Abstract
PURPOSE AND BACKGROUND: Intersex cortical and functional asymmetry is an ongoing topic of investigation. In this pilot study, we sought to determine the influence of acoustic scanner noise and sex on auditory and language cortical activation patterns of the dominant hemisphere.
MATERIALS AND METHODS: Echoplanar functional MR imaging (fMRI; 1.5T) was performed on 12 healthy right-handed subjects (6 men and 6 women). Passive text listening tasks were employed in 2 different background acoustic scanner noise conditions (12 sections/2 seconds TR [6 Hz] and 4 sections/2 seconds TR [2 Hz]), with the first 4 sections in identical locations in the left hemisphere. Cross-correlation analysis was used to construct activation maps in subregions of auditory and language relevant cortex of the dominant (left) hemisphere, and activation areas were calculated by using coefficient thresholds of 0.5, 0.6, and 0.7.
RESULTS: Text listening caused robust activation in anatomically defined auditory cortex, and weaker activation in language relevant cortex of all 12 individuals. As a whole, there was no significant difference in regional cortical activation between the 2 background acoustic scanner noise conditions. When sex was considered, men showed a significantly (P < .01) greater change in left hemisphere activation during the high scanner noise rate condition than did women. This effect was significant (P < .05) in the left superior temporal gyrus, the posterior aspect of the left middle temporal gyrus and superior temporal sulcus, and the left inferior frontal gyrus.
CONCLUSION: Increase in the rate of background acoustic scanner noise caused increased activation in auditory and language relevant cortex of the dominant hemisphere in men compared with women where no such change in activation was observed. Our preliminary data suggest possible methodologic confounds of fMRI research and calls for larger investigations to substantiate our findings and further characterize sex-based influences on hemispheric activation patterns.
Functional MR imaging (fMRI) is a technique frequently used to study the neuronal activity in the cerebral cortex by detecting changes in regional cerebral blood flow and local deoxyhemoglobin concentrations (1–3). The technique has been used extensively to study functional cortical activity in response to a variety of stimuli, including auditory and semantic stimuli (4–8). One of the proposed applications of fMRI is the preoperative mapping of auditory and language cortex in patients with cerebral tumors and as a technique to establish language dominance in patients with epilepsy (9–19); however, one large investigation revealed errors in establishing hemispheric language dominance in 9% of epilepsy patients (20). The source of such errors is unknown.
A potentially serious confounding variable in fMRI investigations of the auditory and language system is the presence of background acoustic noise created by high-speed gradients. Although these gradients make it possible to detect subtle changes in deoxyhemoglobin concentration (4–8, 21), the fast switching of gradients causes acoustic noise (21–23). Because there is considerable overlap of the frequency distribution of echoplanar acoustic scanner noise and conversational speech (22), it is not surprising that scanner noise can activate both auditory cortex and language relevant cortex in some individuals (21). The influence of background acoustic scanner noise on fMRI studies of auditory and language cortex induced by spoken language has not been elucidated. The problem is further complicated by sex-based structural and functional differences in the cortical and precortical auditory pathways.
Numerous investigations have focused on sex-based differences in the functional organization of the brain (24–42). It has been suggested that different tasks are handled differently in men compared with women (24). Evidence suggests that language functions are more likely to be lateralized in men compared with women, such as past tense verb generation (25), phonology (26), and semantic tasks (27). Strong left hemisphere (LH) auditory responses to pure tones have been noted in men (28). Others have suggested increased sensitivity to phonology in the right hemisphere (RH) in women (29, 30). Women appear to be superior in verbal fluency and speech production tasks (31).
One fMRI investigation found stronger left hemispheric dominant activation of the posterior language area in the superior and middle temporal gyri of men compared with women (32). Another fMRI investigation supported this concept showing sex-based differences in temporal lobe activation to passive text listening (33). Other investigators, however, have reported a lack of sex-based differences to stimuli, including speech perceptual, lexical and semantic tasks (34), phonology and semantic aspects of reading (35), and word-stem completion and verb generation (36). Morphologic cortical studies have also shown discordant results (37–41).
Sex-based differences have been observed in precortical auditory pathways as well. Cochlear length is longer in men than in women (43). Differences also exist in tonotopic organization of the cochlear basilar membrane (44), auditory sensitivities at different frequencies (45), maturation dynamics of cochlear function (46), and possible efferent inhibitory activity on outer hair cells (47). The extent to which structural and functional differences may interact with acoustic background scanner noise and an auditory task is unclear at this point. The influence of acoustic scanner noise on auditory attention between the genders is also unknown.
The focus of this pilot investigation was to characterize the influence of echoplanar acoustic background scanner noise on left hemispheric activation, in response to a passive text listening task. Specifically, the aim of this study was to determine any change in activation patterns that may occur in the presence of differing acoustic scanner noise rates and may be influenced by sex.
