Abstract
Summary: A 28-year-old man with long-standing right proptosis presented with an extensive multilobulated partially cystic orbital mass thought to be a lymphangioma. Because of concern that excision or debulking of the lesion was likely to be complicated by excessive bleeding, the lesion was injected with a mixture of ethiodized oil (Ethiodol) and cyanoacrylate glue under direct observation. The mixture caused the injected lobules to assume a firm, rubbery texture, allowing them to be excised without bleeding.
Orbital vascular malformations can be purely venous, purely arterial, or arteriovenous in nature. Another form of vascular malformation, a lymphangioma, is sometimes referred to as a “low- or no-flow malformation” (1, 2). Lymphangiomas are benign hamartomas that are generally diagnosed in early childhood. Although 20% of lymphangiomas involve the orbit and ocular adnexa (3–5), they account for less than 2% of orbital biopsies (3, 4).
Patients with orbital lymphangiomas may be asymptomatic, or they may experience symptoms such as pain, blurred vision, or double vision. Presenting signs include slowly progressive proptosis, vertical globe displacement, restriction of eye movements, and ptosis (5, 6). In addition, spontaneous hemorrhage into vascular channels can result in acute proptosis, compressive optic neuropathy, and loss of vision (3, 6–8).
Although pure lymphangiomas without significant vascular flow exist, many of these lesions are mixed vascular malformations, either continuous with the venous circulation or associated with an arteriovenous malformation (8–10). In addition, these lesions can be diffuse, well circumscribed, or multilobulated. The clinical characteristics of the lesions, their imaging appearance, and their anatomic features (particularly their relationship to the vascular supply of the orbit) may overlap those of other orbital vascular lesions (6, 8–12).
In imaging lymphangiomas, plain skull radiographs and venograms were routinely obtained before 1974 (13); however, CT and MR imaging have largely replaced venography because they provide superior tissue detail and some information regarding vascular flow without the potential complications of venography, such as vascular stasis, hemorrhage, and loss of vision (14–16). In addition, lymphangiomas do not enhance with gadolinium, suggesting that they are isolated from the systemic circulation. Thus, the risks of intralesional embolization are minimal compared with the low but significant risks of venography.
Because of the variable anatomic features of orbital lymphangiomas, their management is problematic. Observation is the preferred method of management for many of these lesions. Complete surgical resection of diffuse lesions is impossible (3, 6, 7, 9, 17), and even attempts at partial resection can result in significant bleeding and tissue injury, with loss of vision or double vision (18).
Alternatives to simple surgical resection include carbon dioxide laser ablation, injection of sclerosing agents, and irradiation of the lesion (19–23). Intralesional injection of a liquid polymer can facilitate surgery. Recently, navigation assistance for precise needle placement has proved to be a valuable adjunct to percutaneous sclerotherapy for the treatment of low-flow vascular malformations with difficult access and high risk of compromise of the draining veins (2).
We describe the use of a previously unreported mixture of 50% ethiodized oil (Cordis, Johnson & Johnson, Miami, FL) and 50% N-butyl-2-cyanoacrylate glue combined with surgical resection to treat a large complex orbital lymphangioma.
Case Report
A 28-year-old man presented to our institution with long-standing right proptosis. Fourteen years earlier, he had been diagnosed with a right orbital tumor after he developed significant proptosis of his right eye. The neuroimaging features of the lesion were thought to be consistent with a lymphangioma, and the patient was observed. With time, he experienced further proptosis and eventually underwent attempted removal of the lesion via a frontal craniotomy at another institution. Histologic examination of the tissue removed at the time of surgery revealed only fibrous tissue and skeletal muscle. Further surgery was discouraged given the degree of intraoperative bleeding encountered during the first surgery, and the patient was followed without intervention. During the next 4 years, his proptosis worsened, and he presented to our institution requesting a second opinion.
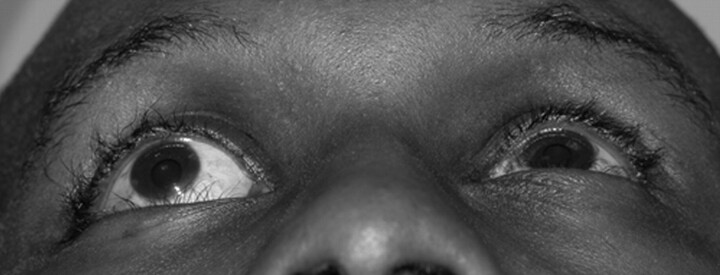
On examination, the patient’s visual acuity without correction was 20/20 in both eyes. Visual fields were full to confrontational testing. His pupils were equally round and reactive to light without a relative afferent pupillary defect. He was orthophoric in primary gaze, with minimal limitation of ductions in all directions on the right. External examination showed 13.5 mm of right proptosis, with marked superior displacement of the globe (Fig 1). Results of the remainder of the ophthalmologic examination, including dilated funduscopy, were normal in both eyes.
