Abstract
PURPOSE: To assess the concordance between data from functional MR imaging (fMRI) guidance and the intraoperative electrical cortical mapping (iCM) in targeting selective motor cortex areas in refractory neuropathic pain.
METHODS: Twenty-one patients (11 women and 10 men; mean age, 55.6 years) with refractory central (ischemic, 8 cases) and neuropathic pain (trigeminal neuropathy, 6 cases; syrinx/amputation/plexus trauma, 7 cases) underwent surgery for the implantation of an epidural electrode for chronic motor cortex stimulation (MCS) with general anesthesia and a frameless neuronavigation system used for the image-guided targeting procedure. All patients were studied by preoperative fMRI and epidural iCM with somatosensory evoked potentials and motor cortex stimulodetection. fMRI investigated systematically motor tasks of both hands and that related to the somatic area (foot or tongue) affected by pain. fMRI data were analyzed with the Statistical Parametric Mapping99 software (initial analysis threshold [AT] corresponding to P < .001), registered in the neuronavigation system and correlated intraoperatively with iCM. Matching of fMRI and iCM was specifically examined, focusing the study on hand mapping.
RESULTS: Concordance between contours of fMRI activation area and iCM in precentral gyrus (mean distance, 3.8 mm) was found in 20/21 patients (95%). Because precision of iCM was suboptimal in 7 patients, concordance for more restrictive values of the AT (P < .0001) was found in only 13 of these 20 patients. Concordance was not found in one patient, as result of image distortion and residual motion artifact.
CONCLUSIONS: In this study, fMRI guidance provides information that matches those of an independent functional method. These data illustrate the functional accuracy of fMRI guidance for the operative targeting of selective motor cortex areas in neuropathic pain.
For operating on brain lesions located in eloquent cortical areas, functional MR imaging (fMRI) guidance is today considered as a highly valuable functional targeting method for risk assessment, therapeutic decision making, and surgical planning (1–15). In functional neurosurgery for neuropathic pain, however, fMRI guidance remains mostly untested as a clinical tool (16–18). Although there is no displacement of the anatomic structures, the functional reorganization secondary to the deafferentation in the cortex may cause functional displacement (4, 19, 20). This particular issue and the functional accuracy of fMRI guidance require, therefore, to be verified and validated in this indication before its routine clinical application in image-guided surgical procedures.
The functional accuracy of fMRI guidance in targeting selective cortical areas in neuropathic pain is of particular interest because the functional targeting method currently used for neurosurgical interventions alleviating pain might be suboptimal. Indeed, the intraoperative electrical cortical mapping (iCM) of the primary sensorimotor cortex including motor bipolar stimulodetection (iBS) and somatosensory evoked potentials (iSEP) may present technical limitations, partially because of the underlying lesion of the somatosensory tracts. Diffused iBS response, iSEP wave attenuation, hypersensitivity to electrical artifacts, or impaired reproducibility of the recordings can reduce significantly the quality of the operative targeting.
From the perspective of improving the quality of the functional targeting method used for surgical interventions in neuropathic pain, we compared data from fMRI guidance with those from iCM in the surgical procedure of epidural chronic electrical stimulation of the motor cortex (MCS), particularly in the detection of the hand area. The MCS procedure was first described by Tsubokawa et al in 1991 for alleviating refractory neuropathic pain; significant and long-lasting pain relief is obtained in a portion of patients ranging from 45% to 75% (21–28). The procedure consists in positioning an epidural electrode for chronic stimulation above the contralateral cortical motor projection of the somatic segment with neuropathic pain. The best results are observed in central poststroke pain and trigeminal neuropathy (23–28). The success rate may depend on not only patient selection, but also the appropriate positioning of the electrode. Therefore, there is a need for improving targeting precision in MCS. Although presenting some limitations, as described above, iCM remains the most accurate method to localize the central sulcus (CS) and the functional motor target to stimulate (iCM-guided MCS procedure) (25, 28–31). MCS needs, however, to improve functional targeting and fMRI may play a role in this, at least for the hand mapping in MCS.
The aim of the present study was to evaluate the functional accuracy of the fMRI guidance in the operative targeting of selective cortical motor areas, for electrode positioning in neuropathic pain. We took the opportunity offered by the image-guided procedure of MCS to compare data from the fMRI technique and iCM. Indeed, the independent and complementary information provided by fMRI and iCM could be accurately compared in a clinical prospective series. In this study, because some technical and methodologic issues must be addressed before the reliable application of fMRI guidance, we performed iCM-guided MCS with image guidance by using a frameless neuronavigation system and compared intraoperatively the data obtained by both techniques. We studied the detection of not only the CS, but also the motor target to stimulate. We focused this study on hand mapping. We assessed, regardless of the postoperative clinical result, the concordance between targets obtained by both techniques as well as the respective limitations.
Methods
Patient Population
Since 1998, 21 consecutive patients—11 women and 10 men, aged 33–73 years (mean, 55.6 years)—with chronic refractory pain syndrome secondary to central (ischemic [8 cases]) and radicular or peripheral somatosensory lesions (trigeminal neuropathy [6 cases]; syrinx/amputation/plexus trauma [7 cases]) underwent surgery for the implantation of an epidural MCS device with general anesthesia and with a frameless surgery navigation system used for the image-guided targeting procedure. The underlying lesion and somatic distribution of pain are summarized in the Table. All patients gave their informed consent to the procedure and were treated according to the ethical guidelines of our institution (25, 32).
