Abstract
BACKGROUND AND PURPOSE: The size of the primary lesion and the lymph node metastasis are critical indicators for the patient prognosis. Here we attempted to assess the correlation between these 2 prognostic parameters in patients with oropharyngeal, hypopharyngeal, oral, or maxillary sinus cancer.
METHODS: We retrospectively studied 66 patients with oropharyngeal or hypopharyngeal (n = 24), oral (n = 35), or maxillary sinus (n = 7) cancer. Of these patients, 25 (10 with oral or maxillary sinus and 15 with pharyngeal cancers) had lymph node metastases. We measured the volumes of the primary lesions as sums of gadolinium-enhanced areas on fat-suppressed, spectral presaturation with inversion recovery (SPIR) T1-weighted images. Histologically confirmed metastatic nodes were mapped to the neck levels.
RESULTS: The tumor volumes were well correlated with the clinical T-category for the primary lesions in oral, maxillary sinus, and oropharyngeal and hypopharyngeal cancers. The volumes of the oropharyngeal and hypopharyngeal cancers were significantly greater (P < .05) in patients with metastatic nodes than in patients without metastasis, whereas there was no significant correlation between the tumor volume and nodal metastasis in patients with oral or maxillary sinus cancer (Mann-Whitney U test). Furthermore, the correlation between tumor volume and the distribution of metastatic nodes in the neck was observed to be weak in patients with oral or maxillary sinus cancer.
CONCLUSION: The MR image–based tumor volume measurement proved to be clinically feasible. We observed a good correlation between tumor volume and lymph node metastasis in patients with oropharyngeal or hypopharyngeal cancer but not in those with oral or maxillary sinus cancer.
Tumor volume is known to be a significant prognostic indicator for the treatment of cancers arising in the head and neck regions (1–4). CT provides good references in measuring tumor volume. Most volumetric studies of head and neck cancers are based on measurements that are carried out by using serial CT images via the summation-of-area technique (2–4); however, the low tissue contrast between the tumor areas and surrounding tissues and artifacts from dental fillings hampered the usefulness of this technique in measuring tumor volumes to a great extent (5).
As compared with CT, MR imaging affords better tissue contrast and can depict cancer invasion more accurately (6). Furthermore, a recent study on pharyngeal cancer showed that the measurements of MR image–based tumor volumes are accurate and reproducible (7).
The presence of regional lymph node metastasis is another factor that significantly affects patient prognosis. Several studies revealed that the tumor volume is a dominant determinant for patient prognosis over the nodal stages (7–9). These findings indicate that an increase in tumor volume is closely correlated with the occurrence of lymph node metastasis. Most of these studies, however, were performed on pharyngeal cancers, and there exists a lack of reports on volumetric measurements of oral cancers.
MR imaging has been proved to be more accurate than CT in evaluating soft-tissue or bone extension of head and neck tumors (5, 10, 11). Therefore, the purpose of the present study was to assess the correlation between the T-stage and the presence of lymph node metastasis. We also compared the tumor volumes of the primary lesions of oral, maxillary sinus, and pharyngeal cancers with the incidence and distribution of lymph node metastasis.
Patients and Methods
Patients
The study cohort comprised 66 patients with oral (n = 35; 10 women and 25 men; average age, 67 ± 12 years), maxillary sinus (n = 7; 7 women; average age, 59 ± 10 years), or oropharyngeal or hypopharyngeal (n = 24; 1 woman and 23 men; average age, 67 ± 9 years) cancer. Primary lesions of the oral cancers were located in the tongue (n = 13), floor of the mouth (n = 10), or mandibular (n = 7) and maxillary (n = 5) gingiva. Primary lesions of the pharyngeal cancers were located in the oropharynx (n = 15) and hypopharynx (n = 9). In this study, we omitted nasopharyngeal cancers because most of the cancers arising in this region are hypo- or undifferentiated types of squamous cell carcinomas; they are reportedly prone to metastasize to the regional lymph nodes and frequently exhibit different behavior in response to radio- and chemotherapy, as compared with those arising in the other pharyngeal regions (12). The initial study cohort comprised 68 patients; however, 2 of these were excluded from the study because of the poor quality of the tumor image.