Materials and Methods
Subjects
Twelve adult monolingual English-speaking volunteers (6 women and 6 men) were studied, ranging in age between 28 and 49 years. All subjects were in good health, were not on any medications, and reported no history of auditory/language abnormalities or other neurologic pathologies. All subjects were right handed as determined by the Edinburgh handedness inventory (48). They gave written informed consent according to institutional standards and received a stipend for their participation. Subjects were positioned within the scanner gantry following placement of tightly occlusive earplugs and were instructed not to move during image acquisitions.
Auditory Stimuli
Two separate narratives of taped spoken text, each consisting of an encyclopedia passage about an animal, were played to the subjects at an equivalent intensity and through an air-conduction system. Text listening tasks were randomized in order of delivery to counteract any effects of learning or habituation. The subjects were instructed to listen to the text and to remain motionless during the scanning. Each task was delivered in the presence of acoustic background noise created by the scanning pulse sequence. The pulse sequence was altered by changing the number of sections for a given TR (ie, 2000 ms) to change the rate of acoustic scanner noise produced during the data acquisition. Therefore, the background acoustic noise condition was determined by scanner noise accompanying 2 section acquisition rates, 6 Hz (12 sections/2 seconds, TR) and 2 Hz (4 sections/2 seconds, TR). As background information, the effects of scanner noise on hearing thresholds are rate dependent, and at high noise rates threshold effects parallel frequency distributions of conversational speech (22). The section acquisition rates and the accompanying scanner noise have been shown elsewhere to cause a nonlinear threshold effect on normal hearing subjects (22). The 2 background acoustic scanner noise conditions were randomized in order.
Equipment and Pulse Sequences
A 1.5T scanner was used, equipped with a birdcage prototype volume echoplanar local gradient 3-axis head coil. An experimental program provided a single shot blipped echo planar sequence with multisection and multirepetition capabilities, with a gradient-recalled echo. Chemical shift saturation was employed before the excitation pulse to diminish ghost artifact from the fat-containing tissues in the head. The following parameters were used: TR/TE, 2000/40 ms; section thickness, 10 mm; number of sections, 4 or 12; matrix, 64 × 64; and field of view, 24 cm.
Keeping the repetition time and other parameters constant while increasing the number of the sections acquired has the effect of increasing the rate of noise production (22). Thus, scanner noise rates for 12 and 4 sections acquired were 6 Hz and 2 Hz, respectively. Imaging began in the lateral aspect left hemisphere, and the order of acquiring either 12 or 4 sections was randomized. For purposes of comparison between the 2 noise conditions, the area of activation contained in 4 contiguous sagittal sections (4 cm) of the left hemisphere was quantified. This covered all auditory and language relevant perisylvian cortex of the left dominant hemisphere.
Regional Activation and Data Analysis
Regional activation areas were calculated as the number of pixels activated in the left hemisphere as a whole and in anatomically defined subregions of auditory and language relevant cortex, including the superior temporal gyrus, middle temporal gyrus (MTG), cortex lining the superior temporal sulcus (STS), angular gyrus (AG), supramarginal gyrus (SMG), inferior frontal gyrus (IFG) and dorsolateral prefrontal cortex (PFC). Reference waveforms were generated by using principle component analysis, to correlate to activated pixels. After motion correction, cross-correlation analysis was used to generate activation maps with coefficient thresholds of 0.5, 0.6, and 0.7, designated on activation maps as red, orange, and yellow pixels, respectively. Analysis of variance was used to determine differences in activation between the genders and among the subregions of auditory and language relevant cortex.
Results
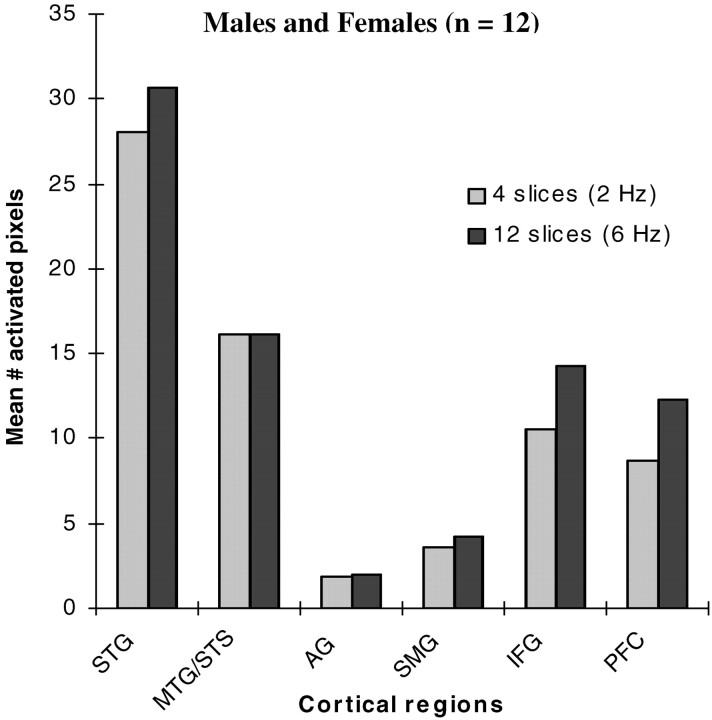
Robust activation was seen in auditory cortex of all 12 individuals, especially in the STG and MTG/STS subregions. These regions contain primary and auditory association cortex and some posterior language relevant cortex as well. Other language relevant cortices showed more variable activation, including the AG, SMG, and IFG. Some individuals showed robust activation within the PFC, but activation in this region was more variable than the other regions, by the nature of the task. The greatest mean activation area was seen within the STG and MTG/STS regions (Fig 1). Other areas in decreasing order of mean activation area included the IFG, PFC, SMG, and AG, respectively (Fig 1).
