Abstract
BACKGROUND: Endovascular therapy (ET) of internal carotid artery (ICA) stenosis is equivalent to carotid endarterectomy for stroke prevention; however, patients with ICA occlusion and acute symptoms are traditionally not candidates for ET. We report our experience in endovascular recanalization of acute stroke patients with ICA occlusion.
PATIENTS AND TECHNIQUES: We reviewed our registry for acute stroke patients treated with ET who had (1) ICA occlusion by digital subtraction angiography (thrombolysis in myocardial ischemia=0) with location of type II (above ophthalmic artery involving M1 or A1 but not both) or type III (proximal to the ophthalmic artery but distal to the bifurcation); (2) acute stroke symptoms from the index lesion presenting 3 hours after onset of symptoms; (3) minimal ischemic changes on brain CT scan (less than one third of the MCA territory); (4) attempted ET. Neuroradiologists reviewed angiograms for thrombolysis in cerebral infarction. A blinded vascular neurologist reviewed postprocedural brain imaging for Alberta Stroke Program Early CT (ASPECT) scoring. Outcome scales were assessed.
RESULTS: We identified 14 patients, 10 of whom were men (mean age, 58 ± 14 years; median age, 54 years; age range, 40–74 years). There were 8 left ICA occlusions, 3 type II; and 6 right ICA occlusions, one type II. Median baseline National Institutes of Health Stroke Scale score was 17 (range, 11–25; mean, 18 ± 4.9). Mean time to ET was 389 ± 103 minutes (median, 306 minutes; range, 197–1290 minutes). Immediate recanalization occurred in 64%. Decrease in expected stroke volume by brain imaging occurred in 50% with mean ASPECT score of 4 ± 2.9 (median, 3; range, 0–8; 21% ≥ 8). Two hemorrhages occurred, one symptomatic; 3 patients died. Good outcome was achieved in 64% of cases.
CONCLUSION: Endovascular therapy of carotid occlusion in hyperacute stroke patients is feasible and may help to reduce stroke volume and increase good outcome in some patients.
Acute ischemic stroke resulting from cardioembolic or atherothrombotic occlusion of the internal carotid artery (ICA) results in high rates of morbidity and mortality. Of patients with acute ICA occlusion presenting with severe neurologic deficits, 16%–55% will die, 40%–60% will have severe neurologic disability, and only 2%–12% will have good functional recovery (1). The addition of intravenous recombinant tissue plasminogen activator (rtPA) treatment may improve outcome (2), but only 8% of patients with ICA occlusion receiving this treatment have shown early recanalization (3, 4).
Alternative treatment for these patients includes both surgical thrombectomy and endovascular thrombolytic and/or mechanical revascularization. In the case of atherothrombotic ICA occlusion, additional therapy consisting of emergency carotid endarterectomy (CEA) with distal thrombectomy or endovascular angioplasty with or without stent placement may be necessary to decrease underlying chronic vessel stenosis. Although cases of emergency CEA have been reported as technically successful, the clinical benefit is controversial (1, 5–7). Nonemergent endovascular angioplasty and stent placement of ICA stenosis has been reported to be technically feasible and equivalent to CEA for stroke prevention (8–11). Patients with ICA occlusion and acute symptoms, however, are traditionally not candidates for this intervention. Here we report a retrospective study to investigate feasibility and outcome of endovascular recanalization in acute stroke patients with ICA occlusion at our institution.
Patients and Techniques
From November 1996 to January 2005, 156 acute stroke patients underwent CEA for possible intra-arterial (AA) thrombolysis. Vascular neurologists and fellows comprising a veteran stroke team at a university-based, tertiary care center, in conjunction with an interventional neuroradiologist, determined patient eligibility. On admission, the vascular neurologist assessed neurologic status by using the National Institutes of Health Stroke Scale (NIHSS). A cerebral CT scan was performed in all cases before treatment with thrombolytics. IA thrombolysis was considered according to an institutional review board–approved protocol after obtaining informed consent from a responsible family member. Per protocol, patients considered for IA thrombolytics met the following criteria: (1) presentation after 3 hours from symptom onset; (2) minimal ischemic changes on brain CT scan (less than one third of the MCA territory); (3) disabling neurologic deficit (NIHHS ≥ 12); (4) clinical suspicion or evidence on transcranial Doppler sonography of large vessel arterial occlusion or stenosis; and (5) no evidence of intracranial hemorrhage. Per protocol, IA therapy was also considered in selected patients who received intravenous tissue plasminogen activator within 3 hours of symptom onset, and also met criteria.