fMRI activation areas and intraoperative cortical mapping target in the precentral gyrus of 21 patients
| Pain* | Neurological Status |
Quality of iCM |
fMRI |
Comparison of iCM and fMRI |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypoesthesia Allodynia | Motricity | iCM Target Obtained† | Limitations of iSEP | Limitations of iBS | fMRI Target Obtained† | Analysis Threshold (P Values)‡ | Extent of fMRI Activation, Both Sides§ | Distance between Targets (mm)‖ | Concordance between Targets, Both Techniques¶ | |||
| 1/40/F | Face | TN | + | Normal | Hand + | Electrical artifacts | Electrical artifacts | Hand +++ | <.0001 | Similar extent | 5 | Good: fMRI helped iCM targeting |
| Face 0 | Electrical artifacts | Electrical artifacts | Face not studied | |||||||||
| 2/62/M | UL | ScS | ++ | Plegic | Hand +++ | Plegic | Hand ++ | <.0001 | Healthy side > painful side | 3 | Excellent | |
| 3/65/F | LL | SS | + | Paretic | Hand +++ | Hand +++ | <.0001 | Similar extent | 5 | Excellent | ||
| Foot ++ | Foot ++ | <.001 | Not studied | 8 | Good: fMRIhelped iCM targeting | |||||||
| 4/44/M | UL | ScS | ++ | Plegic | Hand +++ | Plegic | Hand ++ | <.0001 | Healthy side > painful side | 3 | Excellent | |
| 5/33/F | UL | ScS | + | Normal | Hand +++ | Hand +++ | <.0001 | Similar extent | 3 | Excellent | ||
| 6/66/F | UL | ScS | + | Paretic | Hand +++ | Hand +++ | <.0001 | Similar extent | 4 | Excellent | ||
| 7/34/M | UL | PA | + | Plegic | Hand + | Wave attenuation | Plegic | Hand ++ | <.0001 | Healthy side > painful side | 5 | Good: fMRI helped iCM targeting |
| 8/38/F | Face | TN | + | Normal | Hand +++ | Hand +++ | <.0001 | Similar extent | 6 | Excellent | ||
| Face 0 | No wave | No response | Face not studied | |||||||||
| 9/70/M | UL | SS | + | Paretic | Hand +++ | Hand +++ | <.0001 | Similar extent | 3 | Excellent | ||
| 10/65/F | UL | PRP | +++ | Plegic | Hand + | Wave attenuation | Plegic | Hand +++ | <.0001 | Similar extent | 3 | Good: fMRI helped iCM targeting |
| 11/50/F | Face | TN | + | Normal | Hand +++ | Hand +++ | <.0001 | Similar extent | 5 | Excellent | ||
| Face ++ | Diffused response | Face not studied | 5 | |||||||||
| 12/73/M | UL | A | +++ | None | Hand + | Wave attenuation | Amputation | Hand + | <.001 | Healthy side > painful side | 6 | Good: fMRI helped iCM targeting |
| 13/70/M | Face | TN | + | Normal | Hand +++ | Hand +++ | <.0001 | Similar extent | 3 | Excellent | ||
| Face 0 | Wave attenuation | Not studied | Face not studied | |||||||||
| 14/40/M | UL | A | +++ | None | Hand + | Wave attenuation | Amputation | Hand +++ | <.0001 | Similar extent | 3 | Good: fMRI helped iCM targeting |
| 15/54/F | LL | ScS | + | Paretic | Hand + | Wave attenuation | Hand ++ | <.0001 | Similar extent | 3 | Good: fMRI helped iCM targeting | |
| Foot 0 | No wave | Not studied | Foot ++ | <.0001 | Not studied | 5 | Good: fMRI helped iCM targeting | |||||
| 16/56/F | Face | TN | + | Normal | Hand +++ | Hand + | <.01 | Not studied | 4 | Good: low fMRI significance | ||
| Face ++ | Face not studied | |||||||||||
| 17/45/M | Face | TN | +++ | Normal | Hand +++ | Hand +++ | <.0001 | Similar extent | 3 | Excellent | ||
| Face 0 | No wave | Face ++ | <.0001 | Not studied | 5 | Good: fMRI helped iCM targeting | ||||||
| 18/66/F | UL | ScS | + | Normal | Hand +++ | Hand +++ | <.0001 | Similar extent | 1 | Excellent | ||
| Face | Face 0 | No wave | No response | Face ++ | <.001 | Not studied | ||||||
| 19/59/F | UL | ScS | ++ | Paretic | Hand +++ | Hand +++ | <.0001 | Similar extent | 3 | Excellent | ||
| Face | Face 0 | No wave | No resposne | Face 0 | <.001 | |||||||
| 20/43/M | UL | PA | ++ | Plegic | Hand + | Wave attenuation | Plegic | Hand +++ | <.0001 | Healthy side > painful side | 4 | Excellent |
| 21/72/M | UL | ScS | + | Paretic | Hand +++ | Artifacts | Hand +++ | <.0001 | Similar extent | 5 | Excellent | |
| LL | Foot + | Wave attenuation | No response | Foot ++ | <.0001 | Not studied | ||||||
Note.—iCM indicates intraoperative epidural cortical brain mapping; fMRI, functional magnetic resonance imaging; iSEP, intraoperative somatosensory-evoked potentials; iBS, intraoperative epidural motor cortex bipolar stimulodetection.
UL indicates upper limb; LL, lower limb; TN, trigeminal neuropathy; ScS, subcortical stroke; SS, spinal syrinx; PA, plexus avulsion; PRP, postradic plexopathy; A, amputation.
Quality scales for iCM and fMRI targeting are: 0, no significant target; +, fair and ambiguous target; ++, unambiguous target altered by artifacts/wave attenuation; +++, unambiguous and precise target.
P values correspond to the analysis threshold of fMRI activation areas. Values less than .001 correspond to the initial analysis threshold; some targets remained significant for values less than .00001.
Not studied indicates that comparisons between both sides were not studied because of alterations resulting from residual motion artifacts.
Distances reported between targets were measured intraoperatively by means of the neuronavigation microscope. They are purely indicative and do not reflect the resolution of fMRI and iCM.
Excellent/good indicate important/partial overlap between fMRI target or unambiguous/ambiguous iCM target (note that ambiguous iCM targets impede correlations for both targets at more restrictive P values).
Frameless Neuronavigation System for Image Guidance
We combined epidural iCM with image guidance as reported elsewhere (25, 32). We used the Zeiss-MKM microscope (Carl Zeiss, Oberkochen, Germany) as a frameless navigation system for the first 12 patients and the Treon-Stealthstation (Medtronic SNT, Louisville, CO) for the last 9. Navigation was based on MR anatomic images (Philips Intera 1.5T, Best, the Netherlands) of the brain acquired in stereotactic conditions with the skin-based markers. Axial 3D T1-weighted MR images (130 sections) were transferred into the Leibinger/Fischer STP4.0 (Freiburg, Germany) or the Treon-Stealthstation planning workstation allowing multiplanar visualization of the brain structures, especially the cortical sulci. The preoperative navigation planning procedure started with the localization (33) on anatomic MR images and registration in the 3D planning workstation of the CS and its precise shape (Fig 1). The functional target of the hand on the motor cortex strip was grossly estimated on 3D MR views.
Fig 1.
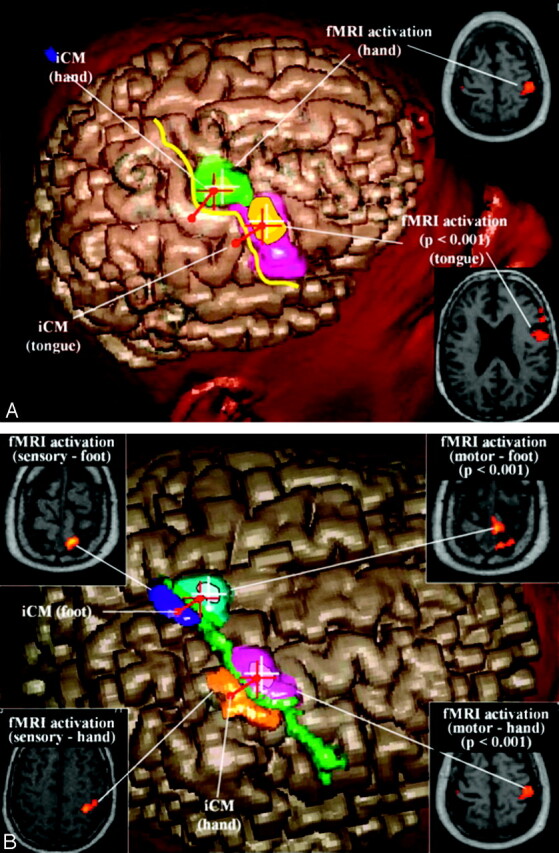
A, Virtual 3D reconstruction (cortex surfacing method) of the right hemisphere in the navigation workstation showing the integration of data from iCM and fMRI in the case of patient 8. The iCM-defined central sulcus (yellow line), the iCM-defined sensorimotor target of the hand (red diabolo), and the fMRI-activated area after motor tasks of the hand (at initial analysis threshold, green area; at more restrictive values, white cross), the fMRI-activated area after motor of the tongue (at initial analysis threshold, orange area; at more restrictive values, yellow area) projected in the portion of the precentral gyrus anatomically devoted to the face (pink area). The iCM-defined motor target of the hand (red cross) corresponds spatially with the fMRI precentral activation (green area).