Fig 1.
Left hemispheric mean number of activated pixels in specific subregions of auditory and language cortex of 12 normal hearing subjects (6 men and 6 women), to passive text listening at 2 different background acoustic scanner noise conditions; 4 sections/2 seconds TR (2 Hz) and 12 sections/2 seconds TR (6 Hz). Activity is greatest in the STG and MTG/STS region. The STG includes the transverse temporal gyrus, planum polare, and planum temporale. The MTG/STS region includes primarily the posterior aspect of the MTG and cortex lining of the posterior aspect of the STS. Activity in the IFG includes primarily Broadman’s areas 44 and posterior 45. Activity in the PFC, AG, and SMG was inconsistent among individuals. Note that there is no significant difference in regional cortical activation of the left hemisphere in the presence of changing scanner noise rates.
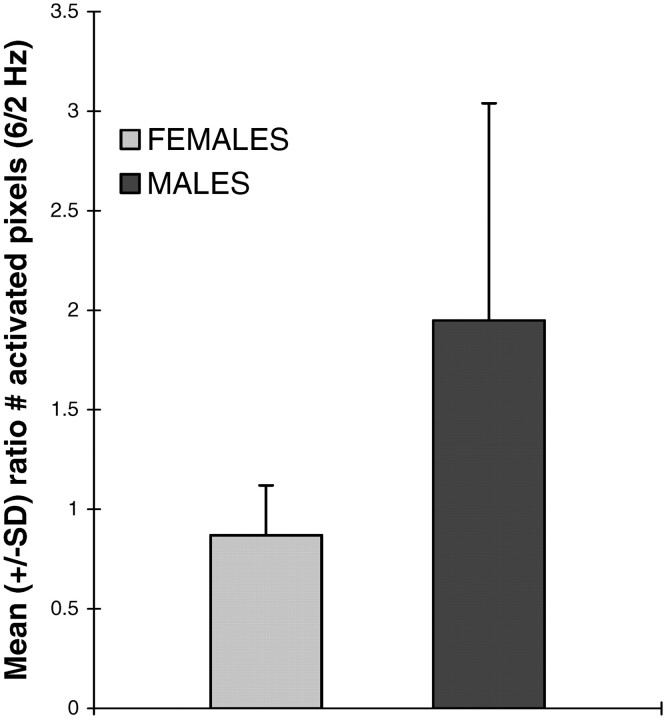
For the group as a whole, there was no significant difference in left hemispheric activation in response to text listening in the 2 background acoustic scanner noise conditions (4 sections = 2 Hz and 12 sections = 6 Hz; Fig 1). When the subjects were divided by sex, however, a sex-based pattern of activation response to the 2 background noise conditions emerged (Fig 2). All 6 men showed a 20% or greater increase in total number of activated pixels within the left hemisphere in response to text listening, when the high scanner noise rate background condition (6 Hz) was present compared with the low noise rate condition (2 Hz). Conversely, none of the women showed an increase in activation >5% in the same conditions. Also, the variance in proportion to the mean among the men in the number of activated pixels was increased compared with the women (Fig 2). There was a significant (P < .01) difference in the change of activation across the 2 background acoustic noise conditions between men and women (Fig 2).
Fig 2.
Ratio of total left hemispheric activation in response to passive text listening between the scanner noise conditions of 12 sections (6 Hz) and 4 sections (2 Hz). There is no change in activation between the 2 background noise conditions in women (n = 6), but a significant (P < .01) increase in left hemispheric activation in men (n = 6) as the rate of scanner noise is increased, compared with that of women. All 6 men showed a 20% or greater increase in activation with the more rapid rate of scanner noise. Also noted is the increased variance among the men.
In light of the individual subregions of auditory and language relevant cortex, women showed very similar patterns of activation between the 2 background acoustic scanner noise conditions (Fig 3A). Compared with women, however, the men showed a statistically significant (P < .05) increase in activation change within the STG, the MTG/STS, and the IFG (Fig 3A). This effect was seen primarily in pixels with lower correlation coefficient thresholds, which raised the possibility that subthreshold activated regions were brought suprathreshold by the increased scanner noise condition. The activation within the IFG corresponded to speech cortical regions (Broadman’s areas 44 and posterior 45). The STG included primary and association auditory cortex, as well as the posterior aspect of the planum temporale, implicated in receptive language function. Activation in the MTG/STS included the posterior aspect of the MTG, as well as cortex lining the STS posteriorly, containing auditory association and posterior language relevant cortex. Increased activation was also seen in the AG and SMG in men, but the difference was not statistically significant for a small sample. There was an increase in dorsolateral PFC mean activation area in men compared with women in response to the increasing noise rates, but the effect was variable and was not statistically significant.
