Abstract
BACKGROUND AND PURPOSE: It is desirable to develop a bedside method for assessing cerebral development in the very premature infant to monitor the effectiveness of interventions aimed at improving cerebral development. Our aim was to describe the growth trajectory of the corpus callosum (CC) on cranial sonography in very premature infants.
METHODS: We recruited 100 very-low-birth-weight infants admitted to a single regional level III neonatal intensive care unit from November 1998 to November 2000. Cranial sonography images of the CC were obtained for 64 (32 boys) infants (mean gestational age, 28 weeks; range, 23–33 weeks) in the first week of life and at term equivalency. The growth rate of the CC was compared in the 64 study infants to the expected growth rate of 0.20–0.27 mm/day from antenatal data and correlated with clinical outcome at 2 years of age by using Mental Development Index (MDI) and Psychomotor Development Index (PDI).
RESULTS: The average growth rate of the CC was half of that expected from antenatal data. Mean growth rates were similar for all gestational ages (mean, 0.11 mm/day; range, 0.05–0.29; P = .4). The CC at term equivalency was longer for those in MDI class 2 (mean, 44.3 mm) compared with MDI class 3 (mean 40.2 mm; P = .003) as well as for PDI class 2 versus 3 (P = .017).
CONCLUSION: Measurement of the length of the CC at cranial sonography is reproducible. Those with poorer neurodevelopmental outcomes have a shorter CC at term equivalency. The CC grows at a much lower rate postnatally than in utero among very premature infants.
Much current research activity is directed toward finding methods for protecting the premature brain from injury and optimizing normal cerebral structural development (1–4). The clinical manifestation resulting from brain injury or impaired development in the preterm infant is frequently not apparent until 2 years of age or older (5–7). Although MR has greater sensitivity than cranial sonography at detecting periventricular leukomalacia (PVL), particularly the noncystic form (8), the portability, ease of use, and lower cost of sonography mean that it is likely to be the preferred imaging technique in the early postnatal period for very premature infants. It is therefore desirable to develop a sonography method for assisting in the evaluation of cerebral structural development. This would assist in monitoring the effectiveness of any future neuroprotective strategies aimed at improving cerebral growth and development.
We chose to study the corpus callosum (CC) for several important reasons. The first is that it is readily visible at cranial sonography and has been shown to be a rapidly growing brain structure, with almost linear growth from 20 weeks to 40 weeks gestation (9, 10), thus enhancing our sensitivity to change in structural development. The second major reason for selecting the CC for investigation was that the CC is a major white matter pathway, and thinning of the CC is a recognized corollary of white matter injury (7, 11, 12). White matter injury or periventricular leukomalacia (PVL) is the major neuropathologic form of brain injury in the premature infant (13), particularly in those with a birth weight <1500 grams. Oligodendroglial cell precursors in the periventricular white matter seem to be very susceptible to injury (13), probably mediated by free radicals, leading to necrosis or apoptosis depending on the magnitude of the injury (14). The absolute size of CC in adolescents who were premature is smaller than controls but does not correlate well with neurodevelopmental outcome. We hypothesized that the rate of growth particularly in the early postnatal period may be more important than the absolute size of the CC. Thus, the aim of this study was to determine how CC growth could be measured at cranial sonography and to detect any trends in growth in very premature infants.
Methods
Subjects.
