Abstract
BACKGROUND AND PURPOSE: There is early evidence that diffusion-tensor imaging (DTI) is useful in demonstrating subtle white matter alterations in different diseases of brain. We hypothesize that DTI in several brain regions in human immunodeficiency virus–positive (HIV+) patients is useful in the early detection of HIV-related brain injury.
METHODS: MR imaging and DTI were performed in 60 HIV+ patients and in 30 controls. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC; mm/s2) maps were generated and coregistered on T2-weighted images. Regions of interest were placed in the splenium and genu of the callosum, the frontal white matter, and the hippocampus. HIV+ patients were divided into those whose CD4 count were <250 cells/mm3 or >250 cells/mm3. According to plasma viral loads, patients were divided into those whose viral loads were <50 copies/mL, 50–100,000 copies/mL, or >100,000 copies/mL.
RESULTS: Statistically significant decrease of FA was found in the genu of the corpus callosum in HIV+ patients compared with controls. FA was reduced in the frontal white matter and hippocampi in HIV+ patients compared with controls. Differences, however, were not statistically significant. No statistically significant differences were found between the HIV+ groups for FA of the splenium or between these groups and the controls. ADC values were significantly increased in the genu of HIV+ patients when compared with controls and were also increased in other locations, but without statistical significance.
CONCLUSION: As used in this study, DTI was not helpful in identifying patients with early HIV infection.
Central nervous system (CNS) involvement is an early feature of infection with the human immunodeficiency virus (HIV). The precise mechanism of the HIV-related changes in the brain is incompletely understood. CSF analysis demonstrates that HIV enters the CNS soon after exposure, even before antibodies are detectable in blood (1, 2). The neuropathologic features of AIDS dementia (HAD) include multigiant cell encephalitis (MGCE) and HIV leukoencephalopathy (3). Although MR imaging is a sensitive technique for depicting the effects of HIV in the brain, it cannot show early pathologic involvement. MR imaging features of brain HIV infection include bilateral symmetric increased T2 signal intensity in the white matter and cerebral atrophy (4). MR spectroscopy may show acutely a mild elevation of choline (probably due to an inflammatory response) and low N-acetyl-asparatate due to neuronal dysfunction (5). Unfortunately, even with multiple volume techniques, the assessment of the overall involvement of the brain is difficult due to restrictions of voxel size. Whole-brain NAA measurements may potentially be useful in this regard, but this technique is not widely available. Measurement of magnetization transfer may also help in detecting subclinical disease, but this technique has not been widely incorporated into clinical imaging (6).
Data from recent studies suggest that diffusion-tensor imaging (DTI) may be sensitive in detecting early CNS involvement of HIV (7–9). Studies demonstrate abnormal fractional anisotropy (FA) in frontal white matter and internal capsules of HIV-positive (+) patients (8). In another study, the largest decreases in FA were found in the corpus callosum and the largest elevations in mean diffusivity were seen in the subcortical white matter in patients who had advanced disease, high viral loads, and low CD4 counts (7). Also, pathologic studies have shown that hippocampal injury is common in HIV encephalitis and may play a role in the development of HAD (10–13).
The purpose of this prospective study was to determine whether DTI obtained in several brain regions in HIV+ patients with and without dementia and compared with healthy volunteers might be helpful in the detection of early HIV infection of the brain.
Methods
Subjects
During a 16-month period (2000–2002), a total of 60 HIV+ patients (48 men and 12 women; 21–64 years of age; mean age, 42 years) and 30 healthy age-matched controls (22 men and 8 women; 13–64 years of age; mean age, 40 years) were studied. Inclusion criteria were HIV seropositive on the basis of self-report and confirmed by enzyme-link immunosorbant assay and Western blot, either meeting the Centers for Disease Control and Prevention criteria for AIDS or demonstrating cognitive impairment. Patients with current or past opportunistic CNS infection, schizophrenia, severe affective disorder, or a history of chronic neurologic disorder (such as multiple sclerosis) or uncontrolled epilepsy were excluded. The patients were not receiving steroids or other medication that could have altered the measurements.