B, Virtual 3D reconstruction (cortex surfacing method) of the right hemisphere in the navigation workstation showing the integration of data from iCM and fMRI in the case of patient 21. The iCM-defined central sulcus (green line), the iCM-defined sensorimotor target of the hand (red diabolo), the fMRI-activated area after motor tasks of the hand (at initial analysis threshold, violet area; at more restrictive values, white cross), and the fMRI-activated area after motor of the foot (at initial analysis threshold, azure area; at more restrictive values, white cross) projected in the portion of the parasagittal precentral convexity. The iCM-defined motor target of the hand (red cross) corresponds spatially with the fMRI precentral activation (violet area). The significant postcentral activations obtained after sensory activation paradigms of the hand (orange area) and foot (blue area) enable validation of the precentral motor activations of the same segments.
fMRI Study
In all patients, we identified the motor cortex activation by preoperative BOLD (blood oxygenation level–dependent) fMRI pulse sequences acquired within the same imaging session as the anatomic MR imaging scanning. The activation paradigm consisted of an alternation of 30-second rest periods with 30 seconds of motor task; this cycle was repeated 6 times. All patients performed a hand motor task (cyclic finger tapping). In addition, 3 patients performed a foot movement task, and a facial movement task (mouth and lips) was tested in 3 patients. During the application of the paradigm, echoplanar T2-weighted images were acquired in multisection, single-shot mode (32 axial sections were obtained every 3 seconds with TE of 40 ms, echoplanar imaging train length of 63 echoes, and voxel size of 3.15 mm × 3.15 mm × 4.8 mm; the frequency encoding was chosen in the anteroposterior direction, and axial plane imaging was used to obtain lower geometric distortion in the direction of the CS). fMRI images were realigned and coregistrated on 3D T1-weighted MR anatomic images and smoothed, and functional parametric maps were obtained by using the SPM99 software tool (Statistical Parametric Mapping, University College London, UK; 34). Sites of neuronal activation were identified by statistical analysis of the signal intensity time course thresholded at Z > 4 (P < .001; P values not corrected in the entire study). We systematically acquired data corresponding to right- and left-hand movement, not only in patients with upper limb pain (n = 13), but also in others (n = 8) as a reference target for further comparison with iCM. Activation paradigms of foot motor tasks were also studied in cases with pain in lower limbs (n = 3). In plegic (n = 5) or amputated (n = 2) patients, activation signal intensity of the painful hand could be obtained by the mental (or virtual) movement (in all 5 plegic patients; in one of the 2 amputated patients) and was thereafter compared with the activation signal intensity obtained after motor tasks on the other side (Fig 2). The contours of the precentral fMRI-activated area were then registered in the neuronavigation planning, and the center of this contour was defined as the fMRI-defined motor target.
Fig 2.
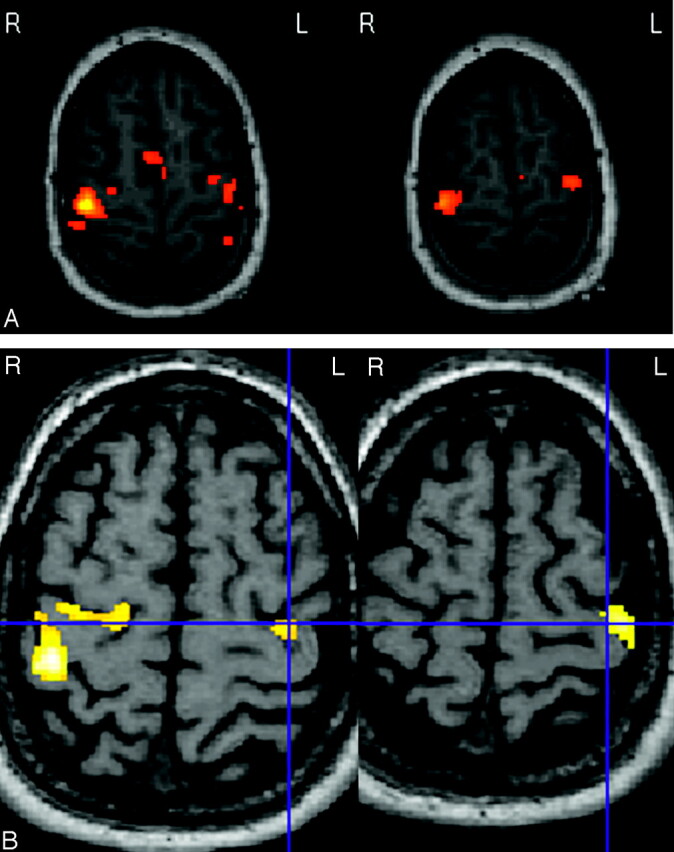
Axial functional MR imaging sequences showing the bilateral precentral cortical activation after motor tasks of the left hand in patients 14 (A) and 20 (B), amputated from the right upper limb (blue cross, enabling correlation between both images on B). This activation is obtained for analysis threshold corresponding to P values much greater than .0001. Minor differences are observed in surface and distribution of the activation between both sides.