Subjects consisted of 100 very-low-birth-weight (VLBW) infants admitted to a single regional level III neonatal intensive care unit from November 1998 to November 2000 recruited into a longitudinal study of neuroimaging in the preterm infant. This comprised 91% of infants eligible for recruitment. Cranial sonogram was performed routinely on day 4–7 of life and then at 6 weeks of age. Other sonograms were performed for clinically indicated reasons, such as prolonged episodes of desaturation, sudden drop in hemoglobin level, and follow-up of intraventricular hemorrhage. A further cranial sonogram was performed at 40 weeks corrected gestational age, at the same time as a volumetric MR image. Of the 100 infants, we excluded 36 (18 boys) because the radiographs were missing (13), scans had not been performed at appropriate time (22), or the CC was inadequately imaged for measurement purposes (1). The study group comprised 64 (32 boys) subjects with a mean gestational age of 28 weeks (range, 23–33 weeks). The mean birth weight was 1086 grams (range, 630–1475 grams). There was no statistical difference in birth weight or gestational age between those included and those excluded from the study. Full neurologic and psychomotor assessment was performed for 55 infants (one later died, and 8 were lost to clinical follow-up). Each of these 55 subjects underwent mental and psychomotor assessment at 2 years of age. Mental Development Index (MDI) and Psychomotor Developmental Index (PDI) based on Bayley scoring (15) were assigned. The MDI evaluates memory, problem solving, and language. The PDI tests control of gross muscle groups and fine motor manipulations. MDI and PDI scores are grouped into classes based on SD of 15. Class 1 is >100, class 2 is 85–100, class 3 is 70–85, and class 4 is <70. Of the 55, 24 were male; steroids were administered antenatally to 47; 12 showed intrauterine growth retardation; 17 had premature rupture of membranes; 18 were from multiple births; and 19 were delivered by caesarean section. Postnatally, 33 needed intermittent positive pressure ventilation for as long as 62 days (mean, 7 days); 17 were still oxygen dependent at 36 weeks corrected gestational age; 19 were treated with indomethicin for patent ductus arteriosus; and one developed necrotizing enterocolitis. Ten control babies born at term were assessed by measuring the length of CC at cranial sonography. For controls, MDI and PDI scores were assessed at 2 years of age.
Measurement of the CC.
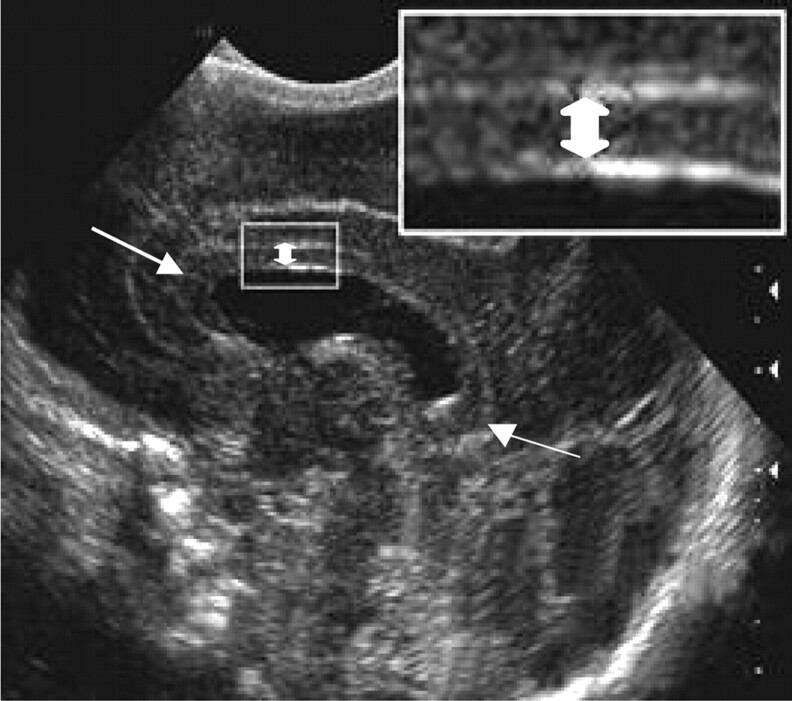
We obtained 2D measurements of the CC, because they do not require complex image processing and can be easily measured by using standard image viewing software. Only midline sagittal images of the CC were used in this study. All images were digitized by using a Vidar Diagnostic Pro Plus digitizer (Wise Healthcare Systems, Wiltshire, UK) at a resolution of 150 dots per inch in 12-bit gray-scale format. The length and height of the CC were measured at one third of the distance between the extreme margins of the genu and splenium (Fig 1). This point was selected rather than the splenium because we had found poor correlation between splenium depth at sonography and white matter volume assessed at MR in previous work (16). The digitized images were calibrated according to the scale on each image, and the CC length and height were measured by using ImageJ software (National Institutes of Health, Bethesda, MD).
