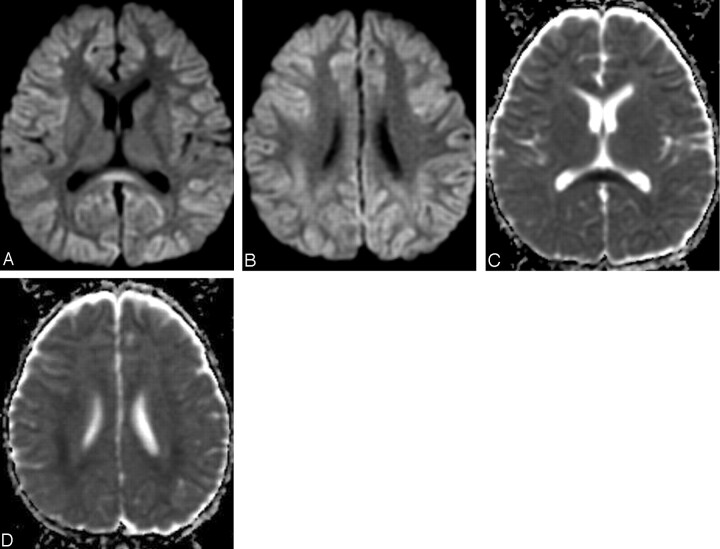
Because adverse effects from intravenous immunoglobulin therapy were suspected, intravenous immunoglobulin was abandoned and steroid pulse therapy (methylpredonisolone sodium succinates, 400 mg intravenous administrated once daily) was started. The patient’s neurologic symptoms and conscious level gradually improved. Two days after intravenous immunoglobulin treatment, the abnormally high signal intensity on diffusion-weighted MR images in the white matter and corpus callosum improved, as did the ADC value (Fig 4A–C). The returned ADC value in the parietal white matter increased gradually and reverted to normal 11 days after termination of intravenous immunoglobulin therapy (Fig. 5). Incidentally, the transient ADC value decreased in the white matter in the frontal lobe, in which diffusion-weighted MR images had previously visualized no abnormal signal intensity.
Fig 4.
MR images obtained after discontinuance of intravenous immunoglobulin (9 days after onset).
A, -B, Diffusion-weighted MR images show reduction of the high signal intensity of the white matter and the corpus callosum.
C, -D, Apparent diffusion coefficient (ADC) maps show recovery of ADC value.
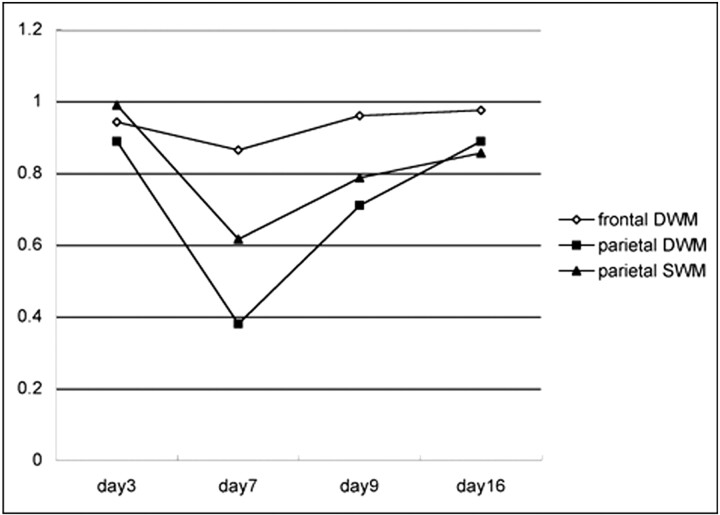
Fig 5.
Graph shows apparent diffusion coefficient (ADC) value change in the frontal and parietal white matter. Abscissa indicates days after onset. Intravenous immunoglobulin therapy was performed on day 5. ADC value decrease in parietal lobe is more distinct in the deep white matter (DWM) than in the superficial white matter (SWM). This remarkable ADC value decrease is transient and recovers to a normal value at day 16 from onset (11 days after intravenous immunoglobulin therapy). The frontal DWM in which no abnormal high signal intensity is recognized on diffusion-weighted MR images also shows transient ADC value decrease.
