Abstract
BACKGROUND AND PURPOSE: The neural basis of mental retardation is poorly understood. This study aimed to characterize structural anomalies of the brain in mental retardation and the relationship between them and the degree of mental retardation.
METHODS: Eighty adolescents receiving educational support and 40 controls underwent MR brain imaging and intelligence quotient (IQ) assessment. MR images were evaluated according to a checklist of qualitative brain anomalies by a neuroradiologist blind to group membership. All scans were assessed by a second neuroradiologist to measure interobserver agreement. Ten percent of the studies were randomly selected for assessment of intraobserver agreement.
RESULTS: Evaluation of MR images by using the checklist generated results with a high degree of interobserver and intraobserver agreement. Intraclass correlations were 0.93 and 0.75 for interobserver agreement on the total abnormality score and the entire checklist, respectively, and 0.97 and 0.85 for intraobserver agreement on the total abnormality score and the entire checklist, respectively. IQ is negatively correlated with the total abnormality score (P < .001). Subjects with an IQ <70 have a significantly greater total score (P = .003) and a significantly greater score for 12 specific anomalies, including thinning of the corpus callosum (P = .001) and abnormalities of the lateral ventricles.
CONCLUSION: Mental retardation is associated with demonstrable brain anomalies, particularly thinning of the corpus callosum and ventricular abnormalities, and with a high total abnormality score. Greater levels of brain anomalies are associated with greater levels of mental retardation as evidenced by IQ.
Mental retardation is defined as subaverage intellectual and adaptive functioning (1). In clinical and research practice mental retardation is generally taken as corresponding to an intelligence quotient (IQ) <70, and this degree of impairment has a prevalence of about 3% in school-age children (2). The neural basis of mental retardation is poorly understood, and a consensus conference on the evaluation of mental retardation stated the need for large controlled MR imaging studies into structural abnormalities (3).
The purpose of this study was to characterize the relationship between the degree of mental retardation and any structural brain anomalies in people with unexplained (idiopathic) mental retardation. We evaluated MR images according to a novel checklist of structural anomalies. In so doing, we sought to assess the degree of interobserver and intraobserver agreement in the qualitative assessment of structural brain anomalies on MR images in adolescents with mental retardation and normal controls.
Methods
Subjects
Subjects 13–22 years of age receiving educational support for learning disabilities were recruited from 99 schools and colleges of education from 18 of the 19 education authorities in Scotland as part of a longitudinal cohort study into the mental health needs of adolescents with learning disabilities. Exclusion criteria were severe cerebral palsy, severe learning disability, lack of speech, known brain injury, and Down syndrome. An initial evaluation of the first 300 subjects to be recruited to the cohort study has already identified, through the Child Behavior Checklist (4), levels of psychiatric morbidity significantly in excess of age-appropriate population norms. The excess is particularly marked within the domains of anxiety and depression, aggressive behavior, inattention, social difficulties, and social withdrawal (P. Hoare, unpublished data). Controls were recruited from nonmentally retarded siblings and age- and sex-matched nonmentally retarded individuals within the social networks of the subjects and their siblings. All subjects and their parents or legal guardians provided informed consent, and ethical permission for the study was received from the Multi-Centre Research Ethics Committee for Scotland.
Imaging studies from the first 120 subjects who underwent MR imaging were selected for inclusion in this study, comprising 80 adolescents with unexplained mental retardation and 40 controls. IQ assessments were performed on all subjects by psychologists within the research team. IQ assessment was performed by using the Wechsler Intelligence Scale for Children (5) in the case of subjects <16 years of age and the Wechsler Adult Intelligence Scale (6) in the case of subjects >16 years of age. The baseline characteristics of the subjects are as follows: (1) subjects receiving learning support (n = 80): males/females, 50/30; mean age at scan, 15.7 years (range, 13.1–22.4 years); mean IQ, 73 (range, 42–131; (2) controls (n = 40): males/females, 18/22; mean age at scan, 16.7 years (range, 13.0–21.2 years); mean IQ, 97 (range, 65–140). It is evident that the IQ range of the subjects receiving learning support indicates that this group includes subjects with high IQs. We established that there were in fact 5 subjects receiving educational support with IQs >100, and, when examined, the individuals were shown to be autistic or severely obsessional. If these 5 subjects are excluded, the mean IQ of the remaining group is <70. It is also evident that the IQ range of the control subjects indicates the inclusion of individuals with low IQs. We established that 2 controls had IQs <70, and this is not unexpected in light of the normal distribution of IQ.