The T-category of the primary lesions was clinically determined on the basis of the International Union Against Cancer (UICC) criteria (13). In some cases, particularly in the T4 cases, however, we used MR gadolinium-enhanced T1-weighted MR images to determine precise tumor invasions, such as, invasions into the thyroid/cricoid cartilage, pterygoid process, and deep portion of the tongue.
MR Imaging
MR imaging was performed by using a 1.5T MR imager (Gyroscan Intera 1.5-T Master; Philips Medical System, the Netherlands) with a head neck coil (Synergy Head Neck coil, Philips Medical Systems) or a surface coil (Synergy Flex L coil, 22 cm; Philips Medical Systems). Gadolinium-enhanced, fat-suppressed (spectral presaturation with inversion recovery [SPIR]) T1-weighted images (TR/TE/numbers of signal intensity acquisition, 500/15/4) of the primary lesions were obtained by using a conventional spin-echo sequence. The section thickness was 4 mm. The MR imaging was performed with a 204 × 256 matrix, a 20-cm field of view, and a 0.8-mm interslice gap. Gadolinium was injected intravenously at a dose of 0.2 mL/kg body weight.
Nonenhanced axial T1-weighted images (TR/TE/numbers of signal intensity acquisition, 500/15/4) and fat-suppressed (SPIR) axial T2-weighted images (TR/TE/numbers of signal intensity acquisition, 4674/80/4) were obtained from all the patients by using a conventional spin-echo sequence and a turbo spin-echo sequence, respectively.
Tumor Volume Measurement
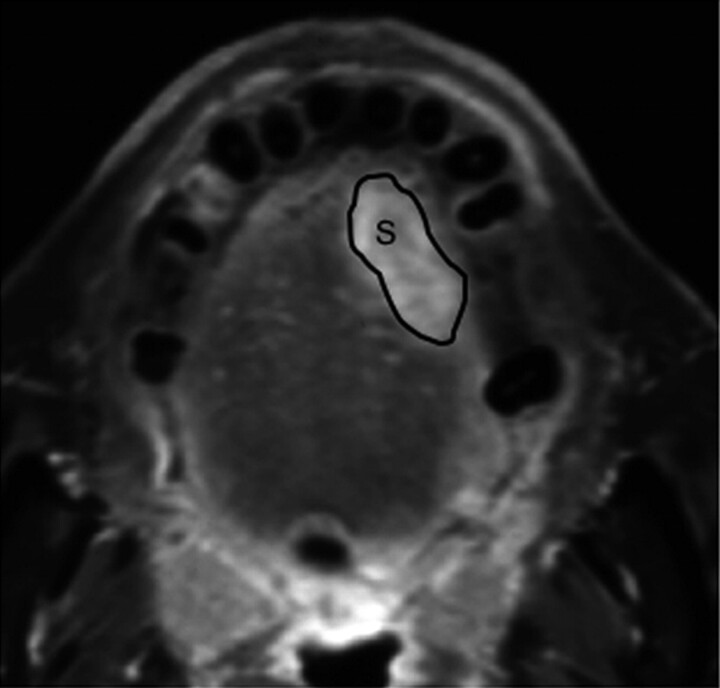
The tumor area was manually outlined from axial MR images as gadolinium-enhanced areas on fat-suppressed T1-weighted images (Fig 1). The tumor volume was then determined by adding the extracted areas multiplied by the sum of the section and gap thicknesses. The results obtained by 2 radiologists (M.S. and Y.I.) were averaged. For interobserver precision, 3 observers examined the gadolinium-enhanced T1-weighted images obtained from 5 patients. The precision of the MR-based tumor volume measurements was determined as the coefficient of variance on the basis of the following formula:
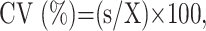 |
where s is the SD and X is the mean of the volume measurements obtained from the 3 observers (M.S., Y.I., and Y.K.). The resultant precision was expressed as an averaged %CV for the 5 patients.
Fig 1.
Manual tracing of tumor area on gadolinium-enhanced, fat-suppressed T1-weighted (TR/TE, 500/15) image. The outlines show tumor areas (S) on MR image of 69-year-old man with squamous cell carcinoma in the tongue.