Discussion
Intravenous immunoglobulin therapy is the intravascular administration of immunoglobulin extracted from the blood pool of healthy human donors and was first introduced in 1952 by Bruton (1) as a treatment for patients with X-linked agammaglobulinemia. Other applicable disorders now include immune thrombocytopenia peliosis, Kawasaki disease, acute and chronic inflammation–related demyelinating polyneuropathy, primary immunodeficiency, acquired immunodeficiency syndrome, and viral infections. Low-dose (100–200 mg/kg) intravenous immunoglobulin is used as a replacement therapy for antibody deficiencies and high-dose (400–1000 mg/kg) intravenous immunoglobulin is used as an immunomodulatory agent in many immune and inflammatory disorders. In the case of infectious disease, intravenous immunoglobulin therapy helps boost the immune system, reduce the number of sick days, shorten hospital stays, and reduce antibiotic consumption and the incidence of serious infection. Adverse reaction to intravenous immunoglobulin is unusual, with the most frequently reported being headaches, fatigue, nausea, and vomiting. The incidence of these symptoms is less than 5%, with the symptoms usually occurring during drip infusion and vanishing simply by a slowing of the infusion rate.
Aseptic meningitis is one of the infrequent severe adverse effects of high-dose intravenous immunoglobulin therapy. Within 48 hours of intravenous immunoglobulin infusion, the patient complains of severe headache, fever, vomiting, nuchal rigidity, drowsiness, and photophobia. Cerebrospinal fluid findings indicate an elevation of protein without organisms. After discontinuance of intravenous immunoglobulin administration, most cases result in remission within several days without further complication. The mechanism of aseptic meningitis associated with intravenous immunoglobulin therapy is not well understood. However, several mechanisms have been proposed, such as direct irritation, cerebrovascular sensitivity, and immunologic hypersensitivity reaction to infused immunoglobulins, stabilizing agents, and released cytokines (2).
Intravenous immunoglobulin preparation is typically made from pooled human blood plasma collected from between 3000 to 10,000 healthy donors; thus it includes various antibodies. Allogenic immunologic reaction in the intravenous immunoglobulin recipient to the antibodies, especially to IgG gamma globulin, is one of the more generally accepted causes of aseptic meningitis following intravenous immunoglobulin therapy. The dynamics of IgG in the cerebrospinal fluid correspond to the course of neurologic symptoms of aseptic meningitis, and IgG is an active ingredient of intravenous immunoglobulin capable of crossing the blood-brain barrier. IgG remains in the cerebrospinal fluid for up to 48 hours after termination of intravenous immunoglobulin administration, decreasing in concentration as the neurologic symptoms of aseptic meningitis simultaneously improve (3). This evidence seems to indicate that IgG not only penetrates the meninges but also enters and affects the brain parenchyma.
Our patient was initially diagnosed as having aseptic meningitis following his intravenous immunoglobulin therapy on the basis of characteristic cerebrospinal fluid findings. However, MR imaging revealed the additional extension of adverse effects to the brain parenchyma and meninges.
Diffusion-weighted MR images and MR spectroscopy suggested that the neuropathic mechanism of the white matter lesion was cytotoxic edema, which is a result of cellular osmoregulation disturbance and may accompany various processes including toxic metabolic disease. Indications are a prominent restriction of water diffusion on diffusion-weighted MR imaging and an increase of glutamate and glutamine complex on MR spectroscopy (4). The edema occurs in glial cells, axons (axonal swelling), and myelin sheaths (intramyelinic edema) in the white matter (5). Intramyelinic edema may show partial or complete recovery with ADC value decreasing to normal. This characteristic ADC value change occurred in our patient, suggestive of the role of intramyelinic edema as a mechanism of white-matter injury following intravenous immunoglobulin therapy.
Glutamate, an important neurotransmitter in the brain, is, however, toxic when excessive and causes cytotoxic edema because of its excitotoxic effect on neurons and astrocytes.
The increase of glutamate and glutamine complex peak on MR spectroscopy in our patient suggests that the excitotoxic effect in the neurons and astrocytes is another mechanism of cytotoxic edema in acute encephalopathy following intravenous immunoglobulin therapy.