Fig 1.
Sagittal midline cranial sonographic image shows the method for measuring height (double-headed arrow), and length (single-headed arrow) of the CC.
A subgroup of 16 infants was selected for initial study to determine the most useful method of measuring the CC. The 16 infants had a mean gestational age at birth of 27 weeks (range, 25–31 weeks). Each of the 16 infants had between 2 and 5 sonograms between birth and term equivalency. A total of 65 sonograms were assessed. In this sample of 16 infants, the length of CC correlated much better with gestational age (R = 0.782; P < .001) than the height of the CC (R = 0.062; P = .624). The CC length was therefore selected for measurement in the complete study group.
For the whole study group of 64 infants, CC length on the first postnatal scan was plotted against gestational age and compared with the published values of normal CC length obtained at endovaginal obstetric sonography (9, 10), calculated as the slope of the published data points (0.20–0.27 mm/day). The CC length was measured at sonograms obtained within 5 days of birth and within 7 days of term equivalency. The growth rate in mm/day was calculated by dividing the difference in length by the number of days between scans. Nine infants were born at 23–25 weeks, 35 at 26–29 weeks, and 20 at 30–33 weeks gestation. Growth rates in different age groups were compared by using analysis of variance. A second examiner measured length of CC on all sonograms of all infants (including the sample group of 16), from the digitized images on the PACS. Intraclass correlation coefficients and Bland-Altman plot (17) within SPSS version 12.0 (SPSS, Chicago, IL) software were used to assess intraobserver and interobserver variation. Statistical analysis was by Student t test and analysis of variance.
The regional ethics committee approved all aspects of this study, and informed consent was obtained from all parents.
Results
Measurement of the CC.
The most reproducible dimension of the CC to measure at cranial sonography was its length. For the intraobserver reliability, the intraclass correlation (ICC) was high—0.993 (95% confidence interval [CI] 0.988, 0.997)—which indicates good intraobserver reliability. Interobserver correlation was also high; mean agreement was −0.35 mm (SD = 0.96 mm). The 95% limits of agreement were −2.2 mm (95% CI −2.42, −2.06) to 1.5 mm (95%CI 1.36, 1.72). The difference between the 2 readers was 1 mm or less for 85 of the 93 measurements performed.
CC length was also a discriminating feature among premature infants of different ages. CC length correlated moderately well with gestational age at birth over the 23–33 weeks age range (R2 = 0.48). The slower the CC grew, the shorter it tended to be at term equivalency (R2 = 0.39; Fig 2). The length of CC at term equivalency had a mean of 41 mm (range, 37–47 mm; mode, 38 mm) for infants born at 23–25 weeks gestation, a mean of 42 mm (range, 37–47 mm; mode, 41 mm) for infants born at 26–29 weeks gestation, and a mean of 44 mm (range, 35–57 mm; mode, 42 mm) for infants born at 30–33 weeks gestation. The length of the CC at term equivalency was significantly greater for control babies (range, 44.4–49.9 mm; mean, 45.6 mm) than for infants born at 23–29 weeks (P = .003). The mean length of the CC at term equivalency was greater for those who had antenatal steroids (mean length 42.9 mm vs 40.1 mm; P = .02). There was no difference for any of the other antenatal or postnatal clinical variables.