Fig 1.
Preoperative appearance of the patient demonstrates marked right proptosis and superior displacement of the globe. Also note enlarged right orbit compared with left orbit.
Imaging
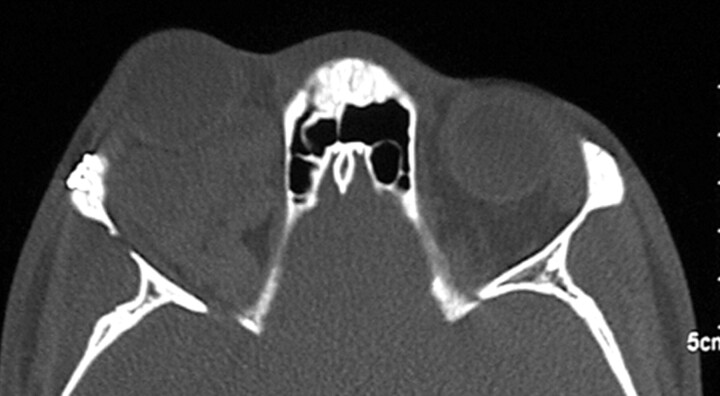
A CT scan showed a large, multilobulated, extra- and intraconal lesion surrounding the right optic nerve, with major components laterally and inferiorly. The bony orbit was enlarged, consistent with the chronicity of the lesion (Fig 2). Doppler sonography of the right orbit revealed a large predominantly echogenic mass with only minimal internal vascularity. MR imaging revealed a heterogeneous multilobulated mass with multiple fluid-fluid interfaces present within each lobule (Fig 3). Interestingly, there were also intracerebral findings consistent with an angiomatous malformation in the right hemisphere (not shown). Neither MR angiography nor CT angiography were performed because it was thought on the basis of the patient’s history and examination as well as the findings on CT scanning, MR imaging, and Doppler sonography, that the information they would provide would be unlikely to affect the patient’s treatment.
Fig 2.
Preoperative CT scan, axial view, bone window settings, demonstrates marked enlargement of the right orbit compared with the left orbit. Note large, multilobulated mass in the right orbit with thinning of lateral orbital wall.
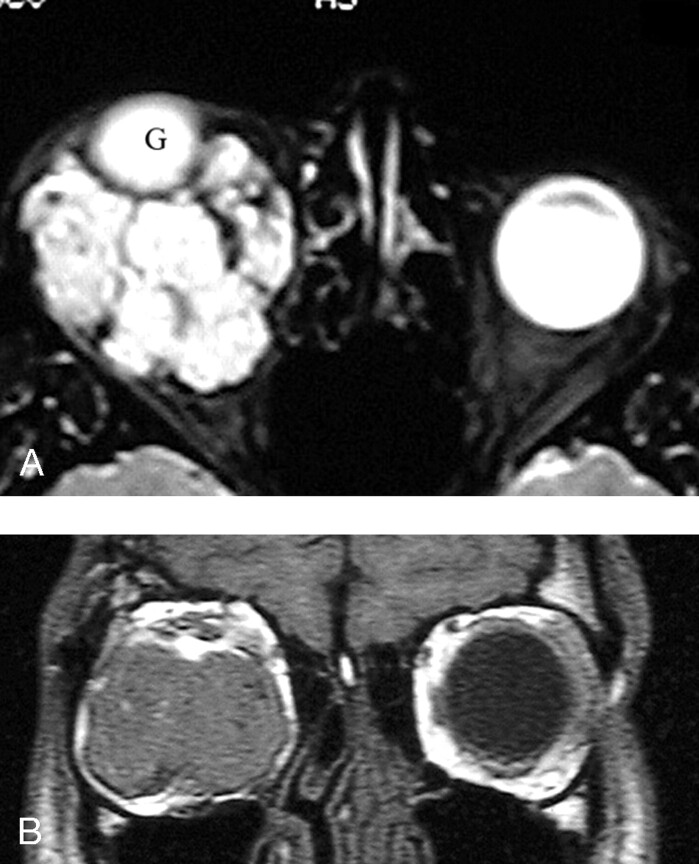
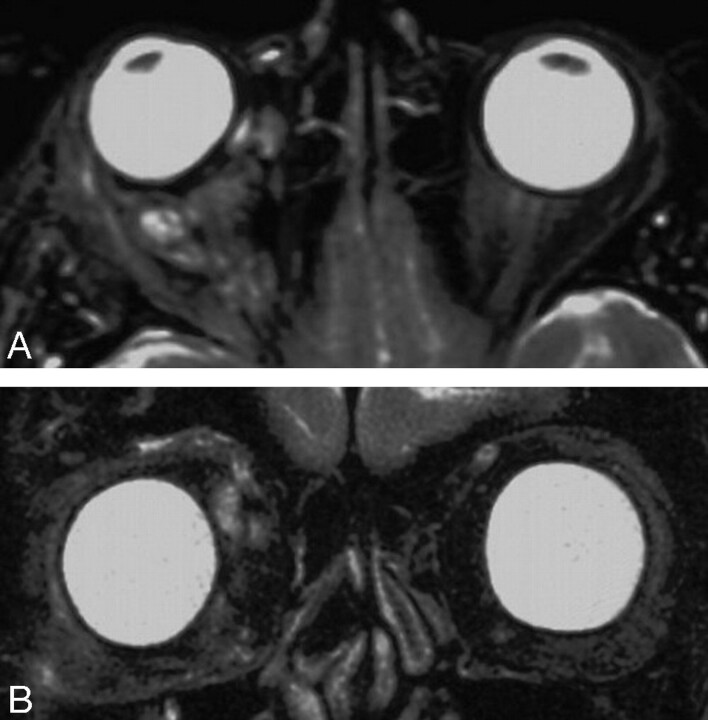
Fig 3.
Preoperative MR imaging. A, Axial MR image obtained at the level of the mid orbit shows a multilobulated mass surrounding and superiorly displacing the right globe (G). B, Coronal MR image obtained in the plane of the left globe shows a large lesion occupying most of the right orbit. The right globe is so proptosed that it is not seen on this image.