Mapping of the Metastatic Lymph Nodes in the Neck
All patients underwent either unilateral or bilateral radical dissection of the neck. The surgeon mapped the excised nodes relative to the surrounding structures of the neck. The nodes were then processed for histologic examination. On the basis of the imaging-based classification proposed elsewhere (14), histologically confirmed metastatic nodes were categorized into 6 neck levels (I–VI). We did not distinguish separately the nodes on the left and right sides of the neck; for example, if a patient had a single metastatic node at level II on the left side of the neck and 2 metastatic nodes at level II on the other side, it was determined that he or she had 3 metastatic nodes at level II.
Statistical Analysis
The Mann-Whitney U test was used to evaluate the significance of the differences in tumor volumes between any 2 separate groups.
Results
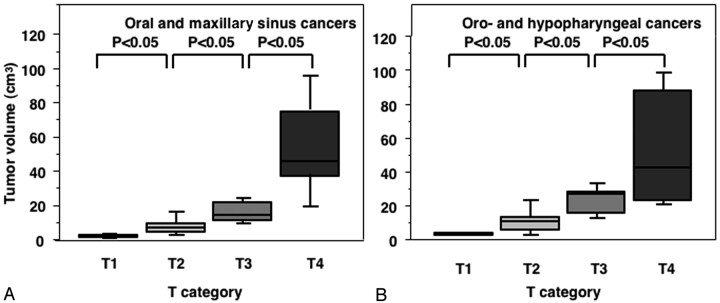
We found that the precision was 2.0% (range, 1.0–4.1%) among the 3 observers for the gadolinium-enhanced, fat-suppressed T1-weighted images. Figure 2 shows a good correlation between the T-category and MR image-based tumor volumes for the oral, maxillary sinus, oropharyngeal and hypopharyngeal cancers. The T4-category pharyngeal cancers exhibited a certain amount of overlap with the T3-category cancers for the 75th percentile box because the T4-category hypopharyngeal cancers contained relatively small tumors with cartilage involvement.
Fig 2.
Graph (box plots) shows primary tumor volumes of oral and maxillary sinus (A) and pharyngeal (B) cancers categorized at T1–T4. The horizontal line is a median (50th percentile) of the measured volumes, the top and bottom of the boxes represent 25th and 75th percentiles, respectively, and whiskers indicate the range from the largest to smallest observed data points within 1.5 interquartile range presented by the box. P, Mann-Whitney U test.
We summarized the results of the T-categories of the primary tumors and of the incidences of metastatic nodes in the necks of these patients (Table). The T4-category primary lesions were associated with an average of 4.0 metastatic nodes on average for the oropharyngeal and hypopharyngeal cancers and 2.7 for the oral and maxillary sinus cancers, whereas the T1- to T3-category primary lesions were associated with an average of 1.0 metastatic node on average for the oropharyngeal and hypopharyngeal cancers and 0.4 for the oral and maxillary sinus cancers.
Summary of 66 patients with oral, maxillary sinus, oropharyngeal, and hypopharyngeal cancers
| Patient No. | T1 | T2 | T3 | T4 | n | |
|---|---|---|---|---|---|---|
| Oropharynx | 15 | 2 | 6 | 4 | 3 | 9 |
| Hypopharynx | 9 | 0 | 3 | 3 | 3 | 6 |
| Maxillary sinus | 7 | 0 | 0 | 0 | 7 | 2 |
| Maxillary gingiva | 5 | 0 | 3 | 2 | 0 | 2 |
| Mandibular gingiva | 7 | 3 | 3 | 0 | 1 | 1 |
| Tongue | 13 | 1 | 9 | 3 | 0 | 2 |
| Oral floor | 10 | 3 | 4 | 2 | 1 | 3 |
| Total | 66 | 9 | 28 | 14 | 15 | 25 |
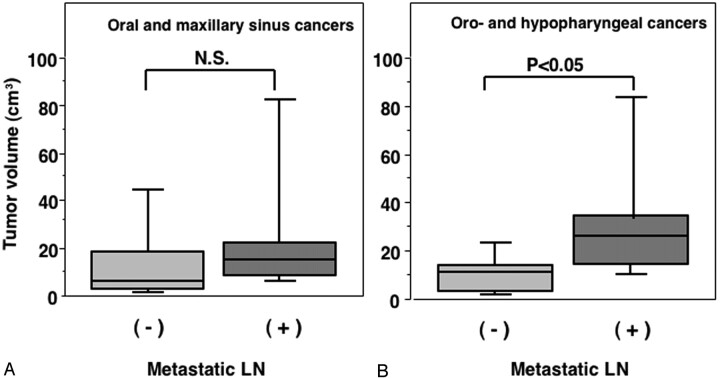
When comparing the tumor volumes between the patients with and without metastatic nodes, the volumes for the oropharyngeal and hypopharyngeal cancers were significantly greater for patients with metastatic nodes (33.2 ± 27.2 cm3) as compared with those without metastasis (10.5 ± 8.4 cm3); however, no significant correlation was observed between the tumor volume and nodal metastasis for patients with oral or maxillary sinus cancer, which was 26.8 ± 31.1 cm3 in those with metastasis and 15.5 ± 21.4 cm3 in those without metastasis (Fig 3).