The subset of IA patients who met the following criteria were included in this analysis: (1) ICA occlusion by digital subtraction angiography (thrombolysis in myocardial ischemia [TIMI]=0) with location of type II (above ophthalmic artery involving M1 or A1, but not both) or type III (proximal to the ophthalmic artery but distal to the bifurcation); (2) acute stroke symptoms from the index lesion of either cardioembolic or atherothrombotic cause; and (3) attempted endovascular therapy (ET).
The treatment team consisted of interventional neuroradiologists, an interventional neurologist, and the treating vascular neurologist working in various combinations with at least 2 physicians present making consensus treatment decisions. Patients who met standard criteria for IV rtPA were treated with 0.9 mg/kg IV rtPA. ET was performed if patients presented after 3 hours from symptom onset or had rapid symptom worsening despite IV rtPA.
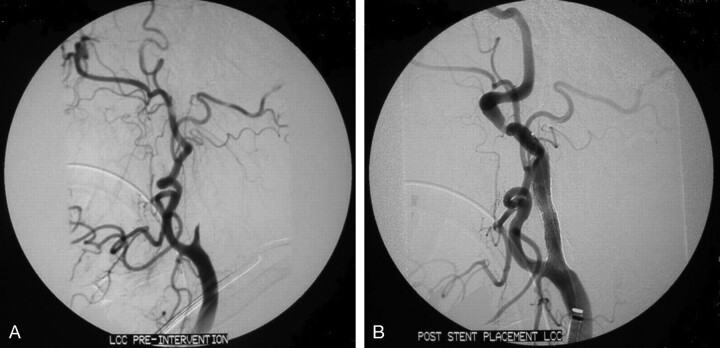
All patients received similar initial endovascular treatment regardless of stroke etiology. Diagnostic cerebral angiography was performed via a femoral artery approach. After initial diagnostic angiography, patients were anticoagulated with IA heparin (1000–2000 U) if they had not received IV rtPA. A 6F guide catheter was placed proximal to the occlusion site. A microcatheter and microguidewire were advanced through the guide catheter and navigated to the occluded vessel segment in proximity to the thrombus. The microcatheter tip was imbedded into the thrombus for thrombolytic infusion. Reteplase was manually infused by slow hand injection at an approximate rate of 0.1 U aliquots diluted in 1 mL normal saline for 1–2 minutes. Urokinase and rtPA were injected in the same manner at a rate of 50,000–200,000 U and 1–4 mg given at 10-minute intervals. Control angiography was performed approximately every 10 minutes to evaluate the status of recanalization. Thrombolytics were administered until recanalization was obtained, 6 hours after the onset of symptoms, or maximum dose limits were achieved (reteplase, 6U; urokinase, 2,000,000 U; rtPA, 45 mg). Aggressive mechanical clot disruption defined as the use of at least one of the following interventional techniques: (1) aggressive microcatheter/microwire clot maceration; (2) percutaneous angioplasty; (3) stent deployment; or (4) use of a snare device was permitted (12). In addition, following thrombolytic administration in those patients with suspected atherothrombotic etiology of stroke, balloon angioplasty with stent placement was performed for flow-limiting ICA stenotic lesions of ≥90% (Fig 1). ICA stent placement was performed by using S.M.A.R.T. stents (Cordis Endovascular, Miami Lakes, FL) or self-expandable Easy Wall-Stents (Boston Scientific, Natick, MA) sized to match the largest vessel diameter in the anticipated deployment area.
Fig 1.
Digital subtraction angiography of a 57-year-old treated with standard dose IV rtPA, IA reteplase, angioplasty, and stent placement with resulting TICI of 2c.
Angiograms were analyzed by either an interventional neurologist or interventional neuroradiologist. For the purpose of this study, we used a thrombolysis in cerebral ischemia (TICI) scale based on the modified TIMI criteria to define cerebral perfusion. Immediate recanalization was defined as TICI grades 2 or 3 achieved immediately after intervention.