MR Examinations
All MR studies were performed on a clinical 1.5T scanner. The following sequences were obtained in controls and patients: 2-mm-thick coronal T2-weighted images (TR/TE/flip angle, 4000 ms/90 ms/90°), 4-mm-thick axial fluid-attenuated inversion recovery images (TR/TE/IR, 11000 ms/80 ms/2800 ms), and 1.25-mm-thick axial T1-weighted images (TR/TE/flip angle, 20 ms/1.76 ms/35°). A single-shot, multisection, spin-echo, echo-planar pulse sequence (TR/TE/flip angle, 1935 ms/89 ms/90°) was used for DTI. We performed diffusion-weighted imaging in 7 directions (X, Y, Z, X + Y, X + Z, Y + Z, X + Y + Z) with the maximum b value of 1000 s/mm2, field-of-view of 230 mm, 128 × 128 matrix reconstructed to 256 × 256 (acquisition time, 34 seconds). We calculated FA, which is defined as
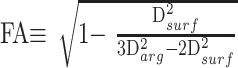 |
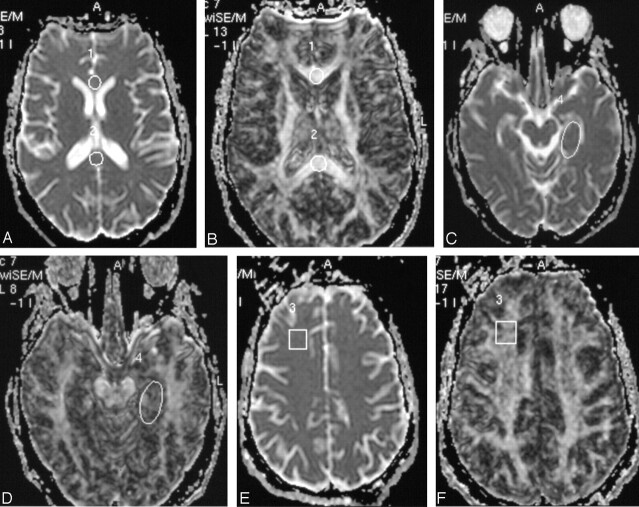
FA and apparent diffusion coefficient (ADC) maps were generated from DTI datasets and coregistered onto T2-weighted images. Round regions of interest of uniform size (100 mm2) were placed in the splenium and genu of the corpus callosum. Square regions of interest of uniform size (200 mm2) were placed in the left frontal white matter, and an oval region of interest was placed (∼250 mm2) in the region of left hippocampus (Fig 1). The differences in shape of the region of interest were chosen to provide the best fit in the structure analyzed.
Fig 1.
ADC (A) and FA (B) images in axial plane with region of interest placed in the genu and splenium of the corpus callosum.
C and D, Region of interest placed in the frontal white matter on ADC and FA.
E and F, Region of interest placed in the hippocampus.
Two experienced reviewers (M.M.T. and A.S.) who were blinded to the HIV status of the patients performed the measurements. Inter- and intraobserver variability were not measured.
Clinical Examinations
Symptomatic HIV+ involvement was confirmed by a neurologist and a neuropsychologist at baseline. The following data were obtained: clinical history (demographic data); neurologic signs (including presence of HAD), and symptoms. CD4+ T-lymphocyte counts (cells/mm3) and HIV-1 RNA level (viral load, copies/mL) in plasma were obtained.
HIV+ patients were divided into those with CD4 counts <250 cells/mm3 or >250 cells/mm3. According to plasma viral loads, patients were divided on the basis of clinical literature into those with viral loads <50 copies/mL, 50–100,000 copies/mL, or >100,000 copies/mL. Viral load <50 is considered under the detectable level, the second group represents patients with mild increase of the viral load, and the last group patients with high level of viral load.
Statistical Analyses
Statistical analysis was performed by using commercially available software (SPSS version 12; SPSS Inc., Chicago, IL). Descriptive statistical analysis included the calculation of means and SDs of the obtained data. Normality was tested by using the Kolmogorov-Smirnov test. We compared the mean FA and ADC values obtained in each of the 4 locations per individual (total of 240 measurements in HIV+) with patients grouped in different categories depending on their viral loads and CD4 cell counts. All FA and ADC measurements obtained in HIV+ patients were then compared with those of the controls. Statistical analysis was performed by using the t test. The level of statistical significance was established at P < .05.