Fig 3.
Graph (box plots) shows primary tumor volumes of oral and maxillary sinus (A) and pharyngeal (B) cancers in patients with (+) or without (−) metastatic lymph node in the neck. P, Mann-Whitney U test.
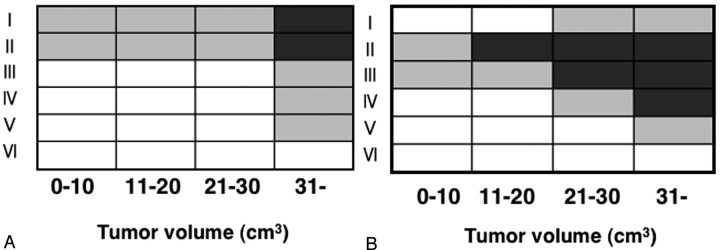
Finally, we assessed the metastatic node distributions in the neck relative to the primary tumor volumes and found that, for the oropharyngeal and hypopharyngeal cancer patients, the number of involved neck levels and neck levels with multiple metastases increased with the size of the primary tumor volume (Fig 4B). In this type of cancer, metastatic nodes only appeared at neck levels II and III when the tumor volume was equal to or less than 20 cm3. In cases of patients with larger tumor volumes, the condition was associated with metastatic nodes at the lower as well as the upper levels of the neck and was also associated with an increase in the number of neck levels with multiple metastases. In patients with oral and maxillary sinus cancer, however, the metastatic nodes were confined to levels I and II, and the incidence of multiple metastases at each neck level was <50% until the tumor volume reached 31 cm3 (Fig 4A).
Fig 4.
Matrix patterns show distributions relative to primary tumor volumes, of metastatic nodes at neck levels (I–VI) of patients with oral and maxillary sinus (A) or pharyngeal (B) cancer. The multiplicity of metastatic nodes at each level of the neck is expressed as >50% (dark box) or ≤50% (gray box) of patients with corresponding tumor size range (cm3) having multiple nodes at that neck level or as no patient having metastatic nodes at that level (white box).
Discussion
We have presented in this study that the volume of the primary lesion and the extent of nodal metastasis in the neck were well correlated in patients with oropharyngeal or hypopharyngeal cancer, but not in those with oral or maxillary sinus cancer. Furthermore, there was a stepwise increase in the number of nodal metastases and the number of the neck levels involved by metastases in the patients with oropharyngeal or hypopharyngeal cancer, but not in those with oral or maxillary sinus cancer. Collectively, these results imply that oral and maxillary sinus cancers and oropharyngeal and hypopharyngeal cancers may have distinct biologies, thereby exerting different behaviors during the development of lymph node metastasis.
The tumor volume was found to be the dominant factor when determining the local control of patients with head and neck cancer; multivariate analysis showed that the tumor volume was independently important and that the state of nodal metastasis, evaluated by the staging based on the absence or presence and extent of regional lymph node metastasis, was not independently significant (15). A more recent study that applied the multivariate analysis to a large cohort of pharyngeal and laryngeal cancer patients, however, showed that the burden of metastasis was an independent prognostic factor (16). Nodal metastasis was previously found to be a significant prognostic factor for patients with oral or oropharyngeal cancer (17), but not for patients with nasopharyngeal cancer (18). Although we did not test whether tumor volume and nodal metastasis are independently important as prognostic factors, these previous studies, in conjunction with the present report, suggest that the difference in the type of cancer or the difference in anatomic location of the primary lesion significantly affects the metastatic potential. Therefore, in some types of the head and neck cancers, nodal metastasis may occur independent of the tumor size and may significantly affect the patient’s prognosis.