Surgical Technique
Because of the history of significant bleeding at the time of the patient’s previous surgery to remove the lesion and despite the imaging findings suggesting that the lesion was a low-flow malformation, we elected to combine excision of the lesion with intralesional injection of cyanoacrylate glue under direct visualization. The lesion was approached through a right lateral orbitotomy and an inferior forniceal transconjunctival incision. A large inferior lateral component of the lesion was identified and partially isolated from surrounding tissue, including the lateral rectus and inferior oblique muscles, which were directly visualized. The lobule was soft and compressible but immediately expanded after release of compression, suggesting a significant vascular component. Using C-arm fluoroscopy, the lobule was entered through direct puncture with a 21-gauge butterfly needle attached to a 1-mL syringe containing a mixture of 50% Ethiodol and 50% N-butyl-2-cyanoacrylate. The lesion was then injected with the solution under fluoroscopic visualization until some back pressure was felt in the syringe (Fig 4). A total of 0.6 mL was injected. After several minutes, the lesion developed a firm and rubbery consistency, and the needle was removed. A cryoprobe was used to manipulate this portion of the tumor, which was removed by using a tonsil snare with care to protect the adjacent lateral rectus and inferior oblique muscles. No significant bleeding was noted.
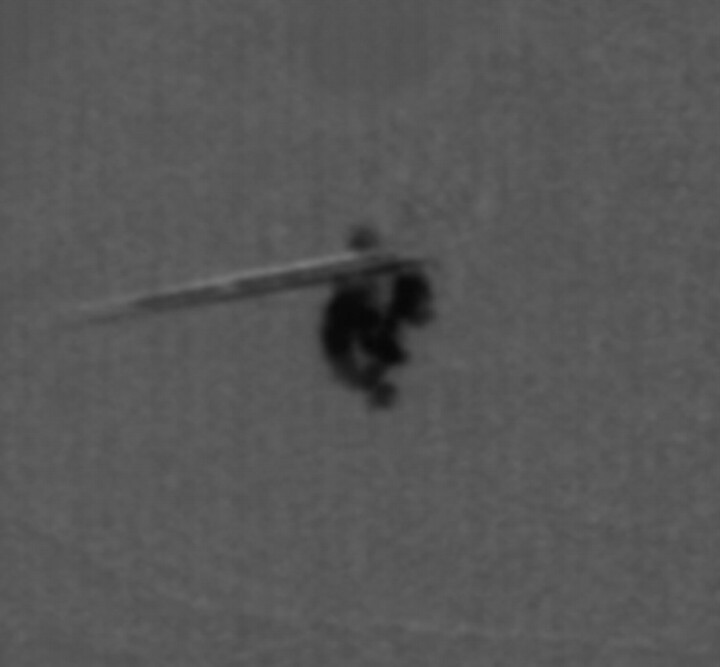
Fig 4.