Fig 3.
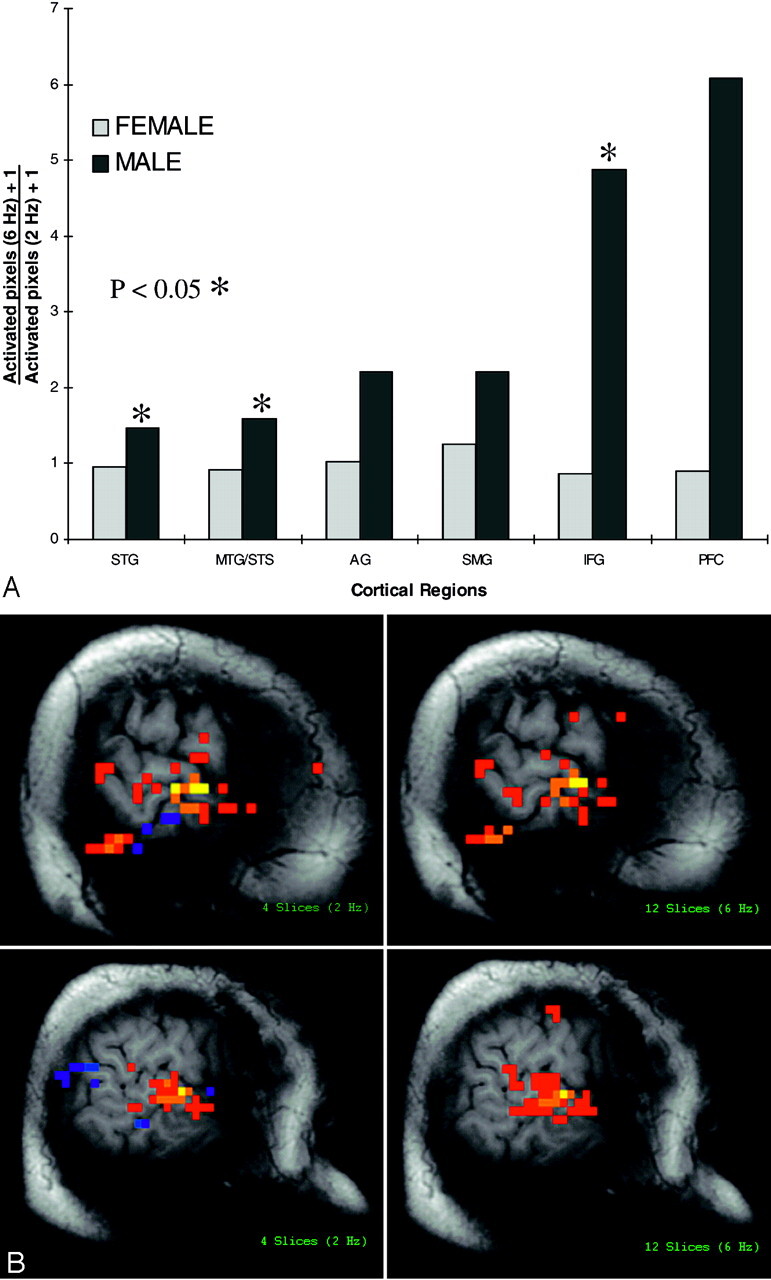
A, Change in activation of left hemispheric auditory and language relevant cortex across 2 background noise conditions (12 sections = 6 Hz; 4 sections = 2 Hz). Note significantly increased activation (P < .05) within the STG, MTG/STS, and IFG subregions to text listening in the presence of more rapid acoustic scanner noise production, in men (n = 6) compared with women (n = 6). Nonsignificant increased activation is also seen within the AG, SMG, and PFC of men. There is no change in activation to the more rapid noise rate in women.
B, Activated pixels in auditory and language cortex in response to text listening with 2 underlying background acoustic scanner noise conditions (2 Hz and 6 Hz). Left hemispheric activation in response to text listening in a woman is shown to be similar between the 2 underlying background acoustic noise conditions (upper images). Left hemispheric activation to text listening in a man is significantly increased by increasing the rate of data acquisition and associated increased rate of background acoustic scanner noise (lower images).
Discussion
There is ongoing debate concerning sex-based differences in the organization of the human brain and, in particular, of language functions (24–42), but functional differences have been observed. A study by Kimura suggested that sex-based differences in intellectual function lay in patterns of ability rather than in the overall level of intelligence (24). Men performed better in tasks such as imagining rotating or manipulating objects in 3D, navigating through a route, mathematical reasoning, target-related motor skills, and disembedding tests. Women, on the other hand, were better in rapid identification of matching items (perceptual speed), arithmetic calculation, recalling landmarks from a route, certain precision manual tasks, and demonstrated greater verbal (listing words beginning with the same letter) and ideational (listing objects with the same color) fluencies.