At present, we do not know why the tumor volume is not well correlated with the extent of nodal metastasis for patients with oral or maxillary sinus cancer. One of the keys to this question may be the lymphangiogenesis around the tumor margin. It is not well understood whether the lymphatic-vessel attenuation is related to metastatic spread (19). In this context, Padera et al showed that the lymphatic vessels at the periphery rather than those within the tumor are responsible for lymph node metastasis (20). Furthermore, a significant correlation exists between the lymphatic vessel attenuation and lymph node metastasis for oropharyngeal cancers, but not for oral cancers (21). Taken together, these findings suggest a critical contribution of lymphangiogenesis to the nodal metastasis of cancer cells and further imply intrinsic or extrinsic differences in reactive lymphangiogenesis between cancers arising in the oral and maxillary sinus regions and those arising in the oropharyngeal and hypopharyngeal regions. Future development in imaging technology of lymphatic vessels might contribute to the prediction of metastasis from head and neck cancers.
The tumor size is one of the critical factors necessary for accurate tumor staging. Currently, the TNM classification is the most commonly used system for describing tumor size and regional lymph node metastasis (22). There are, however, limitations with respect to tumor staging that is based only on subjective criteria, particularly in defining the extent of tumor invasion into the deep portions, such as thyroid/cricoid cartilage, pterygoid process, and the deep portions of the tongue. CT and MR imaging are useful in evaluating tumor extensions into such structures. Tumor volume measurements on the basis of section images obtained by CT and MR imaging may further compensate for such limitations in tumor staging. Several pros and cons exist for tumor volume measurements by using CT or MR imaging (6, 7); for example, MR imaging was more effective than CT at identifying obliteration of the fascial spaces, invasion into the skull base, and lymph node metastasis. Although CT is effective in identifying submucosal diseases, the definition of tumor boundaries is often inaccurate because distinguishing edema and tumors from each other is difficult. MR imaging is not effective in differentiating edema from tumors; however, MR imaging is more sensitive than CT in distinguishing tumors (and peritumoral edema) from normal adjacent structures. A recent study of oropharyngeal and hypopharyngeal cancers showed that CT and MR imaging did not exhibit significant differences (5).
The measurement of tumor volume is, however, a laborious process that is performed by manually tracing the tumor outline and then calculating the tumor volume by using the summation-of-area technique. Therefore, inter-reader or intrareader errors are unavoidable. To overcome this, some investigators appraised the use of a semiautomated method, which provides a higher interobserver reliability and reduced interobserver error (22). Another group showed that MR imaging–based tumor volume measurements with tumor area extraction by using manual tracing resulted in a 13% mean percentage measurement error for primary lesions (7). We experienced interobserver error at levels lower (2%) than those of the preceding report.
It is plausible that manual tracing is error-prone if peritumoral edema is included as part of the tumor. Chong et al (22) showed that the average volumes obtained from semiautomated measurements were smaller than those obtained from manual tracing. This may result from the ability of the semiautomated method to measure a more detailed outline of the irregular tumor margin (23). Therefore, the peritumoral edema may significantly affect the results obtained from manual tracing.
Conclusion
The MR imaging–based tumor volume measurement proved to be clinically feasible. A good correlation was observed between tumor volume and lymph node metastasis in patients with oropharyngeal or hypopharyngeal cancer, but not in those with oral or maxillary sinus cancer.