Results
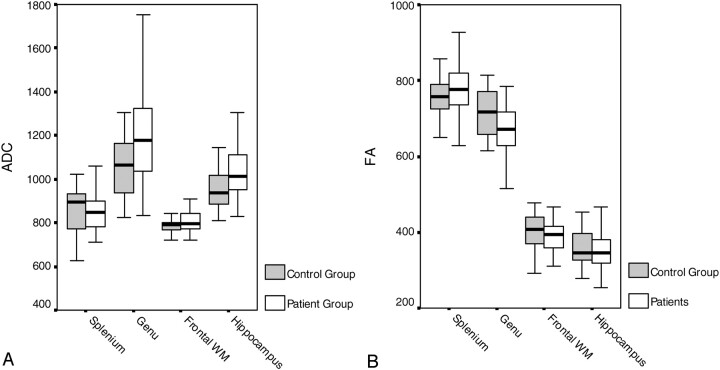
The results of the ADC and FA measurements in all 4 locations in HIV+ patients and controls are summarized in (Table 1) and the related Fig 2A, -B.
TABLE 1:
FA and ADC values in all four locations in HIV-positive patients and controls
| Brain Region | HIV+ Patients (n = 60) | Controls (n = 30) | Significance (P < .05) | |
|---|---|---|---|---|
| FA | Splenium | 774 ± 76 | 752 ± 56 | NS |
| Genu | 673 ± 75 | 717 ± 68 | .037 | |
| Frontal WM | 389 ± 45 | 404 ± 52 | NS | |
| Hippocampus | 358 ± 63 | 366 ± 65 | NS | |
| ADC | Splenium | 867 ± 111 | 859 ± 114 | NS |
| Genu | 1191 ± 216 | 1054 ± 146 | .019 | |
| Frontal WM | 809 ± 58 | 792 ± 44 | NS | |
| Hippocampus | 1043 ± 147 | 968 ± 129 | NS |
Note.—FA indicates fractional anisotropy; ADC, apparent diffusion coefficient (mm/s2); NS, not significant.
Fig 2.
A, B Graphic presentation of data found in (Table 1).
FA was statistically significantly reduced in the genu (mean 673 in HIV+ patients vs mean 717 in controls). FA was reduced in the frontal white matter (mean 389 in HIV+ patients vs mean 404 in controls), and hippocampi (mean 358 in HIV+ patients vs mean 366 in controls) in HIV+ patients compared with controls. FA was increased in splenium of the corpus callosum in HIV+ patients compared with controls. The differences were not statistically significant for splenium, white matter, or hippocampi.
ADC values were significantly higher in the genu of HIV+ patients (mean, 1191) when compared with controls (mean, 1054). ADC values were higher in the other 3 locations, but not statistically significant.
(Table 2) summarizes FA and ADC values measured in 4 locations of the brain in 3 groups of patients according to their viral load level in plasma.
TABLE 2:
FA and ADC measurements in all four locations in HIV-positive patients and controls according to the viral load in plasma
| Controls(n = 16) | HIV+ Patients: Viral Load (Copies/mL) |
||||||
|---|---|---|---|---|---|---|---|
| <50 (n = 12) | P Value | 50–100 000 (n = 19) | P Value | >100 000 (n = 22) | P Value | ||
| Splenium FA | 753 ± 56 | 770 ± 92 | 791 ± 80 | 755 ± 63 | NS | ||
| Genu FA | 717 ± 68 | 680 ± 54 | 679 ± 68 | 679 ± 93 | NS | ||
| White matter FA | 404 ± 52 | 367 ± 35 | 381 ± 39 | 402 ± 50 | NS | ||
| Hippocampus FA | 366 ± 65 | 364 ± 101 | 350 ± 54 | 357 ± 46 | NS | ||
| Splenium ADC | 858 ± 114 | 942 ± 155 | .023 | 836 ± 96 | NS | 858 ± 72 | NS |
| Genu ADC | 1054 ± 146 | 1286 ± 255 | NS | 1135 ± 192 | NS | 1159 ± 208 | NS |
| White matter ADC | 792 ± 44 | 851 ± 70 | .028 | 807 ± 42 | NS | 796 ± 57 | NS |
| Hippocampus ADC | 968 ± 129 | 1081 ± 189 | NS | 1018 ± 123 | NS | 1029 ± 122 | NS |
Note.—FA indicates fractional anisotropy; ADC, apparent diffusion coefficient (mm/s2); NS, not significant.