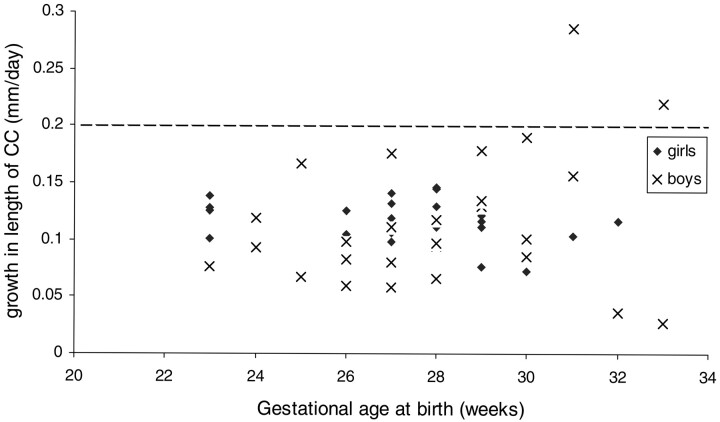
Fig 2.
Plot of postnatal growth rate of length of CC related to gestational age at birth in 64 very premature infants (32 boys) born at 23–33 weeks gestation. Dotted line represents the lower of 2 published growth rates of length of CC at prenatal sonography (13, 14). Growth rates are much lower (as much as 4-fold) in the very premature infant. Growth rate is from birth to term equivalency. The CC was measured by using cranial sonography.
Growth Rate of CC.
The average growth rate of the CC of 0.11–0.13 mm/day from birth to term equivalency was only half of that expected of 0.20–0.27 mm/day derived from published data on corpus callosal growth in utero (9, 10). The mean growth rate of the length of the CC was 0.11 mm/day (range, 0.07–0.17 mm/day; SD, 0.03 mm/day) for infants born at 23–25 weeks gestation, mean of 0.11 mm/day (range, 0.06–0.18 mm/day; SD, 0.03 mm/day) for those born at 26–29 weeks, and mean of 0.13 mm/day (range, 0.05–0.29 mm/day; SD, 0.07 mm/day) for those born at 30–33 weeks (Fig 3). There was much greater variance in growth rate among the 30–33-week group compared with the remainder (P < .001), though there was no statistical difference in mean values across these 3 age ranges (P = .4). Some of the infants >30 weeks gestation at birth had average growth rates approaching the expected normal range (0.20–0.27 mm/day; 9, 10). In all the rest, the growth rate was well below, sometimes as much as 4-fold lower (Fig 3). The growth rate of the CC was greater in those who had steroids antenatally (mean growth 0.15 mm/day vs 0.11 mm/day; P = .02) or who had intrauterine growth restrictions at birth (mean growth rate 0.14 mm/day vs 0.11 mm/day, P = .04). There was no association with sex, multiple birth, premature rupture of membranes, or mode of delivery. In the postnatal period, there was no association of growth rate of CC with oxygen dependency at 36 weeks corrected age or with indomethicin treatment.
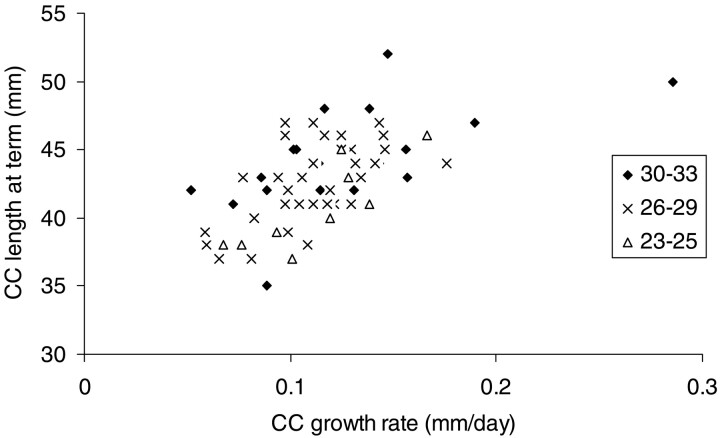
Fig 3.
Plot of postnatal growth rate of length of CC related to length of CC at term equivalency in 64 very premature infants born at 23–33 weeks gestation. Growth rate is from birth to term equivalency. The CC was measured at cranial sonography. The slower the CC grows, the shorter it tends to be at term equivalency (linear regression R2 = 0.39). Symbols correspond to n-week gestation.
Length of CC and Psychoneuromotor Outcome.