All patients were admitted to the neurology/neurosurgery intensive care unit or stroke unit, where their care was managed by the University of Texas—Houston Stroke Treatment Team. All patients received aspirin 325 mg and clopidigrel 75 mg (Bristol-Myers Squibb, New York, NY) daily beginning after review of the 24-hour cerebral CT for evidence of hemorrhage. No patients were treated with additional anticoagulation or GpIIb/IIIa inhibitors. The standard guidelines of blood pressure management for IV rtPA therapy were followed pre- and postprocedure. If successful recanalization was achieved (TICI ≥ 2), a systolic blood pressure goal of <160 was targeted. Repeat cerebral CT scan was performed at 24 hours after IA thrombolysis and at any time when the patient experienced neurologic deterioration (increase in NIHSS ≥ 2). A blinded vascular neurologist reviewed all postprocedural CTs and provided Alberta Stroke Program Early CT Score (ASPECT) grading (13). Decrease in expected stroke volume by brain imaging was defined as ASPECT score ≥4. Hemorrhagic transformation (HT) was defined as areas of increased attenuation on nonenhanced brain CT scans. Symptomatic hemorrhage was defined as ≥2-point increase in NIHSS attributable to HT by the vascular neurologist. Parenchymal hematoma 2 (PH-2) was defined as an intracerebral hematoma that involved >30% of the infarcted area with substantial mass effect. NIHSS and MR spectroscopy were performed at day 7. Favorable outcome was defined as discharge home or to inpatient rehabilitation (MR spectroscopy ≤ 3).
STATA (College Station, TX) statistical software was used for statistical analysis. All values are presented as mean ± SD or median values.
Results
In this study, 156 patients with acute ischemic stroke underwent emergent cerebral angiography for possible IA thrombolysis treatment, and 14 of them qualified for this analysis. Mean age was 58 ± 14 years (median, 54 years; range, 40–74 years). There were 10 men. Eight experienced left ICA occlusions (3 type II and 5 type III), and 6 had right ICA occlusions (one type II and 5 type III). Median baseline NIHSS was 17 (range, 11–25; mean, 18 ± 4.9; Table). Stroke etiology was 2 cardioembolic, 11 atherothrombotic, and a single unspecified case.
Demographics, endovascular treatments, and outcomes of treatment group
| Demographics | |
|---|---|
| Gender | 10 men, 4 women |
| Mean age (ys) ± SD | 58 ± 14 |
| Median baseline NIHSS (range) | 17 (11–25) |
| Mean time (min) from stroke onset to endovascular therapy ±SD | 389 ± 103 |
| Thrombolytics | 9 IV/IA, 3 IA, 1 IV, 1 none |
| Mean total IA dose ± SD | Reteplase (n=8): 3 ± 1.2 mg (median 3 mg, range 1–4.9 mg) |
| Urokinase (n=2): 250,000–650,000 U | |
| rtPA (n=2): 4–19 mg | |
| Occlusion location | 8 left, 6 right |
| Occlusion type | 4 type II, 10 type III |
| Adjunctive therapy | 2 simple mechanical, 5 angioplasty, 6 angioplasty/stenting |
| Immediate recanalization (TICI ≥ 2) | 64% |
| Decrease in expected infarct volume | 50% |
| ASPECT scores | Mean 4 ± 2.9, median 3, range 0–8, 21% ≥ 8 |
| Symptomatic hemorrhage | 7% |
| Mortality | 21% |
| Favorable outcome (discharge home or inpatient rehabilitation) | 64% |
Note.—NIHSS indicates National Institutes of Health Stroke Scale; IA, intraarterial; IV, intravenous; TICI, thrombolysis in cerebral infarction; ASPECT, Alberta Stroke Program Early CT.
Thrombolytic regimens were as follows: 9 combined IV rtPA/IA thrombolytics, 3 only IA thrombolytics, one only IV thrombolytics (+ mechanical intervention), and one only mechanical intervention. Mean time from symptom onset to IV thrombolytic administration (n=10) was 134 ± 27 minutes (median, 119 minutes; range, 95–188 minutes). Of the 12 who received intra-arterial thrombolytics, 2 received urokinase (250,000–650,000 U), 8 received reteplase (mean dose, 3 mg ± 1.2; median, 3; range, 1–4.9), and 2 received rtPA (4–19 mg). Thirteen patients underwent mechanical intervention (2 simple mechanical, 5 angioplasty, and 6 angioplasty/stent placement). Mean time to initiation of endovascular therapy was 389 ± 103 minutes (median, 306 minutes; range, 197–1290 minutes).