No correlation was found between viral load level in plasma and FA values; FA was reduced in genu, white matter, and hippocampi in all viral load groups compared with the controls, but it did not reach the level of statistical significance. Furthermore, no statistically significant differences were found in FA values between the 3 groups of patients. FA values were higher in splenium in all viral load groups compared with controls, without statistical difference.
High ADC values in genu of the corpus callosum and hippocampi of HIV+ patients compared with controls did not correlate with increased viral loads. Increased ADC was found in all viral load groups compared with controls, with statistical significance only when controls were compared with the HIV+ group with the lowest viral load (P = .028). No differences were found between HIV+ groups. The highest ADC values in splenium compared with controls were found in the lowest viral load group (P = .023).
No statistically significant correlation was found between FA and ADC values and CD4 counts (Table 3).
TABLE 3:
FA and ADC measurements in all four locations in HIV-positive patients and controls according to the CD+ count
| Controls | HIV+ Patients: CD4+ Count (cells/mm3) |
|||
|---|---|---|---|---|
| >250 | <250 | P Value | ||
| Splenium FA | 753 ± 56 | 757 ± 102 | 778 ± 63 | NS |
| Genu FA | 717 ± 68 | 678 ± 62 | 680 ± 80 | NS |
| White matter FA | 404 ± 52 | 383 ± 49 | 388 ± 43 | NS |
| Hippocampus FA | 366 ± 65 | 344 ± 57 | 362 ± 66 | NS |
| Splenium ADC | 858 ± 114 | 884 ± 159 | 863 ± 82 | NS |
| Genu ADC | 1054 ± 146 | 1173 ± 243 | 1182 ± 208 | NS |
| White matter ADC | 792 ± 44 | 816 ± 63 | 811 ± 57 | NS |
| Hippocampus ADC | 968 ± 129 | 998 ± 109 | 1055 ± 149 | NS |
Note.—FA indicates fractional anisotropy; ADC, apparent diffusion coefficient (mm/s2); NS, not significant.
Discussion
Research on the pathogenesis of HIV infection of the CNS has reached a pivotal stage. HIV commonly invades the brain soon after the peripheral infection. The virus has been found in the brain as early as 15 days after accidental intravenous inoculation (1). It is known that the neuron dysfunction or death that underlies the clinical symptoms of HIV/CNS disease does not result from direct infection of neurons. Thus, the mechanism of HIV-related brain injury remains poorly understood. It is believed that the predominant pathogenesis involves a combined influence of both HIV infection and activation of immune-competent cells and their subsequent release of toxins leading to neuron and astrocyte dysfunction (2). Neuropathologic hallmarks of HIV brain infection are MGCE and progressive diffuse leukoencephalopathy (3). The chief cellular targets of HIV infection within the CNS are the microglia/macrophages (14). After the early entry of HIV into the brain, the key question remains: what happens to the virus in the CNS? Most studies support the concept of autonomous infection, which suggests that the CNS is an independent reservoir for HIV (2). The CNS infection is persistent and self-sustaining and does not depend on subsequent spread of the virus from systemic circulation. Clinically, HIV brain infection manifests as neuropsychologic impairment that ranges from mild cognitive motor disorder to HAD (2, 15).
Although MR imaging is the most sensitive imaging technique for depicting the effects of HIV in the brain, it is nevertheless not sensitive enough to show early pathologic involvement. The characteristic MR imaging features of HIV infection in the brain include bilateral symmetric white matter disease (hyperintensities on T2-weighted MR images), as well as cerebral atrophy (4).
In the present study, we used DTI in an attempt to detect abnormalities in the brain of HIV+ patients. DTI detects diffusion-driven displacement of molecules during random motion through axonal fibers, which reflects the tissue structure and geometry found at a microscopic level. ADC values, MD and FA are specific DTI indices describing water diffusion in tissue (16–18). FA is a scalar measure of tissue structure; tissues with highly regular fibers (eg, white matter) have higher FA compared with less-organized tissues (eg, gray matter). White matter is highly organized in fiber bundles that restrict diffusion of water, and thus ADC is lower than in CSF and anisotropy is higher. The ADC of gray matter falls in between that of CSF and white matter. Recent studies show the ability of DTI to demonstrate subtle white matter alterations in patients with Alzheimer disease, migraine, chronic alcoholism, malignant brain tumors, and multiple sclerosis (19–27). Therefore, our purpose was to determine whether DTI as a relatively fast imaging technique could be used in daily clinical work in the HIV+ population. We have used parameters obtainable from DTI to try to establish a relationship between HIV+ patients on the basis of their CD4 cell counts and viral loads when compared with normal individuals. Information about the usefulness of DTI measurements in early detection of HIV-related brain injury may be important to neuroradiologists working with this particular patient population. The earlier we can detect HIV-related brain injury with imaging techniques (especially when the patient is still asymptomatic), the sooner clinicians can consider starting antiretroviral therapy. With the knowledge that CD4 count and viral load do not correlate well with clinical symptoms, and at the moment no other reliable clinical method for early detection of HIV-related brain injury exists, the search for a reliable imaging technique is, thus, still necessary.