In one study, damage to either hemisphere in women has been shown to affect vocabulary test scores, but this effect was seen only with left-dominant hemisphere injuries in men, which suggests that there is more balanced hemispheric processing for such tasks in women than in men (24). In a cortical lesion–based study, McGlone also reported that only men showed more depressed verbal intelligence and verbal memory after left compared with right hemispheric damage (49). Women showed no significant difference in verbal scores with left or right hemispheric damage, though they had significantly impaired verbal intelligence compared with nonbrain-damaged controls. Functional sex-based differences in processing auditory and language stimuli in the presence of background scanner noise remains to be elucidated.
Cortical and subcortical structural asymmetries may correlate with sex-based functional differences observed in auditory and language processing, and could, in some way, have influenced the activation patterns observed in our study. A surface-rendering morphometric MR imaging study showed significantly larger left versus right planum temporale (PT) in men, whereas it was not significantly different among women, which suggests a relationship between morphologic and functional attributes of superior temporal cortex (50). Whereas this was consistent with a prior study (51), others have failed to support this conclusion (52). Other investigators have also failed to find sex-related differences in the length (as opposed to volume) of the PT (40, 41).
Significantly longer cochlear length in men compared with women has been observed with 3D (42) and 2D (53) measurements, which suggests that the frequency map of the basilar membrane might differ depending on sex. This has been proposed to account for significantly longer distortion product otoacoustic emission phase delay measures in adult men compared with women (43). Pure-tone auditory sensitivity is better for low frequencies in men and for high frequencies in women (45). In addition, significant sex-based differences have been reported in the level of maturation of cochlear function in both preterm (46) and full-term (47) neonates, and it has been suggested that these differences might be the result of the outer hair cell (OHC) population characteristics. The OHCs of the organ of Corti are thought to enhance hearing sensitivity (via cochlear amplification) and fine tuning of frequency discrimination (46). Because the OHCs are innervated by efferents (brain stem to cochlea) (44), it is reasonable to suppose that OHCs are under some element of central influence and efferent input may be contributing to fine pitch discrimination. Thus, sex-based differences in the cochlea may be a reflection of auditory cortex organization rather than a primary determining factor. Women may have more efferent inhibitory activity than men (47) and may be better at discriminating auditory stimuli among increasing background scanner noise rates compared with men.
Greater RH representation of phonologic functions have been observed in women than in men (29, 30). Shaywitz et al (26) used fMRI to isolate areas of activation of orthography (letter case—line), of phonology (rhyme—case), and of semantic task (semantic—rhyme) in 38 right-handed subjects. Although orthographic task evenly activated the extrastriate (visual) cortex bilaterally in both sexes, phonologic task activation lateralized to the left inferior frontal gyrus in men but remained more evenly split in women. Pugh et al also noted a phonologic representation engaging a number of sites within the IFG and temporal lobe, with inferior frontal sites more LH dominant in men but highly bilateral in women (27). Greater left-dominant lateralization of activity to grammatical task (past-tense verb generation) in perisylvian cortex has also been observed in men compared with women with positron-emission tomography (PET) (25).
In one recent fMRI study, Kansaku et al focused on the posterior language area where the subjects were asked to listen to a story with closed eyes and told that they would be asked questions about the story (32). Women showed no significant lateralization in any of the 3 temporal gyri, but men showed strong left hemispheric lateralization in the STG and MTG. When global structures were removed from the auditory stimuli, however, sex differences disappeared, which suggests that the influence of sex became apparent only when the subjects were required to process the global structures of sentences. An additional finding was left lateralization of activity in the anterior IFG in men. Phillips et al studied 20 subjects, with fMRI, by using a passive text listening task and found a higher degree of bilateral temporal lobe activation in women compared with men, a finding that supports the idea that sex-determined activation patterns may be of task-related origin (33). The potential role of background scanner noise in inducing these sex-based asymmetries is unknown.
Not all fMRI studies have supported the concept of sex-based hemispheric language asymmetries. Van der Kallen et al (42) found no marked sex-related difference in language lateralization in a group of healthy volunteers and epilepsy patients using silent word–generation task with fMRI. In another fMRI investigation, the combined activation of several language-related component processors (ie, speech perceptual, lexical and semantic tasks) was strongly lateralized to the left hemisphere in both men and women, without sex-related difference in any region of interest, which argues against substantive sex differences in the large-scale neural organization of language functions (34). Similar results also were provided by 2 preceding PET studies reporting no significant sex-related differences in large-scale activation patterns (35, 36).
Echoplanar imaging used in most fMRI studies provides the rapid acquisition of activation data from the entire brain (4–8); however, the fast switching of gradients in the gradient coil causes acoustic noise that increases in parallel with the gradient switching rate. Acoustic scanner noise frequencies span the optimal hearing range in humans and those of conversational speech (21), with higher noise rates causing more pronounced effects on pure tone hearing thresholds (22). Moreover, the change in threshold effect across increasing scanner noise rates is not linear across the frequency spectrum (22). Acoustic scanner noise can activate both auditory and language relevant cortex, possibly because of similar temporal and spectral properties to spoken language (21–23). The extent to which the effects of background echoplanar acoustic scanner noise suggested in this pilot investigation might influence or account for discrepancies in the literature regarding sex-based hemispheric asymmetries in auditory and language function is unclear and requires further investigation.