C-arm fluoroscopy performed after injection of 50% Ethiodol combined with 50% N-butyl-2-cyanoacrylate glue demonstrates complete filling of the first segment of the lymphangioma.
Another lobule, more inferomedial, was exposed and injected with 1 mL of the mixture. After the glue hardened, the second portion of the tumor was excised without bleeding. Further dissection exposed the optic nerve, and the tumor was seen encircling the nerve. A third injection was attempted but was stopped prematurely because the glue appeared to be traveling toward the orbital apex rather than filling another portion of the tumor. The operation was terminated at this point, and the lateral orbital wall was not replaced.
Immediately following surgery, the patient’s vision remained 20/20 in the right eye, with a marked reduction in proptosis. The right pupil was slightly larger and more ovoid than the left pupil, but there was no relative afferent pupillary defect.
Follow-Up
Pathologic examination of the specimens revealed lymphoid tissue with large vascular channels. Some of the channels contained blood and some contained organized thrombi, extensive hemosiderin deposition, and trace amounts of fibrin. These findings were thought to be most consistent with lymphangioma.
One week after surgery, the patient continued to have 20/20 vision without an afferent pupillary defect. The right eye was slightly hypotrophic, with significant limitation of elevation, moderated limitation of depression and adduction, and mild limitation of abduction. Two months after surgery, the patient had only 4 mm of proptosis compared with 13.5 mm preoperatively (Fig 5). He had a slight right exotropia but could fuse with a right head turn. There was still moderate limitation of adduction and mild limitation of abduction, elevation, and depression. Repeat MR imaging showed residual tumor in the upper right quadrant of the orbit (Fig 6).
Fig 5.
Appearance of the patient 2 months postoperatively shows marked reduction in proptosis and displacement of globe.
Fig 6.
Postoperative MR imaging. A, Axial MR imaging at the level of the mid orbit obtained 2 months after surgery demonstrates significant reduction in the amount of the lesion. B, Coronal MR image, obtained in the same plane as Fig 3B, 2 months after surgery, shows that both globes are now seen and appear to be in the same relative position, indicating that the bulk of the mass has been resected.
Discussion
In 1999, members of the Orbital Society published a consensus statement on the classification of orbital vascular malformations, and lymphangiomas were classified as no-flow malformations. However, it can be difficult before surgery to ascertain the degree of vascularity of a particular lesion. Indeed, Lacey et al (20) emphasized that vascular malformations can be arterial, capillary, venous, lymphatic, or a combination of these elements Before the surgery, we thought it likely on the basis of the results of CT scanning, MR imaging, and Doppler sonography that the patient’s lesion was a low-flow or no-flow vascular malformation (ie, a lymphangioma). Nevertheless, we could not ignore the possibility that the lesion had a significant arterial component. Fortunately, both sclerotherapy and embolization combined with surgical resection have been used with equal success in both high-flow and low-flow vascular malformations. Thus, in choosing treatment options, we considered agents used for both types of lesions.
Arat et al (24) recommend a multidisciplinary approach to orbital venous malformations. These authors suggested that superficial lesions can be treated by carbon dioxide laser ablation or percutaneous alcohol sclerotherapy, whereas more extensive lesions may require embolization with detachable coils, surgery, or a combination of these techniques.
Burrows et al (19) recommend the use of sclerosing agents for the treatment of low-flow vascular malformations of the orbit. Indeed, it is stated by some authors such as Ernemann et al (2) that percutaneous sclerotherapy is the treatment of choice for such lesions, particularly when a surgical navigation device is used for needle placement. Sclerotherapy induces a chronic inflammatory state within the lesion and, thus, obliterates channel lumens. Substances used previously for sclerotherapy include ethanol, Ethibloc (a suspension of ethanol, zein protein, and contrast medium; Ethincon, Cornelia, GA), doxycycline, bleomycin, and OK-432 (Chugai Seiyaku, Tokyo, Japan) (a solution of group A Streptococci organisms in a suspension of penicillin) (19). Sclerosing agents delivered through a percutaneous route also have been used successfully in a few patients with conjunctival and maxillofacial lymphatic abnormalities as well as other nonorbital facial lymphangiomas (22, 23). However, previous studies involving orbital venous malformations have demonstrated that sclerosing agents can cause a compartment syndrome with potential loss of vision (19).