In our study, there was a trend of FA reduction in the frontal white matter, genu of the corpus callosum, and the hippocampi of the HIV+ patients when compared with normal controls. Difference was statistically significant only for the genu of the corpus callosum. This is in contradiction to a previous report in which statistically significant differences were found between FA values in white matter and corpus callosum of HIV+ patients and controls. Significant changes in diffusion anisotropy in frontal white matter and corpus callosum and viral loads were found in a recently published study of 10 HIV+ patients (7). The greatest elevation in the diffusion coefficient was found in the subcortical white matter of the frontal lobes when compared with parieto-ocipital white matter, which suggests a predilection for frontal white matter involvement in HIV infection of the brain. This is in accordance with findings from MR spectroscopy studies of HIV disease (5, 28). A limitation of the study by Filippi et al was not only the small number of patients, but also that all patients were receiving various combinations of highly active antiretroviral therapies (HAART) (7).
Another recently published study demonstrated abnormal ADC and FA in normal-appearing periventricular white matter and corpus callosum in HIV+ patients (9). Six nondemented HIV+ patients showed a reduction in FA in frontal white matter. In that study, 5 of 6 patients received HAART. In the present study, we also found a tendency for increased ADC values in all measured brain regions; however, statistical significance differences when compared with controls were only demonstrated in the genu of the corpus callosum and are in accordance with previous studies. FA and ADC values measured in HIV+ patients did not differ much in different viral load or CD4+ count groups. Furthermore, we did not find statistically significant differences when correlation between different viral load groups and controls were performed. The control values, surprisingly, were nearly identical to the highest (>100,000 copies/mL) rather than lowest (<50 copies/mL) viral load levels. This contradicts previously published reports, though the results of the initial studies were based on much smaller patient numbers. It seems obvious that DTI values measured in our study are widespread in each group, resulting in clinically useless statistical values. Looking at those results, one cannot recommend using DTI measurements (as used in our study) in early detection of HIV-related brain injury. Correlation between DTI and viral load level in the CSF (instead of plasma) may show better results.
Because we have shown that by using DTI ADC values are abnormal but that DTI values were not significantly altered, the sensitivity of the DTI technique needs to be questioned. FA follows a biexponential model—that is, for b values <1000–3000, most of what is measured as water diffusion parallels white matter fibers (fast pool), whereas b values higher than that range measure mostly water diffusion perpendicular to the fibers (slow pool) (29, 30). It is also possible that the slow pool actually is related to intracellular water motion (in the case of white matter, intra-axonal water motion) (31, 32). Because acute HIV infection involves mainly lymphocytes and macrophages, DTI should not be a sensitive technique; more chronic HIV infection affects the white matter (loss of myelin) and the neurons (necrosis, axonal swelling). Thus, DTI should be able to detect abnormalities at least in patients with long-term infections. To detect axonal abnormalities, it seems reasonable to postulate that very high b values will be needed. Because white matter abnormalities are typical also of late infection, it is possible that FA will be altered. A trend toward lower FA was seen in the frontal white matter and hippocampi but unfortunately showed no statistical significance. Thus, it seems that, alone and in individual patients, DTI as we performed it does not reflect the underlying pathophysiologic changes. It is also plausible that the HIV-induced abnormalities do not follow mono- or biexponential functions that a model (such as q-space analysis) that takes into account multiexponential signal intensity decays will improve the performance of DTI, because it is more sensitive to diffusion restriction determination (31). q-Space analysis is based on multiexponential decay of the water signal intensity in MR diffusion experiments allowing the differentiation between physiologic compartments, on the basis of their diffusion characteristics (31). This latter technique is not available at our institution.