Immediate recanalization occurred in 64% of cases (cardioembolic, 0%; atherothrombotic, 64%; P=.001). Two hemorrhages occurred, both atherothrombotic etiology, one asymptomatic and one both symptomatic and PH-2 (IV lytics/mechanical intervention only). Reduction in expected stroke volume by brain imaging occurred in 50% of cases (50% cardioembolic; 55% atherothrombotic; P = .89) with median ASPECT score of 3 (range, 0–8; mean, 4 ± 2.9; 21% ≥ 8). Twenty-four-hour median NIHSS was 20 (range, 9–32; mean, 19 ± 9). Three patients died, 2 from massive cerebral edema (nonrecanalized) and another from hemorrhagic transformation (IV lytics and mechanical intervention). Of the survivors, 7-day median NIHSS was 13 (mean, 14 ± 7; range, 2–23) and median 7-day MR spectroscopy was 3.5 (mean, 3.3 ± 0.8; range, 2–4; 18% ≤ 2). Good outcomes were achieved in 64% of the patients (50% cardioembolic; 55% atherothrombotic; P=.89) with disposition of 14% home and 50% to inpatient rehabilitation centers.
Discussion
Our study showed that emergency endovascular recanalization is technically feasible in patients with acute stroke due to internal carotid artery occlusion. In addition, this intervention may also improve survival and reduce morbidity by restoration of blood flow to penumbral areas and decreasing stroke volume.
Case reports of endovascular treatment of internal carotid artery occlusion also confirm the feasibility of this technique. These studies suggest that intra-arterial thrombolysis may increase recanalization rates to 37%–100% and clinical improvement to 53%–94% without significant increase in hemorrhagic transformation over intravenous therapy alone (14–21). With a recanalization rate of 64%, good outcomes in 64% of cases, and a hemorrhage rate of 7%, our results are similar to those previously reported. These studies, however, are difficult to compare because they vary in techniques used and thrombolytic type and dosing.
Our endovascular approach to treatment of carotid occlusion is similar despite stroke etiology. All patients are initially treated with thrombolytic and simple mechanical intervention. If this is unsuccessful in artery recanalization, we proceed with aggressive mechanical clot disruption. In those patients with atherothrombotic stroke etiology and a severe (>90%) stenosis, we also perform angioplasty and stent placement procedures. Additional treatment is usually necessary with atherothrombotic lesions, because these vessels tend to reocclude without this intervention. We found few significant differences in outcome between the atherothrombotic and cardioembolic subgroups. A larger patient population, however, would be needed to draw conclusions adequately.
Revascularization of carotid occlusion in the acute stroke patient is still controversial and currently is not the standard of care. The potential benefits of recanalization must be balanced against the risks of reperfusion hemorrhage, dissection, and distal embolism. These risks can be minimized by limiting the time from onset to revascularization to limit reperfusion injury, by using experienced operators to reduce the risk of dissection, and by using devices to prevent distal embolization. In addition, careful patient selection is critical. Neuroimaging techniques such as perfusion-weighted/diffusion-weighted MRI and CT angiography may also help in identifying patients who would benefit most from this intervention (22, 23).
In conclusion, endovascular therapy of carotid occlusion in hyperacute stroke patients is feasible and may help to decrease stroke volume and improve outcome in some patients. Whether such intervention is better than thrombolytic therapy alone should be studied in a prospective randomized controlled trial.
Footnotes
R.M.S. and H.M.S. are supported by National Institutes of Health training grant T32NSO7412 to the University of Texas–Houston Medical School Stroke Program.