Our pilot investigation suggests the potential for background acoustic scanner noise to alter the activation patterns in the dominant left hemisphere of men but not women, possibly because of saturating or synergistic effects. Although increasing the rate of acoustic scanner noise production had no effect on activation patterns in women, auditory, posterior language, and anterior language relevant cortical activation increased by 20% or more in each of the 6 men in the same conditions; however, the relatively small number of subjects studied here is insufficient to draw statistically certain conclusions about the influence of acoustic noise, or sex, on auditory and language cortical activation patterns. It is nevertheless intriguing that noise-induced increases in activation observed in men involved the same cortical subregions (ie, left STG, MTG/STS, and IFG) reported in the literature to show sex-based asymmetries. If the phenomenon described here were substantiated with further studies, it would have serious methodologic implications and may account for some of the discrepancies of sex-based lateralization studies by using fMRI as a technique. If the effects of scanner noise in a given experiment were to increase left hemisphere activation in men but not women, this could artificially lateralize language function in men. A review of the imaging parameters of the fMRI literature cited above, however, shows an inconsistent relationship between background acoustic noise and (non)lateralization of language functions, which is not necessarily consistent with our results. Whether this could be explained by other factors such as the study design, type of earplugs used, stimulus delivery system, or acoustic properties of the scanner room remains to be determined.
The effect of background acoustic scanner noise on hemispheric activation is likely to be laboratory dependent. The design of the coils and scanner suite, as well as the effectiveness of ear plugs or occlusive ear devices, certainly could affect the character of the scanner noise perceived by the subject and, therefore, the effects on auditory and language cortical activation. Likewise, the effective background scanner noise on auditory and language activation may depend upon the task design. In our investigation, the control state was background acoustic scanner noise, but a control task may change the impact of scanner noise on activation patterns. Investigators focusing on evaluating the complexities of the auditory cortex by using fMRI have already begun to consider experimental designs that reduce the effect of background acoustic scanner noise on auditory activation (54–56). Also, postprocessing strategies and activation thresholds could influence the likelihood of noise-induced alterations in activation area, particularly if active but subthreshold regions account for the observed effects.
Control of the attention to the preferred stimuli—in other words, inhibition of the nonpreferred stimuli—and perhaps selective behavior in distributing the attention may have contributed to the sex-based differences observed in our experiment. Consciously driven modulation of attention has been shown to operate in the sensory cortex responsible for processing the related stimuli (57). It has been shown that focused auditory attention has selective control in early sensory processing of the auditory cortex in the superior temporal plane (58). Evidence suggests an attention-related enhancement of both activation magnitude and extent in auditory cortex, especially in the association areas (59) and in the primary auditory cortex (60). This may help to explain the significantly increased activation volume in the left hemisphere of men as they directed attention to the text listening tasks during increasing background noise rates. Whether women are better able to attend to an auditory stimulus in the presence of background acoustic noise interference, compared with men, remains to be investigated.
Conclusion
Our preliminary results have several interesting implications. The presence of a sex-specific effect of background acoustic noise on auditory and language cortical activation as suggested by our results could have serious methodologic implications for fMRI investigations. The differences in activation observed may be based in sex-specific functional and structural correlates, but these preliminary results should be confirmed with larger studies and more sophisticated paradigms should be developed to analyze the underlying properties of the auditory and language system that potentiate sex-based differences in performance and activation. The possibility of modulation of consciously driven attention favoring women’s ability to isolate a preferred stimulus, as an explanation for our observed sex-based activation patterns, warrants further investigation. Ultimately, sex specific hemispheric asymmetries may be best considered in the context of background environments.
Acknowledgments
We thank Gokce Kaan Atac, MD, for his very much-appreciated contributions in the references section.
Footnotes
This study was supported by a Radiological Society of North America Research and Education Fund Scholar’s Grant.