Intraoperative Cortical Mapping
The patient’s head was fixed by a Mayfield clamp for neuronavigation. The CS and the zone corresponding grossly to the motor target of the hand on MR imaging were projected on the skin surface by the navigation microscope/probe to center the craniectomy. A 4-cm2 craniectomy was performed with general anesthesia with Propofol and Sufentanyl. A quadripolar electrode (Resume II electrode; Medtronic Inc., Minneapolis, MN) was placed at different locations on the dural surface over the CS region. iSEPs were recorded through this electrode, and the coordinates of every recording contact were registered in the navigation workstation to reproduce a virtual grid covering the CS region. Median nerve stimulation was performed in all patients. The CS was defined by means of the phase reversal of the N20P30 wave on the iSEP after stimulation of the contralateral median nerve according to a protocol described elsewhere (31). With this method, an epidural mapping of the functional CS was designed by iSEP. The motor target of the hand was defined as the coordinates of the electrode recording the highest amplitude of P20 iSEP wave, reproduced on 3 recordings. iSEP after facial stimulation were also used for facial pain according to a technique described elsewhere (31). The location of the motor target of the hand was assessed with iSEP, which is considered the gold standard in functional methods for MCS. The technique of iBS was also used for confirmation of the iSEP-defined motor target: iBS was performed through the Resume II electrode (5-mm space tips bipolar stimulation; isolated square-wave pulses with a duration of 1 ms; 60 Hz; 5–20 mA) with peripheral electromyographic monitoring. We used a Pathfinder Viking IV stimulator and averagor (Nicolet Biomedical, Madison, WI). With this method, an epidural mapping of the functional CS was designed by iCM (25, 26, 31). The accuracy of iCM is based on a resolution >5 mm, depending on the shortest distance between 2 contacts of the recording electrode. The iCM target was estimated as precise and unambiguous only when data were reproducible on 3 repeated recordings. The spatial accuracy of the navigation system and the preoperative shift of cerebral structures were assessed as described elsewhere (32).
Comparison of fMRI Data and Intraoperative Cortical Mapping
In this study, matching of fMRI and iCM was specifically examined, regardless of the clinical result. In all patients, the coordinates of the iCM-defined targets were correlated with the contours of the fMRI-defined activation areas (at initial analysis threshold) in the navigation system (Figs 1 and 3). fMRI data were thresholded on the basis of the P value, and the distances between the fMRI- and iCM-defined targets were determined by using the centroid of the fMRI blob. When targets were unambiguous (focal, reproducible, significant, with no artifact), we estimated that they corresponded spatially only if the contours of the fMRI-activated area included the target of highest iCM wave (Fig 3A). When repeated iCM recordings provided ambiguous results (diffused, not reproducible, altered by artifacts), we designated as the iCM target the one defined by the recording presenting the highest amplitude (Fig 3B). If this target was projected within the contours of the fMRI-activated area, we estimated that targets from both techniques corresponded spatially. When no iCM target was available, no comparison was possible. Finally, when spatial concordance between both targets was obtained with lower thresholds than that corresponding to P < .001, we estimated that the concordance was not significant (Fig 3C).
Fig 3.
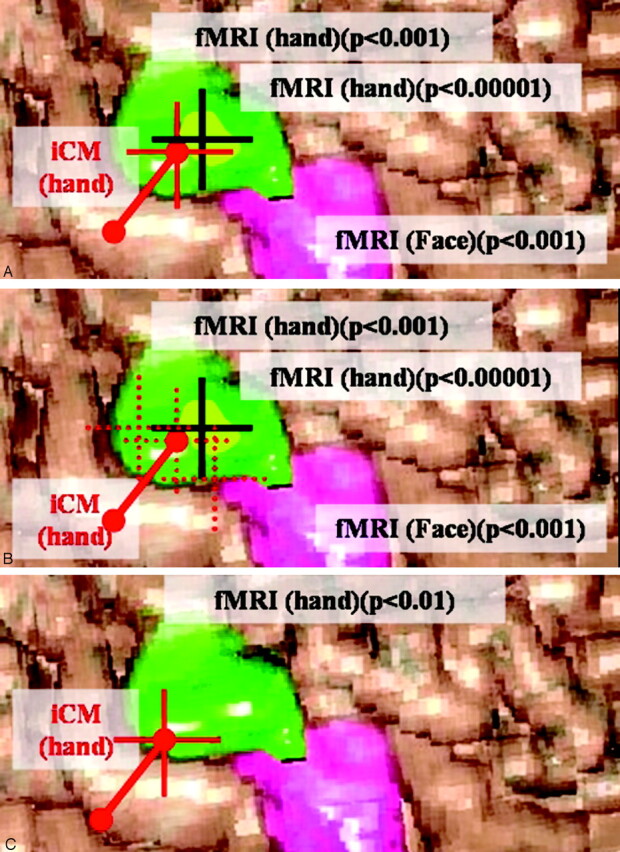
Correlation, in the navigation system, between the iCM-defined targets (center of a 1-cm area between 2 poles of the grid but represented by a red cross) and the contours of the fMRI-defined activation areas (green and pink surfaces for hand and face, respectively, including focus of highest significance [centroid of the blob, black cross] designated as “fMRI target”) at the initial (or more restrictive) analysis threshold corresponding to P < .001 (or P < .0001). These pictures and the surface of cortical activation are only illustrative and do not represent actual data.
A, When targets are unambiguous (focal/reproducible/significant/with no artifact), we estimate that they correspond spatially only if the contours of the fMRI-activated area include the target of highest iCM wave.
B, When repeated iCM recordings provide ambiguous (diffused, not reproducible, altered by artifacts) results (red pointed square crosses), we designate as the iCM target the one defined by the recording presenting the highest amplitude (red cross). If this target is projected within the contours of the fMRI-activated area, we estimate that targets from both techniques corresponded spatially. When no iCM target is available, no comparison is possible.
C, When spatial concordance between both targets was obtained with lower thresholds than that corresponding to P < .001 (ie, when P < .01), we estimate that the concordance is not significant.
We fixed arbitrarily the concordance obtained for fMRI threshold corresponding to P < .001 as sufficient for the clinical purposes. Indeed, this study was not performed in normal subjects, but in pathologic conditions (deafferentation) generating iCM suboptimal precision (somatosensory wave attenuation). Moreover, the neuronavigation used to correlate iCM and fMRI data offered infracentimetric resolution. Finally, the degree of precision required by the surgical technique (eg, regarding the size of the electrode—10 mm between contacts—and the field of cortex bipolar transdural stimulation—within 10 mm) was also infracentimetric. Therefore, we considered distances between targets less than 10 mm as irrelevant.
Results
The results obtained with iCM, fMRI, and the combination of both techniques are summarized here. The detailed data for each patient are provided in the Table.
Intraoperative Cortical Mapping
The phase reversal method allowed us to define the functional CS in all cases. iCM allowed us to identify the hand motor area in all cases; however, although an unambiguous target was obtained in 14 patients, the quality of the iCM for localizing the hand was suboptimal in the other 7 patients.
The 14 patients with an unambiguous hand mapping corresponded to 5 patients (2, 4, 5, 6, and 9) with upper limb pain; 5 patients (8, 11, 13, 16, and 17) with facial pain; 2 patients (18 and 19) with upper limb and facial pain; 1 patient (3) with lower limb pain; and 1 patient (21) with upper and lower limb pain (Table). The motor target of the hand was used to approach the motor area of the face or the foot by displacing empirically the electrode along the CS by 2 cm backward and upward, respectively (according to anatomic landmarks) in 7 of the 8 patients with facial pain and in 1 of the 3 patients with lower limb pain. These targets were, however, suggested by the iCM in 4 patients (11 and 16 in the facial cortical area and 3 and 21 in the parasagittal cortical area) in which iCM-defined target seemed to match data acquired with the technique by using targeting from the hand and electrode displacement.