References
- 1.Bundgaard T, Bentzen SM, Wildt J, et al. Histopathologic, stereologic, epidemiologic, and clinical parameters in the prognostic evaluation of squamous cell carcinoma of the oral cavity. Head Neck 1996;18:142–152 [DOI] [PubMed] [Google Scholar]
- 2.Mukherji SK, O’Brien SM, Gerstle RJ, et al. The ability of tumor volume to predict local control in surgically treated squamous cell carcinoma of the supraglottic larynx. Head Neck 2000;22:282–287 [DOI] [PubMed] [Google Scholar]
- 3.Nathu RM, Mancuso AA, Zhu TC, Mendenhall WM. The impact of primary tumor volume on local control of oropharyngeal squamous cell carcinoma treated with radiotherapy. Head Neck 2000;22:1–5 [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Chen MK, Liu MT, Wu HK. The effect of primary tumor volumes in advanced T-staged nasopharyngeal tumors. Head Neck 2002;24:940–946 [DOI] [PubMed] [Google Scholar]
- 5.Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004;233:93–100 [DOI] [PubMed] [Google Scholar]
- 6.Sakata K, Hareyama M, Tamakawa M, et al. Prognostic factors of nasopharynx tumors investigated by MR imaging and the value of MR imaging in the newly published TNM staging. Int J Radiat Oncol Biol Phys 1999;43:273–278 [DOI] [PubMed] [Google Scholar]
- 7.Gordon AR, Loevner LA, Shukla-Dave A, et al. Intraobserver variability in the MR determination of tumor volume in squamous cell carcinoma of the pharynx. AJNR Am J Neuroradiol 2004;25:1092–1098 [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson CR, Thames HD, Huang DT, Schmidt-Ullrich RK. The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys 1995;33:281–287 [DOI] [PubMed] [Google Scholar]
- 9.Wakisaka M, Mori H, Fuwa N, Matsumoto A. MR analysis of nasopharyngeal carcinoma: correlation of the pattern of tumor extent at the primary site with the distribution of metastasized cervical lymph nodes: preliminary results. Eur Radiol 2000;10:970–977 [DOI] [PubMed] [Google Scholar]
- 10.Sumi M, Izumi M, Yonetsu K, Nakamura T. Sublingual gland: MR features in normal and diseased states. AJR Am J Roentgenol 1999;172:717–722 [DOI] [PubMed] [Google Scholar]
- 11.Kimura Y, Sumi M, Sumi T, et al. Deep extension from carcinoma arising from the gingiva: CT and MR features. AJNR Am J Neuroradiol 2002;23:468–471 [PMC free article] [PubMed] [Google Scholar]
- 12.Shanmugaratnam K. Histological typing of tumours the upper respiratory tract and ear. 2nd ed. Berlin: Springer-Verlag;1991
- 13.Sobin LH, Wittekind C, eds. UICC TNM classification of malignant tumors. 6th ed. New York: Wiley;2002
- 14.Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol 2000;174:837–845 [DOI] [PubMed] [Google Scholar]
- 15.Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, et al. The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys 1995;32:635–641 [DOI] [PubMed] [Google Scholar]
- 16.Jakobsen J, Hansen O, Jorgensen KE, Bastholt L. Lymph node metastases from laryngeal and pharyngeal carcinomas: calculation of burden of metastasis and its impact on prognosis. Acta Oncol 1998;37:489–493 [DOI] [PubMed] [Google Scholar]
- 17.Wenzel S, Sagowski C, Kehrl W, Metternich FU. The prognostic impact of metastatic pattern of lymph nodes in patients with oral and oropharyngeal squamous cell carcinomas. Eur Arch Otorhinolaryngol 2004;261:270–275 [DOI] [PubMed] [Google Scholar]
- 18.Tang SG, See LC, Chen WC, et al. The effect of nodal status on determinants of initial treatment response and patterns of relapse-free survival in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2000;47:867–873 [DOI] [PubMed] [Google Scholar]
- 19.Stacker SA, Achen MG, Jussila L, et al. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002;2:573–583 [DOI] [PubMed] [Google Scholar]
- 20.Padera TP, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002;296:1883–1886 [DOI] [PubMed] [Google Scholar]
- 21.Beasley NJ, Prevo R, Banerji S, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 2002;62:1315–1320 [PubMed] [Google Scholar]
- 22.Chong VFH, Zhou JY, Khoo JBK, et al. Nasopharyngeal carcinoma tumor volume measurement. Radiology 2004;231:914–921 [DOI] [PubMed] [Google Scholar]
- 23.Joe BN, Fukui MB, Meltzer CC, et al. Brain tumor volume measurement: comparison of manual and semiautomated methods. Radiology 1999;212:811–816 [DOI] [PubMed] [Google Scholar]