Another potential adjuvant to resection of orbital lymphangiomas is embolization with one of several polymer glues. In 1999, Lacey et al (20) examined 47 patients with venous malformations of the orbit. Of the 47 patients reviewed in this study, 30 (64%) had clinical or imaging evidence of distensibility. Fifteen (50%) of the 30 patients with distensible lesions underwent surgery. Of these, 6 underwent intraoperative direct-puncture venography with embolization by using a mixture of N-butyl-2-cyanoacrylate, Lipiodol (a chemical combination of 40% iodine in poppy seed oil; Andre Guerbet, Aulnay-sous-Bois, France), and tantalum powder to produce a radiopaque cast of the malformation. The direct intralesional injection of the cyanoacrylate glue mixture greatly facilitated the excision of distensible venous malformations of the orbit.
We extended the use of this technique to the treatment of our patient. Although his lymphangioma showed no significant flow on Doppler sonography or MR imaging, it was, in fact, noted to be distensible at the time of surgery. To minimize the significant potential to bleed during resection, we injected the lesion with a glue mixture. In contrast to the technique describe by Lacey et al (20), a different contrast agent, Ethiodol, was used.
Ethiodol was introduced in 1954 and has generally replaced Lipiodol as the contrast medium of choice. Lacey et al (20) used Lipiodol because it slows the polymerization of the glue mixture, and excessive polymerization may make resection difficult. Additionally, Lacey et al used tantalum powder to provide increased radiopacity in visualizing the lesion. Our Ethiodol mixture provided adequate radiopacity despite the lack of tantalum powder, and the glue mixture was not excessively hard. Thus, this glue mixture is a viable alternative to that used by Lacey et al. Our procedure also differed from that described by Lacey et al in that manual outflow obstruction was not used. The lesion was thought to be primarily lymphatic rather than venous, so the injection of glue was controlled simply by the force of pressure applied to the plunger of the syringe with careful monitoring by fluoroscopy.
The use of liquid polymers in combination with surgery in the treatment of orbital lymphangiomas has several advantages compared with surgery alone or surgery combined with a sclerosing agent. First, the use of glue aided intraoperative handling of the lesion by changing its consistency from soft and malleable to firm and stable. Second, this technique eliminated bleeding following scalpel incision compared with surgery alone. Third, sclerosing agents such as ethanol can diffuse into the surrounding tissue, causing significant swelling, transmural vessel necrosis, peripheral nerve injury, central nervous system depression, and a range of other side effects (19). Although injection of glue could potentially result in distal venous obstruction in lesions with a substantial venous component, the slow deliberate manual injection of glue combined with real-time fluoroscopic monitoring makes such a complication unlikely. In both techniques, the injection of a nonnative substance into the body can elicit an immune reaction.
Our case report supports the multidisciplinary approach to the management of low-flow orbital vascular malformations (12, 20, 24). This approach could also be used in the management of many other head and neck vascular malformations, in which resection of the lesion could be performed more safely when preceded by injection of a polymerizing glue. Finally, development of alternative glue mixtures may improve the dynamics of polymerization, thus providing even more intraoperative control and improved handling of the lesion.