The remaining 7 patients with a suboptimal iCM for localizing the hand corresponded to 5 patients (7, 10, 12, 14, and 20) with upper limb pain; 1 patient (1) with facial pain; and 1 patient (15) with lower limb pain). Indeed, repeated iCM recordings provided ambiguous motor target and a large cortical zone on which to fix the electrode for MCS. Data were altered by electrical artifacts, iSEP wave attenuation, diffused motor response or by the plegic/amputated status (Table). In the first operated patient, intense electrical artifacts in the operating room required us to reoperate on the patient several days later in another room, which was thereafter dedicated to further MCS procedures. A severe iSEP wave attenuation due to the underlying lesion of the somatosensory tracts was observed after median nerve stimulation in 6 patients (7, 10, 12, 14, 15, and 20), tibial nerve stimulation in 2 patients (15 and 21) or facial stimulation in 4 patients (8, 13, 17, 18, and 19). Patients 7, 10, 14, and 20 had brachial plexus avulsion or amputation impeding the accurate peripheral muscular detection. In 6 cases (patients 7, 10, 12, 14, 15, 18), iBS gave little additional localizing information, even for localizing the CS, because diffused muscle contractions were recorded after stimulation (<20 mA) of either the postcentral or the precentral cortex. In this series, iCM provided approximate (patients 8, 13, 17, and 20) or irreproducible (patients 1, 7, 10, 12, 14, and 15) targets for MCS.
Functional MR Imaging Data
Cortical activation during motor tasks of both hands was studied in all patients. Motor tasks of the foot (patients 3, 15, and 21) and of the tongue (patients 17 and 18) were also studied (Fig 1; Table). fMRI data were analyzed with an initial analysis threshold corresponding to P < .001. A highly significant, focal cortical activation area following hand motor tasks was consistently localized to the contralateral precentral gyrus (Figs 1 and 2) and the supplementary motor area in all patients but one (16, in which case the activation was obtained for analysis threshold corresponding to P < .01). In 13 of 20 patients, significant activation was still observed for analysis threshold corresponding to P < .0001 and the cortical activation area whose diameter ranged between 10 and 5 mm, corresponding to 1 or 2 pixels on SPM analysis.
Differences in surface and minor displacement of the precentral activation area on the gyrus was observed between both sides in 14 patients (1, 3, 5, 6, 8, 9, 10, 13, 14, 15, 17, 18, 19, and 21). We also observed a significantly reduced surface of precentral activation (for similar analysis threshold) on gyrus associated with activation in primary sensory areas (postcentral gyrus) and supplementary motor area in 5 patients (2, 4, 7, 12, and 20). Mental or virtual movements were tested in plegic (2, 4, 7, 10, and 20) or amputated (12 and 14) patients (phantom limb pain): they activated a precentral signal intensity of the paralytic hand in 4 patients (7, 10, 14, and 20) showing characteristics grossly analogous to those observed on the healthy side. In addition, the significance thresholds chosen to generate the activation maps in virtual movements (although individual) were globally the same as those used to detect motor activation in the normal side of the patients. Moreover, in patients 10 and 20, testing motor tasks of the healthy hand activated bilateral precentral areas (Fig 2). As discussed elsewhere (20), we tested these data with different levels of statistical analysis, but studying reorganizational changes was beyond the scope of this study. In patients 2, 4, and 12, the activated area obtained after motor tasks of the nonpainful side was considered not significantly displaced and, at least, more reliable to define a target for MCS so that it was projected in the navigation planning to the opposite side to be used as a target. In patients 3, 15, and 21, the cortical activation signal intensity obtained after motor tasks of the foot was located on the parasagittal convexity (Fig 1B). This observation matched with data from iBS in patient 3 (Table). Motor tasks of the tongue provided a significant activation in patient 17. In one patient (21), we tested sensory areas of the hand and foot. The significant postcentral activations allowed us to validate the precentral motor activations of the same segments (Fig 1B).
fMRI showed some limitations related to the patient’s cooperation during the imaging process, mainly during additional mapping sites. The complete protocol of activation paradigms was not achieved in 6 patients (contralateral study of the foot in patients 3, 15, and 21; movement artifacts or diffused cortical activation in patients 12, 16, and 18). Finally, cortical sulci were easily visualized on stereotactic MR images and used to center the craniectomy in all patients.
Comparison of fMRI Guidance with Intraoperative Cortical Mapping
The comparison of targets from both techniques allowed us to calculate the spatial accuracy of the navigation and the mean distance between targets defined by both techniques for the hand (mean, 3.8 mm; SD, 1.3 mm) (Table; Fig 1). These distances, ranging from 1 to 8 mm, are purely indicative because their measurements were performed by means of the neuronavigation microscope and did not reflect the resolution of fMRI and iCM (based on image technique having a spatial resolution of >5 mm).
Concordance between contours of fMRI activation area and iCM in the precentral gyrus was found for the hand in 20/21 patients (95%) (Table). Thirteen of them (patients 2, 3, 4, 5, 6, 8, 9, 11, 13, 17, 18, 19, and 21) still showed concordance for more restrictive values of the analysis threshold (P < .0001) between fMRI contours (less extended) and unambiguous target iCM (found within the fMRI contour; Fig 3A). These data, as well as the nonambiguity of iCM data, suggested an excellent concordance between both techniques (Table). In the other 7 patients (1, 7, 10, 12, 14, 15, and 20), the quality of the iCM was reduced by somatosensory wave attenuation and general anesthesia. The target from the iCM recording that presented the highest amplitude was projected within the contours of the fMRI-activated area so that concordance with fMRI was observed but only at the initial analysis threshold (P < .001). These data suggested a good concordance between both techniques in this group despite the ambiguity of iCM data (Table; Fig 3B). This group included patients with brachial plexus avulsion or amputation (7, 10, 12, 14, and 20). We did not find any difference in surface and distribution of the fMRI activation in these 7 patients compared with the others. Obviously, impairment of the somatosensory wave in these patients had no influence on fMRI activation maps. The combination of both techniques in these 7 patients helped to improve the iCM target selection for the hand (Fig 1). Concordance was not found in one last patient (16) at the initial analysis threshold, though the iCM target matched the activation contours (obtained with lower thresholds than that corresponding to P < .001) (Fig 3C). We estimated such activation as clinically not significant. This mislocalization was found to be a result of image distortion and residual motion artifact in this patient. fMRI activations during motor tasks of the foot or tongue were significant in 4 patients (3, 15, 17, and 21; Table), and the target calculated by the 2-cm projection along the sulcus was found within the fMRI contours. In these cases, combining both techniques also improved the targeting (Table).
Discussion
This study confirms the functional accuracy of fMRI guidance for the operative targeting of selective motor cortex areas in neuropathic pain. Moreover, it suggests that fMRI could be used as a valid adjunct to iCM techniques. Finally, this study emphasizes the interest of combining fMRI guidance and iCM as a tool to potentially improve the quality of the targeting method in functional neurosurgical procedures.