To the best of our knowledge, there are no published data on DTI abnormalities of the hippocampus in HIV+ patients. We have shown reduced FA and increase in the ADC values in the hippocampi of HIV+ patients compared with controls. Unfortunately, the differences between these 2 groups were not statistically significant. No patients had signal intensity abnormalities in the hippocampi on conventional MR imaging. Studies show that hippocampal injury is common in HIV encephalitis. In one study, the authors found HIV type 1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains (12). HIV infection may contribute to neuronal injury and death, and neurons may act as potential viral reservoirs. In another study, 58% of patients with HIV encephalitis had gp41-positive microglial cells in the basal neocortex and hippocampus (10). Selective damage of neurons was found in hippocampi of patients with moderate to severe HIV encephalitis (13). A form of HAD may be similar to Alzheimer disease with abnormalities found in the hippocampi. According to results from previous studies where vulnerability of hippocampal neurons in HIV has been found, reduced anisotropy in the hippocampus in HIV+ patients may be expected, and this was, at least, partially demonstrated in our study. Selective damage to hippocampal neurons in HIV infection most likely results from an indirect mechanism, mediated by factors or virus released by infected microglia. Loss of neurons secondary to activation of inflammatory processes may then manifest decreased anisotropy.
Deep gray matter also could have been used as a location for DTI measurements in HIV+ patients, and the hope still remains for future studies with more promising results.
DTI did not correlate well with virologic and immunologic parameters in our study. No differences in FA were found between the different groups with regard to CD4 T-lymphocyte count and viral load level in plasma. The largest decrease in anisotropy was in the corpus callosum of patients with advanced HIV disease and with the highest viral load level and lowest CD4 counts as were found in a previously published study (7). Recent clinical trials have shown that CD4 count and even viral load levels in plasma are not reliable markers for HIV infection and HAD. Knowing that viral load level in plasma does not correlate with viral load level in CSF, failed statistical significance between different HIV+ group and DTI values is not surprising.
We did not evaluate findings on fluid-attenuated inversion recovery and T2-weighted imaging in this study. Findings on fluid-attenuated inversion recovery and T2-weighted imaging are unspecific in HIV-related brain injury, except if the involvement is diffuse such as in HIV encephalopathy. In those cases, however, the diagnosis is obvious and would not need DTI.
Conclusion
As used in our study, DTI was not able to detect statistically significant abnormalities in HIV+ patients when compared with controls. In addition, there were no correlations between FA in several brain regions and CD4 counts and viral loads. There was, however, a trend to lower FA in the frontal white matter and hippocampi of all HIV+ patients when compared with controls. FA and ADC values were significantly different in the genu of all HIV+ patients when compared with controls. Also, there was a trend toward higher ADC values in the frontal white matter, splenium and hippocampi of all HIV+ patients compared with controls.
Footnotes
Presented in part at the 17th Symposium Neuroradiologicum, Paris, August 18–24, 2002.
References
- 1.Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 1992;42:1736–1739 [DOI] [PubMed] [Google Scholar]
- 2.Rausch DM, Davis MR. HIV in the CNS: pathogenic relationships to systemic HIV disease and other CNS disease. J Neurovirol 2001;7:85–96 [DOI] [PubMed] [Google Scholar]
- 3.Budka H, Constanzi G, Cristina S, et al. Brain pathology induced by infection with the human immunodeficiency virus (HIV). Acta Neuropathol 1987;75:185–198 [DOI] [PubMed] [Google Scholar]
- 4.Olsen WL, Longo FM, Mills CM, et al. White matter disease in AIDS: findings at MR imaging. Radiology 1988;169:445–448 [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Ernst T, Leonido-Yee M, et al. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology 1999;52:100–108 [DOI] [PubMed] [Google Scholar]
- 6.Dousset V, Armand JP, Lacoste D, et al. Magnetization transfer study of HIV encephalitis and progressive multifocal leukoencephalopathy. AJNR Am J Neuroradiol 1997;18:895–901 [PMC free article] [PubMed] [Google Scholar]