The mean length of CC at term equivalency was greater for those in MDI class 2 (mean, 44.3 mm; SD, 3.0 mm) compared with MDI class 3 (mean, 40.2 mm; SD, 2.6 mm; P = .003), and it was also greater for those in PDI class 2 (mean, 44.2 mm; SD, 3.1 mm) compared with PDI class 3 (mean, 41.7 mm; SD, 3.1 mm; P = .017). Gestational age at birth had no influence on these differences: mean gestational age for classes 2 and 3 for MDI and PDI were the same (mean, 27 weeks; P > .8).
CC Growth Rate and Psychoneuromotor Outcome.
There was no correlation between growth rate of the CC and MDI or PDI scores or classes for the study group as a whole or when analyzed by gestational age bands, yet the only infant in PDI class 4 (untestable because of the level of disability) had the lowest CC growth rate (0.05 mm/day).
Discussion
The principle finding in our prospective observational study of very premature infants is that the CC, on average, grows at only half the expected rate from birth to term equivalency. CC growth was in the normal range only in some of the boy infants >30 weeks gestational age, though very low growth rates were seen in all age groups. A second major finding was that those with poorer outcomes had a shorter CC at term equivalency, regardless of gestational age at birth.
Our study also demonstrated that, in the neonatal brain, the most reproducible dimension of the CC at cranial sonography was its length. Thinning of the CC is a feature of adolescents born prematurely, and thinning of the CC has been noticed as early as term equivalency on MR and sonography (7, 11, 12). The length of CC, though significantly shorter than in controls, does not correlate with white matter loss as strongly as cross-sectional measurements in adolescents (11). Our cross-sectional dimensions of the CC in premature infants were not sufficiently reproducible to be of use. We postulated that the length of the CC was likely to be affected by any reduction in white matter tracts during the preterm growth phase, because increasing bulk of CC in this time period is in part due to development of the posterior third of the body of the CC (auditory fibers) and the splenium (visual fibers) (11). Recent data from a study of adolescents suggests that poor verbal skills in boys born very prematurely are associated with poor development of the posterior CC (18).
Possible explanations for our finding of slowing in growth of the CC are, first, that we are detecting a normal phenomenon—namely, that the growth of the CC is slower after 26 weeks gestation or that there is a very wide range of normal. This would contradict evidence from prenatal sonography showing a consistent growth in length of the CC in vivo in the human fetus (9, 10) and laboratory studies based on animal and human brain data that have shown that the cellular growth of the forebrain, brain stem, and cerebellum are similar and logarithmic in the second half of pregnancy (19). Alternatively, it may be that the CC grows at a different rate ex utero than in utero.
Measurement error seems to be an unlikely explanation, because the intraobserver and interobserver variation were very low in our study. There are clear limitations in our 2D measures. Because we were trying to measure the growth of the CC, the most useful measure to have made would be the volume of the entire structure. This would be feasible if a tomographic imaging technique such as MR imaging were being used, but that is impractical, in light of the single-section nature of sonography studies. The next most useful measure, in light of the limitations of sonography, would be to measure the area of the CC in a midline sagittal scan. Various techniques for obtaining an accurate and reliable measure of this area were investigated. The most promising is the method of active contour modeling, or snakes, in which an energy-minimizing spline conforms to the region of interest by finding a stable solution to the balance of internal and external forces (20). We hope to apply this method in the future; however, because of the limitations in image acquisition we have to proceed with a more straightforward measure, which has the potential advantage of being more universally applicable in daily clinical practice.
A third possible explanation for our observation of the reduction in CC growth in preterm infants is that the changes in growth rate of the CC truly reflect growth and maturation of the white matter itself. Our findings support such an explanation but do not prove it. The CC length at term equivalency is related to its growth rate (Fig 2), in that the growth rate of the CC is lower in all babies born at <30 weeks gestation; there is a more restricted range of growth of CC in the babies born at 23–30 weeks than in those born at 30–33 weeks.