Intraoperative Cortical Mapping
Since MCS was first reported, epidural iCM is the targeting method of reference used to localize the functional CS and the somatotopic target on the motor strip (28, 31). Although very precise, iCM often presents practical limitations in neuropathic pain, particularly when performed with general anesthesia (25, 29–31) as was also the case in this series. Indeed, iBS mapping performed epidurally with general anesthesia often requires stimulation intensity >15 mA and yields diffused peripheral responses, especially in plegic or amputated patients. Moreover, such patients often present a significant degree of iSEP wave attenuation secondary to the central or peripheral underlying lesion of the somatosensory tracts. This renders iCM more sensitive to electrical artifacts from the operative room environment. In our series, these limitations reduced the quality of iCM results in 7 patients and required repeated recordings that showed poor reproducibility. Even without somatosensory lesion, the results from iSEP and iBS for localizing the CS do not always match precisely. Therefore, the target defined for MCS may be unreliable or ambiguous. In addition, the N20-P30 iSEP phase reversal used to define the CS after median nerve stimulation is rarely obtained after stimulation of other nerves (30). In our case, the results of the targeting procedure for the lower limb or the face by iSEP or iBS were rarely unambiguous (Table).
Although iCM remains the most accurate functional method to localize neuronal activity on the primary sensorimotor cortex, all of these limitations suggest that, in practice, iCM-guided MCS might be insufficient to provide accurate targeting in numerous patients. Such targeting inaccuracy should be suspected in every patient showing no analgesic effect from MCS (32). This illustrates why fMRI guidance is so much expected to be useful.
Comparison of fMRI Guidance with Intraoperative Cortical Mapping
Technical and Methodological Issues.
The limitations of iCM and the necessity to validate fMRI lead us to compare both methods by means of the currently available operative navigation systems (33–36). This evaluation has already been performed in studies integrating fMRI in the image-guided neurosurgical interventions of tumors or nontumor lesions in eloquent cortical areas (1–15). Almost all authors used iCM as method of reference to compare data from fMRI but also from functional activation PET or magnetoencephalography (8, 13, 37–40). Most procedures were performed by using general anesthesia (1, 2, 8, 12, 13, 39, 40) or conscious sedation (10, 13). Roux et al have applied the fMRI guidance to MCS surgery with comparison to iCM in a short preliminary series (16, 17).
Comparing fMRI and iCM into navigation softwares represents an accurate method to validate the functional and spatial accuracy of fMRI guidance (3, 7, 15). Indeed, the reliability of fMRI is still debated among the MR community, because the sensitivity of the technique is weak and the principles of the BOLD are still not completely understood (41). The genesis of activation is perfusion dependent, based on BOLD echoplanar imaging and not necessarily the faithful reflection of the neuronal activity (41). The functional value of fMRI signal intensity may be altered by different stages. One source of false activation foci comes from large draining veins that could be relatively far from the actual site of activation (42, 43). fMRI signal intensity can be also contaminated by residual motion artifacts, especially in patients with chronic pain such as in this series. The patient’s ability to remain immobile during the imaging process is altered by intractable pain. High-dose drug therapy reduces the level of tolerance to noise, the ability to follow instructions and to repeat motor tasks. Activation paradigms should also be improved and standardized to reduce the variability of the cortical activation, as observed in 5 of our patients (17). Other noise components are generated by cardiac and respiratory motion. In addition, a minimal spatial resolution is needed for the unambiguous identification of cortical activation (44). Finally, the intrinsic distortion of echoplanar images is also of concern. Although magnetic field inhomogeneity is usually not too severe in motor areas, the image fusion and the registration of fMRI data in navigation software are sources of potential inaccuracy and functional mislocalization (1, 2, 12, 17). We carefully excluded significant image distortion by using an appropriate method of acquisition, automated registration, and fusion of functional and anatomic MR images sufficiently accurate and reliable for use in stereotactic neurosurgery (11, 16, 17, 45).
fMRI Data in Refractory Neuropathic Pain.
In the present study, a significant fMRI activation area (P < .001) was obtained on the contralateral precentral gyrus in 95% of the patients. Also, analysis thresholds raised to unnecessary, but more restrictive values (much higher than P < .0001) still revealed a focal target in 13 patients (Fig 1). These results demonstrate the high specificity of the technique. In these patients, ICM presented limitations in 33%; however, the fact that the patients presenting limitations for one technique did not show difficulties in the other illustrated the independence and complementarity of both techniques. Significant activation was also obtained after motor tasks of the foot and tongue, though our experience is still limited. In the future, a similar study correlating iCM to the direct fMRI activation of foot or face will allow us to abandon the technique of assuming these targets on the motor strip (Fig 1). In view of the poor results of iCM, we expect fMRI to provide very useful information for functional targeting of these segments in a near future. Herein, we confirm that the activation for the foot can be found on the parasagittal precentral cortex (Fig 1B) (25).
fMRI study in amputees is particularly useful. These patients, as well as patients with brachial plexus total avulsion, are those for whom iCM presents the most important limitations and who would expect the highest benefit from fMRI guidance. Like others, we found that mental/virtual movement of the missing/paralytic limb easily induced contralateral primary sensorimotor cortex and CS activations (16, 46, 48). This observation suggests that the neural mechanisms involved in the mental representation of an action and in its execution are the same (47). Moreover, the significant bilateral activation after motor tasks of the healthy side illustrates that some adaptation has occurred (Fig 2) (20). In neuropathic pain, although the cortical sulci are not invaded or displaced like in brain tumors, a functional plasticity might take place as the result of significant deafferentation (4). Indeed, fMRI represents an interesting tool to study the mechanisms of neuronal plasticity in functional areas or the cortical reorganization phenomena in phantom limb pain (19, 20). Roux et al have suggested that cortical areas devoted to the missing limb seem to persist for several years after amputation (16, 17). Karl et al and Lotze et al have shown a marked reorganization of motor and somatosensory cortex in upper limb amputees with phantom limb pain in which some fMRI-activated areas were displaced (19, 20). In our series, the activated area of the missing hand was smaller in size (for the same analysis threshold) in 2 of 5 amputees, but its focus of highest significance presented only minor displacement (<5 mm), as compared with the healthy side, in the 5 amputees (Fig 2). It has not been proved that these data are exactly comparable with real motion. In fact, in phantom limb pain (20), it has been shown that during imagined movement of phantom hand, activation in the contralateral precentral gyrus was significantly higher compared with imagination of hand movements in the controls. Moreover, reorganizational changes in phantom limb pain may consist in coactivation of hand and mouth areas and also in a shift of the lip representation. Therefore, different levels of statistical analysis are indicated for assessing whether these activations could be compared with those acquired in other patients. This was, however, beyond the scope of this study. In our patients, the displacement was so irrelevant compared with the spatial resolution of the navigation system and the size of the electrode that we postulated that this displacement was not significant for the technique of electrode positioning. Therefore, when no clear target was individualized directly (on missing side), the use of the mirror-projection activated area of the valid hand as a target for fMRI postulated that plasticity of the motor cortex had not displaced the functional area (16, 46). This method needs, however, to be further validated in a larger group of patients. Finally, we estimated that target definition by using the initial analysis threshold (corresponding to P < .001) was accurate enough for the clinical purpose. Indeed, the variations of fMRI contours at different analysis thresholds were very limited, remained within the range of the spatial resolution of the navigation device, and were insignificant with regard to the size of the Resume electrode.