For a differential diagnosis of our patient, infarct and ischemic change, recurrence of viral encephalitis, postconvulsive encephalopathy, and encephalopathy from other drugs were proposed. However, the reversibility and distribution of MR imaging findings and the clinical course in our patient did not correspond to these other disorders.
In addition, there have been several reports regarding MR imaging findings associated with intravenous immunoglobulin therapy or convulsion. Voltz et al (6) reported on posterior reversible encephalopathy syndrome following intravenous immunoglobulin therapy and suggested that the pathologic mechanism was vasogenic edema, which has a different cause than that proposed in our patient. Lansberg et al (7) and Kim et al (8) reported a transient MR signal intensity change in general tonic convulsion or status epilepticus. An abnormal signal intensity was noted for the cortical gray matter, subcortical white matter, and the hippocampus; such signal intensity distribution did not match that in our patient. For us, the abnormal signal intensity was present in the deep white matter, and its distribution resembled that of adrenoleukodystrophy, which is a representative demyelinating disease in children. Transient MR signal intensity change at the corpus callosum associated with anticonvulsant therapy has also been reported (9, 10). Although association with anticonvulsant drugs cannot be ignored, we believe such treatment to be unrelated because the adverse symptoms appeared after termination of anticonvulsant drug administration.
In conclusion, we presented the MR imaging findings of acute but transient encephalopathy associated with intravenous immunoglobulin therapy. The MR imaging findings indicated 2 primary mechanisms of cytotoxic edema. Reversibility of restriction of water diffusion (low ADC value) on diffusion-weighted MR imaging suggests intramyelinic edema in the myelin sheath, and an increase of glutamate and glutamine complex peak on MR spectroscopy suggests excitotoxic injury in the neurons and astrocytes.
References
- 1.Bruton OC. Agammaglobulinemia. Pediatrics 1952;16:722–728 [Google Scholar]
- 2.Sekul EA, Cupler EJ, Dalakas MC. Aseptic meningitis associated with high-dose intravenous immunoglobulin therapy: frequency and risk factors. Ann Intern Med 1994;121:259–262 [DOI] [PubMed] [Google Scholar]
- 3.Cutler RW, Watters GV, Hammerstad JP. The origin and turnover rates of cerebrospinal fluid albumin and gamma-globulin in man. J Neurol Sci 1970;10:259–268 [DOI] [PubMed] [Google Scholar]
- 4.Mark LP, Prost RW, Ulmer JL, et al. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. AJNR Am J Neuroradiol 2001;22:1813–1824 [PMC free article] [PubMed] [Google Scholar]
- 5.Milhorat TH. Classification of the cerebral edemas with reference to hydrocephalus and pseudotumor cerebri. Childs Nerv Syst 1992;8:301–306 [DOI] [PubMed] [Google Scholar]
- 6.Voltz R, Rosen FV, Yousry T, Beck J, Hohlfeld R. Reversible encephalopathy with cerebral vasospasm in a patient with Guillain-Barre syndrome treated with intravenous immunoglobulin. Neurology 1996;46:250–251 [DOI] [PubMed] [Google Scholar]
- 7.Lansberg MG, O’Brien MW, Norbash AM, Moseley ME, Morrell M, Albers GW. MRI abnormalities associated with partial status epilepticus. Neurology 1999;52:1021–1027 [DOI] [PubMed] [Google Scholar]
- 8.Kim JA, Chung JI, Yoon PH, et al. Transient MR signal change in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am J Neuroradiol 2001;22:1149–1160 [PMC free article] [PubMed] [Google Scholar]
- 9.Polster T, Hoppe M, Ebner A. Transient lesion in the splenium of the corpus callosum: three further cases in epileptic patients and a pathophysiological hypothesis. J Neurol Neurosurg Psychiatry 2001;70:459–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda M, Shiroyama T, Tsukahara H, Shimono T, Aoki S, Takeda K. Transient splenial lesion of the corpus callosum associated with antiepileptic drug: evaluation by diffusion-weighted MR imaging. Eur Radiol 2003;13:1902–1906 [DOI] [PubMed] [Google Scholar]