It is possible that the relative slowing of growth from birth in very premature infants reflects a less benign, ex utero environment with perinatal exposures and diffuse cerebral white matter injury reducing white matter growth. If so, then, from our data, it would appear that the CC in only some of the older premature infants overcomes the insults or deficits in this environment to reflect normal growth.
We have demonstrated that postnatal growth of the CC differs from growth in utero. Is the rate of growth of the CC in the postnatal period normal or abnormal in these very premature infants? We would need to compare the postnatal growth of the CC in preterm and full-term infants to decide this, though there is no obvious reason to think that normal brain growth should differ substantially between preterm and full-term infants. Our finding that those with poorer outcomes had a shorter CC at term equivalency is further evidence suggesting that lower growth of the CC postnatally is an abnormal finding, particularly for those born very early as the length of CC at birth is directly related to gestational age.
We found that antenatal steroid administration was associated with a slightly improved growth rate of CC and with a slightly longer CC at term equivalency. This suggests that antenatal steroids have a mildly beneficial effect on postnatal brain growth in very premature infants, at least up until term equivalency. This finding supports other work suggesting antenatal steroid use is associated with a reduction in intraventricular hemorrhage and improved neuromotor outcome (21, 22).
The disadvantages of our study are that we have not assessed growth of the CC in our control group of full-term infants, we have not established a method for assessing thinness of the CC, and we have not established whether the apparent reduction in growth is temporary or sustained throughout the infant period.
Our aim was to find an easily reproducible measurement of the CC at sonography. We have achieved that aim. We have also demonstrated that marked differences in growth of CC length occur among very premature infants. We have found that premature infants with poorer outcomes at 2 years of age have a shorter CC at term equivalency than those with better outcomes. We have not demonstrated any relationship between CC growth and neurodevelopmental outcome at 2 years of age. It is clear that the relationship between neuromotor outcome and brain growth is not a straightforward quantitative one. The next part of our research is to analyze the patterns of growth of CC in the weeks between birth and term equivalency to determine how early and at what stage we can detect reduced growth and whether there is any critical stage when corpus callosal growth affects outcome.
Not all premature infants with white matter injury develop neurodevelopmental problems, and those with appreciable CC thinning can compensate for early damage to the CC (23) or may be left with specific learning disabilities (24). Despite this, it is desirable to prevent or at least minimize white matter injury by developing effective neuroprotective strategies. These include magnesium sulfate (2, 4), developmental care of the preterm infant (25), selective hypothermia (or head cooling) (26), erythropoietin (1, 27) as well as more specific antioxidants (28), and neuropeptides (29). Measuring the length and rate of growth of the CC at cranial sonography may be a useful bedside technique for assessing early the effectiveness of these strategies.
Acknowledgments
Many thanks to Bobby Henderson, who analyzed the pilot group of 16 cases, and to Matthew Hankins, the biostatistician who performed statistical analysis of observer variation.
Footnotes
Supported in part by grants from the Neurological Foundation of New Zealand and the Health Research Council of New Zealand.