Interest of Combining fMRI Guidance with Intraoperative Cortical Mapping in Targeting Selective Motor Cortex Areas in Neuropathic Pain.
The concordance observed between fMRI and iCM data (for fMRI threshold corresponding to a P value < .001) in 20 of 21 patients (95%) of this series confirmed the functional accuracy of fMRI guidance (Fig 2). The fact that, when statistical stringency for fMRI increased, concordance between fMRI and iCM targets dropped to 13 of 20 patients must not be considered as a demonstration of nonconcordance. This only revealed an impossibility of demonstrating the concordance for more stringent analysis thresholds due to the ambiguity of the iCM target in these 7 cases. This ambiguity was related by some characteristics of the study, not performed in ideal conditions (awake and normal subjects) but in a pathology (deafferentation) in which the attenuation of the somatosensory waves implied suboptimal precision of iCM. In all cases in which the quality of both techniques was optimal; however, excellent concordance for thresholds corresponding to P < .0001 was obtained. Moreover, the distances between targets from both techniques (ranging from 1 mm to 8 mm) fell within the infracentimetric resolution of the neuronavigation and below the precision required by the surgical purposes (eg, regarding the size of the electrode). Thus, although these preliminary results present some limitations and require careful statement, we estimated that the concordance for fMRI threshold corresponding to P < .001 found in 20 of 21 patients allowed surgical fMRI guidance.
Similar observations have been found in almost all patients operated on for brain lesions located in eloquent areas (1, 2, 4, 8, 9, 12, 13). Furthermore, these results allow many authors to consider fMRI as highly valuable preoperatively for risk assessment, therapeutic decision making and surgical planning in eloquent cortical areas (1, 2, 3, 5, 6, 7, 10, 11, 12, 14, 15). To our knowledge, only one team has tested this combination in a short preliminary series of patients with neuropathic pain (16–18). Indeed, Sol and Roux observed a concordance between both techniques. They suggested that fMRI guidance could help in guiding electrode positioning in MCS and could even replace iCM in detection of the CS. The present study, performed on a larger series, allows us to confirm that fMRI guidance represents a valid functional targeting method; however, the numerous issues still under evaluation regarding the reliability of fMRI guidance lead us not to abandon iCM techniques for MCS and to recommend to use both techniques in combination. Indeed, despite practical limitations, iCM remains the most accurate functional method to localize motor or sensory targets on the primary cortex.
This preliminary series illustrates the potential usefulness of combining fMRI guidance with iCM in the targeting procedure of MCS (Fig 1). Combining fMRI guidance with iCM results allowed either to confirm the iCM-defined target or to correct the final targeting for MCS when iCM data are ambiguous by choosing the electrophysiologic target that matched with the activated area on fMRI. This provided a unique and unambiguous final target to stimulate and avoided performing repeat operations on patients showing no analgesic effect. Therefore, combining fMRI guidance with iCM improved the quality of the functional targeting, especially in cases of patients with altered somatosensory tracts (Table).
Conclusions
In this preliminary series, fMRI guidance is able to provide data matching those obtained by an independent functional method. These data validate the fMRI guidance as an accurate functional targeting method in neuropathic pain. Moreover, we recommend combining both techniques in functional neurosurgical procedures because it can improve the quality of the operative targeting of selective motor cortex areas.
References
- 1.Braun V, Dempf S, Tomczak R, et al. Functional cranial neuronavigation: direct integration of fMRI and PET data. J Neuroradiol 2000;27:157–163 [PubMed] [Google Scholar]
- 2.Braun V, Dempf S, Tomczak R, et al. Multimodal cranial neuronavigation: direct integration of functional magnetic resonance imaging and positron emission tomography data: technical note. Neurosurgery 2001;48:1178–1181 [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove GR, Buchbinder BR, Jiang H. Functional magnetic resonance imaging for intracranial navigation. Neurosurg Clin N Am 1996;7:313–322 [PubMed] [Google Scholar]
- 4.Duffau H. Acute functional reorganisation of the human motor cortex during resection of central lesions: a study using intraoperative brain mapping. J Neurol Neurosurg Psychiatry 2001;70:506–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilbrun MP. Optimization of cranial resections. Stereotact Funct Neurosurg 2001;76:140–144 [DOI] [PubMed] [Google Scholar]
- 6.Kim PE, Singh M. Functional magnetic resonance imaging for brain mapping in neurosurgery. Neurosurg Focus 2003. . http://www.neurosurgery.org/focus/july03/15-1-1.pdf [DOI] [PubMed]
- 7.Krings T, Reul J, Spetzger U, et al. Functional magnetic resonance mapping of sensory motor cortex for image-guided neurosurgical intervention. Acta Neurochir (Wien)1998;140:215–222 [DOI] [PubMed] [Google Scholar]
- 8.Nimsky C, Ganslandt O, Kober H, et al. Integration of functional magnetic resonance imaging supported by magnetoencephalography in functional neuronavigation. Neurosurgery 1999;44:1249–1255 [DOI] [PubMed] [Google Scholar]
- 9.Puce A, Constable T, Luby ML, et al. Functional magnetic resonance imaging of sensory and motor cortex: comparison with electrophysiological localization. J Neurosurg 1995;83:262–270 [DOI] [PubMed] [Google Scholar]
- 10.Pujol J, Conesa G, Deus J, et al. Presurgical identification of the primary sensorimotor cortex by functional magnetic resonance imaging. J Neurosurg 1996;84:7–13 [DOI] [PubMed] [Google Scholar]
- 11.Rohlfing T, West JB, Beier J, et al. Registration of functional and anatomical MRI: accuracy assessment and application in navigated neurosurgery. Comput Aided Surg 2000;5:414–425 [DOI] [PubMed] [Google Scholar]
- 12.Schulder M, Maldjian JA, Liu WC, et al. Functional image-guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg 1998;89:412–418 [DOI] [PubMed] [Google Scholar]
- 13.Sobottka SB, Bredow J, Beuthien-Baumann B, et al. Comparison of functional brain PET images and intraoperative brain-mapping data using image-guided surgery. Comput Aided Surg 2002;7:317–325 [DOI] [PubMed] [Google Scholar]