References
- 1.Bandettini PA, Wong EC, Hinks RS, et al. Time course EPI of human brain function during task activation. Magn Reson Med 1992;25:390–397 [DOI] [PubMed] [Google Scholar]
- 2.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 1992;89:5675–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc Brain Metab Rev 1995;7:240–276 [PubMed] [Google Scholar]
- 4.Belliveau JW, Kennedy DN, McKinstry RC, etal. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 1991;254:716–719 [DOI] [PubMed] [Google Scholar]
- 5.Rao SM, Binder JR, Bandettini PA, et al. Functional magnetic resonance imaging of complex human movements. Neurology 1993;43:2311–2318 [DOI] [PubMed] [Google Scholar]
- 6.Yetkin FZ, Mueller WM, Hammeke TA, et al. Functional magnetic resonance imaging mapping of the sensorimotor cortex with tactile stimulation. Neurosurgery 1995;36:921–925 [DOI] [PubMed] [Google Scholar]
- 7.Howard RJ, Brammer M, Wright I, et al. A direct demonstration of functional specialization within motion-related visual and auditory cortex of the human brain. Curr Biol 1996;6:1015–1019 [DOI] [PubMed] [Google Scholar]
- 8.Eden GF, Van Meter JW, Rumsey JM, et al. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature 1996;4:66–69 [DOI] [PubMed] [Google Scholar]
- 9.Hinke RM, Hu Z, Stillman AE, et al. Functional magnetic resonance imaging of Broca’s area during internal speech, Neuroreport 1993;4:675–678 [DOI] [PubMed] [Google Scholar]
- 10.McCarthy G, Blamire AM, Rothman DL, et al. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proc Natl Acad Sci U S A 1993;90:4952–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benson RR, Kwong KK, Buchbinder BR, et al. Noninvasive evaluation of language dominance using functional MRI. In: Proceedings of the Society of Magnetic Resonance in Medicine (SMRM). Berkeley, CA: SMRM;1994. :684
- 12.Desmond JE, Sum JM, Wagner AD, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain 1995;118:1411–1419 [DOI] [PubMed] [Google Scholar]
- 13.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 1996;46:978–984 [DOI] [PubMed] [Google Scholar]
- 14.Yetkin FZ, Akansel G, Swanson SJ, et al. Correlation of functional MR imaging with Wada results in patients with partial complex epilepsy. Radiology 1996;201:132–133 [Google Scholar]
- 15.Bahn MM, Lin W, Silbergeld DL, et al. Localization of language cortices by functional MR imaging compared with intracarotid amobarbital hemispheric sedation. AJR Am J Roentgenol 1997;169:575–579 [DOI] [PubMed] [Google Scholar]
- 16.Hertz-Pannier L, Gaillard WD, Mott S, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology 1997;48:1003–1012 [DOI] [PubMed] [Google Scholar]
- 17.Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole-brain functional MRI in patients with brain lesions. Neurology 1999;52:798–809 [DOI] [PubMed] [Google Scholar]
- 18.Lehericy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 2000;54:1625–1633 [DOI] [PubMed] [Google Scholar]
- 19.Brockway JP. Two functional magnetic resonance imaging fMRI tasks that may replace the gold standard, Wada testing, for language lateralization while giving additional localization information. Brain Cognition 2000;43:57–59 [PubMed] [Google Scholar]
- 20.Woermann FG, Jokeit H, Luerding R, et al. Language lateralisation by Wada-test and fMRI in 100 patients with epilepsy. Neurology2003;61:699–701 [DOI] [PubMed] [Google Scholar]
- 21.Ulmer JL, Biswal BB, Yetkin FZ, et al. Cortical activation response to acoustic echo planar scanner noise. J Comput Assist Tomogr 1998;22:111–119 [DOI] [PubMed] [Google Scholar]
- 22.Ulmer JL, Biswal BB, Mark LP, et al. Acoustic echoplanar scanner noise and pure tone hearing thresholds: the effects of sequence repetition times and acoustic noise rates. J Comput Assist Tomogr 1998;22:480–486 [DOI] [PubMed] [Google Scholar]
- 23.Bandettini PA, Jesmanowicz A, Van Kylen J, et al. Functional MRI of brain activation induced by scanner acoustic noise. Magn Reson Med 1998;39:410–416 [DOI] [PubMed] [Google Scholar]
- 24.Kimura D. Sex differences in the brain. Sci Am 1992;267:118–125 [DOI] [PubMed] [Google Scholar]
- 25.Jaeger JJ, Lockwood AH, van Valin RD Jr, et al. Sex differences in brain regions activated by grammatical and reading tasks. Neuroreport 1998;9:2803–2807 [DOI] [PubMed] [Google Scholar]
- 26.Shaywitz BA, Shaywitz SE, Pugh KR, et al. Sex differences in the functional organization of the brain for language. Nature 1995;373:607–609 [DOI] [PubMed] [Google Scholar]
- 27.Pugh KR, Shaywitz BA, Shaywitz SE, et al. Cerebral organization of component processes in reading. Brain 1996;119:1221–1238 [DOI] [PubMed] [Google Scholar]
- 28.Salmelin R, Schnitzler A, Parkkonen L, et al. Native language, gender, and functional organization of the auditory cortex, Proc Natl Acad Sci U S A 1999;96:10460–10465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukatela G, Carello C, Savic M, Turvey MT. Hemispheric asymmetries in phonological processing. Neuropsychologia 1986;24:341–350 [DOI] [PubMed] [Google Scholar]