References
- 1.Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischaemic brain damage. Acta Paediatr Suppl 2002;91:36–42 [DOI] [PubMed] [Google Scholar]
- 2.Crowther CA, Hiller JE, Doyle LW, Haslam RR. Australasian Collaborative Trial of Magnesium Sulphate (ACTOMgSO4) Collaborative Group: effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA 2003;290:2669–2676 [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Cerebral white matter injury of the premature infant—more common than you think. Pediatrics 2003;112:176–180 [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook A. Challenge of conducting trials of neuroprotection in the asphyxiated term infant. Semin Perinatol 2003;27:320–332 [DOI] [PubMed] [Google Scholar]
- 5.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 2003;111:939–948 [DOI] [PubMed] [Google Scholar]
- 6.Pinto-Martin JA, Whitaker AH, Feldman JF, et al. Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Dev Med Child Neurol 1999;41:826–833 [DOI] [PubMed] [Google Scholar]
- 7.Stewart AL, Rifkin L, Amess PN, et al. Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet 1999;353:1653–1657 [DOI] [PubMed] [Google Scholar]
- 8.Miller SP, Scozzio, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol 2003;24:1661–1669 [PMC free article] [PubMed] [Google Scholar]
- 9.Malinger G, Zakut H. The corpus callosum: normal fetal development as shown by transvaginal sonography. AJR Am J Roentgenol 1993;161:1041–1043 [DOI] [PubMed] [Google Scholar]
- 10.Achiron R, Achiron A. Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study. Ultrasound Obstet Gynecol 2001;18:343–347 [DOI] [PubMed] [Google Scholar]
- 11.Cooke RW, Abernathy LJ. Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child Fetal Neonatal Ed 1999;81:F116–F121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santhouse AM, Ffytche DH, Howard RJ, et al. The functional significance of perinatal corpus callosum damage: an fMRI study in young adults. Brain 2002;125:1782–1792 [DOI] [PubMed] [Google Scholar]
- 13.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 2001;50:553–562 [DOI] [PubMed] [Google Scholar]
- 14.Johnston MV, Trescher WH, Ishidia Nakajima W. Neurobiology of hypoxic–ischemic injury in the developing brain. Pediatr Res 2001;49:735–741 [DOI] [PubMed] [Google Scholar]
- 15.Bayley N. Bayley scales of infant development. 2nd ed. San Antonio, TX: Psychological Corporation;1993
- 16.Anderson NG, Warfield SK, Wells S, et al. A limited range of measures of 2-D ultrasound correlate with 3-D MRI cerebral volumes in the premature infant at term. Ultrasound Med Biol 2004;30:11–18 [DOI] [PubMed] [Google Scholar]
- 17.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310 [PubMed] [Google Scholar]
- 18.Nosarti C, Rushe TM, Woodruff PW, et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 2004;127:2080–2089 [DOI] [PubMed] [Google Scholar]
- 19.Dobbing J. The later development of the brain and its vulnerability. In: Davis JA, Dobbing J, eds. Scientific foundations of paediatrics. 2nd ed. London: Heinemann Medical;1981;744–759
- 20.Pham D, Xu C, Prince J. Current methods in medical imaging segmentation. Annu Rev Biomed Eng 2000;2:315–337 [DOI] [PubMed] [Google Scholar]
- 21.Leviton A, Dammann O, Allred EN, et al. Antenatal corticosteroids and cranial ultrasonographic abnormalities. Am J Obstet Gynecol 1999;181:1007–1017 [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R, Chiswick ML, Rimmer S, et al. Antenatal steroids are associated with a reduction in the incidence of cerebral white matter lesions in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2002;86:F96–F101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercuri E, Jongmans M, Henderson S, et al. Evaluation of the corpus callosum in clumsy children born prematurely: a functional and morphological study. Neuropediatrics 1996;27:317–322 [DOI] [PubMed] [Google Scholar]
- 24.Davatzikos C, Barzi A, Lawrie J, et al. Correlation of corpus callosum morphometry with cognitive and motor function in periventricular leukomalacia. Neuropediatrics 2003;34:247–252 [DOI] [PubMed] [Google Scholar]
- 25.Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics 2004;113:846–857 [DOI] [PubMed] [Google Scholar]
- 26.Tooley JR, Eagle RC, Satas S, Thoresen M. Significant head cooling can be achieved while maintaining normothermia in the newborn piglet. Arch Dis Child Fetal Neonatal Ed 2005;90:F262–F266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strunk T, Hartel C, Schultz C. Does erythropoietin protect the preterm brain? Arch Dis Child Fetal Neonatal Ed 2004;89:F364–F366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Follett PL, Deng W, Dai W, et al. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci 2004;24:4412–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gressens P, Besse L, Robberecht P, et al. Neuroprotection of the developing brain by systemic administration of vasoactive intestinal peptide derivatives. J Pharmacol Exp Ther 1999;288:1207–1213 [PubMed] [Google Scholar]