References
- 1.Meyer FB, Sundt TM Jr, Piepgras DG, et al. Emergency carotid endarterectomy for patients with acute carotid occlusion and profound neurological deficits. Ann Surg 1986;203:82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke: the national institute of neurological disorders and stroke rt-PA stroke study group. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 3.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86 [DOI] [PubMed] [Google Scholar]
- 4.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator: the rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol 1993;14:3–13 [PMC free article] [PubMed] [Google Scholar]
- 5.Kasper GC, Wladis AR, Lohr JM, et al. Carotid thromboendarterectomy for recent total occlusion of the internal carotid artery. J Vasc Surg 2001;33:242–249; discussion 249–250 [DOI] [PubMed] [Google Scholar]
- 6.Sbarigia E, Toni D, Speziale F, et al. Emergency and early carotid endarterectomy in patients with acute ischemic stroke selected with a predefined protocol: a prospective pilot study. Int Angiol 2003;22:426–430 [PubMed] [Google Scholar]
- 7.Brandl R, Brauer RB, Maurer PC. Urgent carotid endarterectomy for stroke in evolution. Vasa 2001;30:115–121 [DOI] [PubMed] [Google Scholar]
- 8.Endovascular versus surgical treatment in patients with carotid stenosis in the carotid and vertebral artery transluminal angioplasty study (cavatas): a randomised trial. Lancet 2001;357:1729–1737 [PubMed] [Google Scholar]
- 9.Carotid revascularization using endarterectomy or stenting systems (caress): phase I clinical trial. J Endovasc Ther 2003;10:1021–1030 [DOI] [PubMed] [Google Scholar]
- 10.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–1501 [DOI] [PubMed] [Google Scholar]
- 11.Brott TG, Brown RD Jr, Meyer FB, et al. Carotid revascularization for prevention of stroke: carotid endarterectomy and carotid artery stenting. Mayo Clin Proc 2004;79:1197–1208 [DOI] [PubMed] [Google Scholar]
- 12.Noser EA, Shaltoni HM, Hall CE, et al. Aggressive mechanical clot disruption: a safe adjunct to thrombolytic therapy in acute stroke? Stroke 2005;36:292–296 [DOI] [PubMed] [Google Scholar]
- 13.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22:1534–1542 [PMC free article] [PubMed] [Google Scholar]
- 14.Nesbit GM, Clark WM, O’Neill OR, Barnwell SL. Intracranial intraarterial thrombolysis facilitated by microcatheter navigation through an occluded cervical internal carotid artery. J Neurosurg 1996;84:387–392 [DOI] [PubMed] [Google Scholar]
- 15.Endo S, Kuwayama N, Hirashima Y, et al. Results of urgent thrombolysis in patients with major stroke and atherothrombotic occlusion of the cervical internal carotid artery. AJNR Am J Neuroradiol 1998;19:1169–1175 [PMC free article] [PubMed] [Google Scholar]
- 16.Du Mesnil De Rochemont R, Sitzer M, Neumann-Haefelin T, et al. Endovascular recanalization of acute atherothrombotic carotid artery occlusion holds up progressive stroke. Neuroradiology 2004;46:583–586 [DOI] [PubMed] [Google Scholar]
- 17.Levy DI. Endovascular treatment of carotid artery occlusion in progressive stroke syndromes: technical note. Neurosurgery 1998;42:186–191; discussion 191–183 [DOI] [PubMed] [Google Scholar]
- 18.Bellon RJ, Putman CM, Budzik RF, et al. Rheolytic thrombectomy of the occluded internal carotid artery in the setting of acute ischemic stroke. AJNR Am J Neuroradiol 2001;22:526–530 [PMC free article] [PubMed] [Google Scholar]
- 19.Eckert B, Kucinski T, Neumaier-Probst E, et al. Local intra-arterial fibrinolysis in acute hemispheric stroke: effect of occlusion type and fibrinolytic agent on recanalization success and neurological outcome. Cerebrovasc Dis 2003;15:258–263 [DOI] [PubMed] [Google Scholar]
- 20.Keisuke I, Mori T, Izumoto H, et al. Emergency carotid artery stent placement in patients with acute ischemic stroke. AJNR Am J Neuroradiol 2005;26:1249–1258 [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe M, Mori T, Imai K, et al. Intra-arterial fibrinolysis combined with balloon angioplasty in patients with serious symptoms due to acute embolic total occlusion of the carotid artery. Stroke 2004;35:298(P232) [Google Scholar]
- 22.Sims JR, Rordorf G, Smith EE, et al. Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV TPA treatment. AJNR Am J Neuroradiol 2005;26:246–251 [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann-Haefelin T, Wittsack HJ, Fink GR, et al. Diffusion- and perfusion-weighted MRI: influence of severe carotid artery stenosis on the DWI/PWI mismatch in acute stroke. Stroke 2000;31:1311–1317 [DOI] [PubMed] [Google Scholar]