- 30.Luh KE, Levy J Interhemispheric cooperation: left is left and right is right but sometimes the twain shall meet. J Expt Psychol Hum Percept Perform 1995;21:1243–1258 [Google Scholar]
- 31.Halpern DF. Sex differences in cognitive abilities. Hillsdale, NJ: Lawrence Erlbaum;1986
- 32.Kansaku K, Yamaura A, Kitazawa S. Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex 2000;10:866–872 [DOI] [PubMed] [Google Scholar]
- 33.Phillips MD, Lowe MJ, Lurito JT, et al. Temporal lobe activation demonstrates sex-based differences during passive listening. Radiology 2001;220:202–207 [DOI] [PubMed] [Google Scholar]
- 34.Frost JA, Binder JR, Springer-Verlag JA, et al. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain 1999;122:199–208 [DOI] [PubMed] [Google Scholar]
- 35.Price CJ, Moore CJ, Friston KJ. Getting sex into perspective. Neuroimage 1996;3:S586 [Google Scholar]
- 36.Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J Neurophysiol 1995;74:2163–2173 [DOI] [PubMed] [Google Scholar]
- 37.Harasty J, Double KL, Halliday GM, et al. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol 1997;54:171–176 [DOI] [PubMed] [Google Scholar]
- 38.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 1996;366:223–230 [DOI] [PubMed] [Google Scholar]
- 39.Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci 1995;15:3418–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. J Comp Neurol 1992;323:326–340 [DOI] [PubMed] [Google Scholar]
- 41.Aboitiz F, Scheibel AB, Zaidel E. Morphometry of the Sylvian fissure and the corpus callosum, with emphasis on sex differences. Brain 1992;115:1521–1541 [DOI] [PubMed] [Google Scholar]
- 42.van der Kallen BF, Morris GL, Yetkin FZ, et al. Hemispheric language dominance studied with functional MR: preliminary study in healthy volunteers and patients with epilepsy. AJNR Am J Neuroradiol 1998;19:73–77 [PMC free article] [PubMed] [Google Scholar]
- 43.Sato H, Sando I, Takahashi H. Sexual dimorphism and development of the human cochlea: computer 3-D measurement. Acta Otolaryngol (Stockh)1991;111:1037–1040 [DOI] [PubMed] [Google Scholar]
- 44.Ulmer JL, Mark LP, Biswal BB, Daniels DL. Functional MR imaging and auditory activation. In: Mukherji SK, Castelijns JA, eds. Modern head and neck imaging. Berlin: Springer-Verlag;1999. :49–78
- 45.Corso JF. Age and sex differences in pure-tone thresholds. Arch Otolaryngol 1963;77:385–405 [DOI] [PubMed] [Google Scholar]
- 46.Morlet T, Perrin E, Durrant JD, et al. Development of cochlear active mechanisms in human differs between gender. Neurosci Lett 1996;220:49–52 [DOI] [PubMed] [Google Scholar]
- 47.Newmark M, Merlob P, Bresloff I, et al. Click evoked otoacoustic emissions: inter-aural and gender differences in newborns. J Basic Clin Physiol Pharmacol 1997;8:133–139 [DOI] [PubMed] [Google Scholar]
- 48.Oldfield RC. The assessment and analysis of handedness: the Edinburg inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 49.McGlone J. Sex differences in the cerebral organization of verbal functions in patients with unilateral brain lesions. Brain 1977;100:775–793 [DOI] [PubMed] [Google Scholar]
- 50.Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl’s gyrus and the planum temporale. Cereb Cortex 1994;4:107–118 [DOI] [PubMed] [Google Scholar]
- 51.Bilder RM, Howei W, Bogerts B, et al. Absence of regional hemispheric volume asymmetries in first episode schizophrenia. Am J Psychiatry 1994;151:1437–1447 [DOI] [PubMed] [Google Scholar]
- 52.Duara R, Kushch A, Gross-Glenn K, et al. Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Arch Neurol 1991;48:410–416 [DOI] [PubMed] [Google Scholar]
- 53.Hardy M. The length of the organ of Corti in man. Am J Anat 1938;62:291–311 [Google Scholar]
- 54.DiSalle F, Formisano E, Seifritz E, et al. Functional fields in human auditory cortex revealed by time-resolved fMRI without interference of EPI noise. Neuroimage 2001;13:328–338 [DOI] [PubMed] [Google Scholar]
- 55.Hall DA, Summerfield AQ, Goncalves MS, et al. Time-course of the auditory BOLD response to scanner noise. Mag Reson Med 2000;43:601–606 [DOI] [PubMed] [Google Scholar]
- 56.Talavage TM, Edmister WB. Measuring and reducing the impact of imaging noise on echo-planar functional magnetic resonance imaging (fMRI) of auditory cortex. In: Abstracts of the 21st Midwinter Meeting of the Association for Research in Otolaryngology, no 138 held in St. Petersburg Beach, FL, February 15–19, 1998.
- 57.Woodruff PW, Benson RR, Bandettini PA, et al. Modulation of auditory and visual cortex by selective attention is modality-dependent. Neuroreport 1996;7:1909–1913 [DOI] [PubMed] [Google Scholar]
- 58.Woldorff MG, Gallen CC, Hampson SA, et al. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc Natl Acad Sci U S A 1993;90:8722–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grady CL, van Meter JW, Maisog JM, et al. Attention-related modulation of activity in primary and secondary auditory cortex. Neuroreport 1997;8:2511–2516 [DOI] [PubMed] [Google Scholar]
- 60.Jancke L, Mirzazade S, Shah NJ. Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett 1999;266:125–128 [DOI] [PubMed] [Google Scholar]