- 14.Tomczak RJ, Wunderlich AP, Wang Y, et al. fMRI for preoperative neurosurgical mapping of motor cortex and language in a clinical setting. J Comput Assist Tomogr 2000;24:927–934 [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson ID, Romanowski CA, Jellinek DA, et al. Motor functional MRI for pre-operative and intraoperative neurosurgical guidance. Br J Radiol 2003;76:98–103 [DOI] [PubMed] [Google Scholar]
- 16.Roux FE, Ibarrola D, Lazorthes Y, Berry I. Virtual movements activate primary sensorimotor areas in amputees: report of three cases. Neurosurgery 2001;49:736–741 [DOI] [PubMed] [Google Scholar]
- 17.Roux FE, Ibarrola D, Lazorthes Y, Berry I. Chronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study: technical case report. Neurosurgery 2001;48:681–687 [DOI] [PubMed] [Google Scholar]
- 18.Sol JC, Casaux J, Roux FE, et al. Chronic motor cortex stimulation for phantom limb pain: correlations between pain relief and functional imaging studies. Stereotact Funct Neurosurg 2001;77:172–176 [DOI] [PubMed] [Google Scholar]
- 19.Karl A, Birbaumer N, Lutzenberger W, et al. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci 2001;21:3609–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotze M, Flor H, Grodd W, et al. Phantom movements and pain: an fMRI study in upper limb amputees. Brain 2001;124:2268–2277 [DOI] [PubMed] [Google Scholar]
- 21.Canavero S, Bonicalzi V, Castellano G, et al. Painful supernumerary phantom arm following motor cortex stimulation for central poststroke pain: case report. J Neurosurg 1999;91:121–123 [DOI] [PubMed] [Google Scholar]
- 22.Carroll D, Joint C, Maartens N, et al. Motor cortex stimulation for chronic neuropathic pain: a preliminary study of 10 cases. Pain 2000;84:431–437 [DOI] [PubMed] [Google Scholar]
- 23.Ebel H, Rust D, Tronnier V, et al. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien)1996; 138:1300–1306 [DOI] [PubMed] [Google Scholar]
- 24.Meyerson BA, B, Lind G, Herregodts P. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien)1993;58:150–153 [DOI] [PubMed] [Google Scholar]
- 25.Nguyen JP, Lefaucheur JP, Decq P, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain: correlations between clinical, electrophysiological and anatomical data. Pain 1999;82:245–251 [DOI] [PubMed] [Google Scholar]
- 26.Nguyen JP, Lefaucheur JP, Le Guerinel C, et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res 2000;32:263–265 [DOI] [PubMed] [Google Scholar]
- 27.Saitoh Y, Shibata M, Sanada Y, Mashimo T. Motor cortex stimulation for phantom limb pain. Lancet 1999;353:212. [DOI] [PubMed] [Google Scholar]
- 28.Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien)1991;52:137–139 [DOI] [PubMed] [Google Scholar]
- 29.King RB, Schell GR. Cortical localization and monitoring during cerebral operations. J Neurosurg 1987;67:210–219 [DOI] [PubMed] [Google Scholar]
- 30.Legatt AD, Kader A. Topography of the initial cortical component of the median nerve somatosensory evoked potentials. J Clin Neurophysiol 2000;17:321–325 [DOI] [PubMed] [Google Scholar]
- 31.Wood CC, Spencer DD, Alisson T, et al. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg 1988;68:99–111 [DOI] [PubMed] [Google Scholar]
- 32.Pirotte B, Voordecker Ph, Joffroy A, et al. The Zeiss-MKM system for frameless image-guided approach in epidural motor cortex stimulation for central neuropathic pain. Neurosurg Focus 2001. . http://www.neurosurgery.org/focus/september01/11-3-3.pdf [DOI] [PubMed]
- 33.Yousri TA, Schmid UD, Schimdt D, et al. The central sulcal vein: a landmark for identification of the central sulcus using functional magnetic resonance imaging. J Neurosurg 1996;85:608–617 [DOI] [PubMed] [Google Scholar]
- 34.Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 1995;2:166–172 [DOI] [PubMed] [Google Scholar]
- 35.McCarthy G, Alisson T, Spencer DD. Localization of the face area of human sensorimotor cortex by intracranial recording of somatosensory evoked potentials. J Neurosurg 1993;79:874–884 [DOI] [PubMed] [Google Scholar]
- 36.Schiffbauer H, Berger MS, Ferrari P, et al. Preoperative magnetic source imaging for brain tumor surgery: a quantitative comparison with intraoperative sensory and motor mapping. J Neurosurg 2002;97:1333–1342 [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Larrea L, Peyron R, Mertens P, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neurosurg 1997;68:141–148 [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain 1999;83:259–273 [DOI] [PubMed] [Google Scholar]
- 39.Peyron R, Garcia-Larrea L, Deiber MP, et al. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain 1995;62:275–286 [DOI] [PubMed] [Google Scholar]
- 40.Peyron R, Frot M, Schneider F, et al. Role of operculoinsular cortices in human pain processing: converging evidence from PET, fMRI, dipole modeling, and intracerebral recordings of evoked potentials. Neuroimage 2002;17:1336–1346 [DOI] [PubMed] [Google Scholar]
- 41.Pouratian N, Sheth S, Bookheimer SY, et al. Applications and limitations of perfusion-dependent functional brain mapping for neurosurgical guidance. Neurosurg Focus 2003; http://www.neurosurgery.org/focus/july03/15–1-2.pdf [DOI] [PubMed]
- 42.Lai S, Hopkins AL, Haacke EM, et al. Identification of vascular structures as a major source of signal contrast in high resolution 2D and 3D functional activation imaging of the motor cortex at 1.5T: preliminary results. Magn Reson Med 1993;30:387–392 [DOI] [PubMed] [Google Scholar]
- 43.Segebarth C, Belle V, Delon C, et al. Functional MRI of the human brain: predominance of signals from extracerebral veins. Neuroreport 1994;5:813–816 [DOI] [PubMed] [Google Scholar]
- 44.Yoo SS, Talos IF, Golby AJ, et al. Evaluating requirements for spatial resolution of fMRI for neurosurgical planning. Hum Brain Mapp 2004;21:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rutten GJ, Ramsey N, Noordmans HJ, et al. Toward functional neuronavigation: implementation of functional magnetic resonance imaging data in a surgical guidance system for intraoperative identification of motor and language cortices. Neurosurg Focus 2003;http://www.neurosurgery.org/focus/july03/15-1-6.pdf [DOI] [PubMed]
- 46.Roux FE, Ibarrola D, Tremoulet M, et al. Methodological and technical issues for integrating functional magnetic resonance imaging data in a neuronavigational system. Neurosurgery 2001;49:1145–1156 [DOI] [PubMed] [Google Scholar]
- 47.Lee L, Siebner HR, Rowe JB, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci 2003;23:5308–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roux FE, Boulanouar K, Ranjeva JP, et al. Cortical intraoperative stimulation in brain tumors as a tool to evaluate spatial data from motor functional MRI. Invest Radiol 1999;34:225–229 [DOI] [PubMed] [Google Scholar]