- 7.Filippi CG, Ulug AM, Ryan E, et al. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol 2001;22:277–283 [PMC free article] [PubMed] [Google Scholar]
- 8.Pomara N, Crandall DT, Choi SJ, et al. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res 2001;106:15–24 [DOI] [PubMed] [Google Scholar]
- 9.Ulug AM, Filippi CG, Ruyan E, et al. Utility of DWI tensor imaging, and MR spectroscopy in HIV patients with normal brain scans. Int Soc Magn Reson Med 2000;8 [Google Scholar]
- 10.Reyes E, Mohar A, Mallory M, et al. Hippocampal involvement associated with human immunodeficiency virus encephalitis in Mexico. Arch Pathol Lab Med 1994;118:1130–1134 [PubMed] [Google Scholar]
- 11.Spargo E, Everall IP, Lantos PL. Neuronal loss in the hippocampus in Huntington’s disease: a comparison with HIV infection. J Neurol Neurosurg Psychiatry 1993;56:487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres-Munoz J, Stockton P, Tacoronte N, et al. Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol 2001;60:885–892 [DOI] [PubMed] [Google Scholar]
- 13.Masliah E, Ge N, Achim CL, et al. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol 1992;51:585–593 [DOI] [PubMed] [Google Scholar]
- 14.Kolson DL, Lavi E, Gonzales-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res 1998;50:1–47 [DOI] [PubMed] [Google Scholar]
- 15.Wesselingh SL, Power C, Glass JD, et al. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol 1993;33:576–582 [DOI] [PubMed] [Google Scholar]
- 16.Ulug AM, Moore DF, Bojko AS, Zimmerman RD. Clinical use of diffusion-tensor imaging for disease causing neuronal and axonal damage. AJNR Am J Neuroradiol 1999;20:1044–1048 [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bihan, D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. Magn Reson Imag 2001;13:534–546 [DOI] [PubMed] [Google Scholar]
- 18.Basser PJ, Jones DK. Diffusion tensor MRI: theory, experimental design and data analysis: a technical review. NMR Biomed 2002;14:456–467 [DOI] [PubMed] [Google Scholar]
- 19.Lim KO, Helpern JA. Neuropsychiatric applications of DTI: a review. NMR Biomed 2002;15:587–593 [DOI] [PubMed] [Google Scholar]
- 20.Buchsbaum MS, Tang CY, Peled S, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport 1998;9:425–430 [DOI] [PubMed] [Google Scholar]
- 21.Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 1999;52:1626–1632 [DOI] [PubMed] [Google Scholar]
- 22.Rocca MA, Colombo B, Inglese M, et al. A diffusion tensor magnetic resonance imaging study of brain tissue from patients with migraine. J Neurol Neurosurg Psychiatry 2003;74:501–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfefferbaum A, Sullivan EV, Hedehus M, et al. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 2000;44:259–268 [DOI] [PubMed] [Google Scholar]
- 24.Lu S, Ahn D, Johnson G, Cha S. Pertumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol 2003;24:937–941 [PMC free article] [PubMed] [Google Scholar]
- 25.Wolkin A, Choi SJ, Szilagyi S, et al. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry 2003;160:572–574 [DOI] [PubMed] [Google Scholar]
- 26.Mori S, Fredriksen K, van Zijl PC, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Am Neurol 2002;51:377–380 [DOI] [PubMed] [Google Scholar]
- 27.Sinha S, Bastin ME, Whittle IR, Wardlaw JM. Diffusion tensor imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol 2002;23:520–527 [PMC free article] [PubMed] [Google Scholar]
- 28.Chang L, Itti I, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: a coregistered (1)H MRS and SPECT study. J Magn Reson Imaging 2000;12:859–865 [DOI] [PubMed] [Google Scholar]
- 29.Yoshiura T, Wu O, Zaheer A, et al. Highly diffusion-sensitized MRI of the brain: dissociation of gray and white matter. Magn Reson Med 2001;45:734–40 [DOI] [PubMed] [Google Scholar]
- 30.Mulkern RV, Gudbjartsson H, Westin CF, et al. Multi-component apparent diffusion coefficients in human brain. NMR Biomed 1999;12:51–62 [DOI] [PubMed] [Google Scholar]
- 31.Assaf Y, Cohen Y. Assignment of the water slow-diffusing component in the central nervous system using q-space diffusion MRS: implications for fiber tract imaging. Magn Reson Med 2000;43:191–199 [DOI] [PubMed] [Google Scholar]
- 32.Cohen Y, Assaf Y. High b-value q-space analysed diffusion-weighted MRS and MRI in neuronal tissues: a technical review. NMR Biomed 2002;15:516–542 [DOI] [PubMed] [Google Scholar]