References
- 1.Harris GJ. Orbital vascular malformations: a consensus statement on terminology and its clinical implications—Orbital Society. Am J Ophthalmol 1999;127:453–455 [DOI] [PubMed] [Google Scholar]
- 2.Ernemann U, Westendorff C, Troitzsch D, Hoffmann J. Navigation-assisted sclerotherapy of orbital venolymphatic malformation: a new guidance technique for percutaneous treatment of low-flow vascular malformations. AJNR Am J Neuroradiol 2004;25:1792–1795 [PMC free article] [PubMed] [Google Scholar]
- 3.Jones IS. Lymphangiomas of the ocular adnexa: an analysis of sixty-two cases. Am J Ophthalmol 1961;51:481–509 [DOI] [PubMed] [Google Scholar]
- 4.Brock ME, Smith RJ, Parey SE, Mobley DL. Lymphangioma: an otolaryngologic perspective. Int J Pediatr Otorhinolaryngol 1987;14:133–140 [DOI] [PubMed] [Google Scholar]
- 5.Krema H, Shields CL, Shields JA, Singh A. Bilateral multiple orbital lymphangiomas in a patient with systemic lymphangiomatosis. J Pediatr Ophthalmol Strabismus 2002;39:118–120 [DOI] [PubMed] [Google Scholar]
- 6.Harris GJ, Sakol PJ, Bonavolonta G, De Conciliis C. An analysis of thirty cases of orbital lymphangioma: pathophysiologic considerations and management recommendations. Ophthalmology 1990;97:1583–1592 [DOI] [PubMed] [Google Scholar]
- 7.Iliff WJ, Green WR. Orbital lymphangiomas. Ophthalmology 1979;86:914–929 [DOI] [PubMed] [Google Scholar]
- 8.Rootman J, Hay E, Graeb D, Miller R. Orbital-adnexal lymphangiomas: a spectrum of hemodynamically isolated vascular hamartomas. Ophthalmology 1986;93:1558–1570 [PubMed] [Google Scholar]
- 9.Wright JE. Orbital vascular anomalies. Trans Am Acad Ophthalmol Otolaryngol 1974;78:OP606–616 [PubMed] [Google Scholar]
- 10.Graeb DA, Rootman J, Robertson WD, Lapointe JS, Nugent RA, Hay EJ. Orbital lymphangiomas: clinical, radiologic, and pathologic characteristics. Radiology 1990;175:417–421 [DOI] [PubMed] [Google Scholar]
- 11.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:412–422 [DOI] [PubMed] [Google Scholar]
- 12.Garrity JA. Orbital venous anomalies: a long-standing dilemma. Ophthalmology 1997;104:903–904 [DOI] [PubMed] [Google Scholar]
- 13.Wright JE, Sullivan TJ, Garner A, Wulc AE, Moseley IF. Orbital venous anomalies. Ophthalmology 1997;104:905–913 [DOI] [PubMed] [Google Scholar]
- 14.Weiss RA, Haik BG, Saint-Louis LA, Ellsworth RM. Advanced diagnostic imaging techniques in ophthalmology. Adv Ophthalmic Plast Reconstr Surg 1987;6:207–263 [PubMed] [Google Scholar]
- 15.Safer JN, Guibor P. Ocular complications of orbital venography. Radiology 1975;114:647–648 [DOI] [PubMed] [Google Scholar]
- 16.Bond JB, Haik BG, Taveras JL, et al. Magnetic resonance imaging of orbital lymphangioma with and without gadolinium contrast enhancement. Ophthalmology 1992;99:1318–1324 [DOI] [PubMed] [Google Scholar]
- 17.Kennerdell JS, Maroon JC, Garrity JA, Abla AA. Surgical management of orbital lymphangioma with the carbon dioxide laser. Am J Ophthalmol 1986;102:308–314 [DOI] [PubMed] [Google Scholar]
- 18.Gurelik M, Ozum U, Erdogan H, Aslan A. Orbital lymphangioma and its association with intracranial venous angioma. Br J Neurosurg 2004;18:168–170 [DOI] [PubMed] [Google Scholar]
- 19.Burrows PE, Mason KP. Percutaneous treatment of low-flow vascular malformations. J Vasc Interv Radiol 2004;15:431–445 [DOI] [PubMed] [Google Scholar]
- 20.Lacey B, Rootman J, Marotta TR. Distensible venous malformations of the orbit: clinical and hemodynamic features and a new technique of management. Ophthalmology 1999;106:1197–1209 [DOI] [PubMed] [Google Scholar]
- 21.Tunc M, Sadri E, Char DH. Orbital lymphangioma: an analysis of 26 patients. Br J Ophthalmol 1999;83:76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrendt S, Bernsmeier H, Randzio G. Fractionated beta-irradiation of a conjunctival lymphangioma. Ophthalmologica 1991;203:161–163 [DOI] [PubMed] [Google Scholar]
- 23.Herbreteau D, Riche MC, Enjolras O, et al. Percutaneous embolization with Ethibloc of lymphatic cystic malformations with a review of the experience in 70 patients. Int Angiol 1993;12:34–39 [PubMed] [Google Scholar]
- 24.Arat YO, Mawad ME, Boniuk M. Orbital venous malformations: current multidisciplinary treatment approach. Arch Ophthalmol 2004;122:1151–1158 [DOI] [PubMed] [Google Scholar]