Protocol
MR imaging was performed on a 1.5T unit consisting of a T1-weighted sagittal sequence with parameters of 16/450/0.75 (TE/TR/excitations) and a T2-weighted axial sequence with parameters of 102/6300/2. Volume data were obtained with a 3D inversion-recovery prepared T1-weighted sequence with parameters of 3.3/8.1/1; TI, 600; flip, 15; section thickness, 1.7 mm (no gap); matrix, 256 × 192; field of view, 220 mm. All studies were assessed by a neuroradiologist (R.J.G.) experienced in pediatric neuroimaging who was blinded to group membership. The volume images were assessed on multiplanar rendering software. Ventricles and other CSF spaces, gray and white matter features, and developmental anomalies were assessed. Within these broad headings, 36 features (Table 1) were defined as normal, moderately abnormal, or markedly abnormal and were scored as 0, 1, or 2, respectively. A moderate anomaly was taken to be a feature subjectively at the upper limit of normality for age and where it was felt that this probably represented an abnormality. A marked anomaly was taken to be a feature subjectively beyond the upper limit of normality for age and where it was felt that the feature was definitely abnormal. Examples are provided in Figs 1 and 2. The scores (0, 1, or 2) for all 36 checklist items were added together to generate total abnormality scores. The structural features assessed in this study include intracranial anomalies drawn from qualitative structural neuroimaging findings reported in neurodevelopmental disorders such as mental retardation (7–11) and schizophrenia (12–14).
TABLE 1:
Structural anomalies assessed on MR imaging and their observed frequencies among subjects and controls
| Observed Frequencies |
||||
|---|---|---|---|---|
| Moderate Anomaly |
Marked Anomaly |
|||
| Subjects (n = 80) | Controls (n = 40) | Subjects (n = 80) | Controls (n = 40) | |
| Lateral ventricle | ||||
| Blunting of lateral angles of midpoint of body of lateral ventricles | 30 | 12 | 13 | 1 |
| Blunting of lateral angles of frontal horns of lateral ventricles | 31 | 12 | 13 | 1 |
| Enlargement of frontal horns of lateral ventricles | 37 | 14 | 7 | 0 |
| Enlargement of body of lateral ventricles | 40 | 12 | 7 | 0 |
| Enlargement of occipital horns of lateral ventricles | 28 | 12 | 10 | 0 |
| Asymmetry of lateral ventricle | 28 | 12 | 3 | 0 |
| Abnormal shape of lateral ventricle | 32 | 10 | 6 | 0 |
| Third ventricle | ||||
| Widening of anterior portion of third ventricle | 22 | 6 | 3 | 0 |
| Widening throughout third ventricle | 21 | 6 | 3 | 0 |
| Cortex | ||||
| Enlargement of cortical sulci | 26 | 9 | 3 | 0 |
| Enlargement of subarachnoid spaces | 22 | 9 | 3 | 0 |
| Enlargement of interhemispheric fissure | 7 | 1 | 1 | 0 |
| Enlargement of subarachnoid cisterns | 23 | 10 | 2 | 0 |
| Temporal lobe | ||||
| Abnormal shape of hippocampus | 1 | 0 | 4 | 0 |
| Abnormal gyral pattern of hippocampus | 4 | 0 | 1 | 0 |
| Reduced size of hippocampus | 2 | 0 | 3 | 0 |
| Enlargement of temporal horns | 12 | 10 | 5 | 0 |
| White matter abnormalities | ||||
| Corpus callosal dysgenesis | 0 | 0 | 0 | 0 |
| Corpus callosal thinning | 17 | 2 | 6 | 0 |
| Corpus callosal notching | 17 | 9 | 2 | 0 |
| Focal high-intensity white matter lesions | 4 | 0 | 3 | 0 |
| White matter high intensity of myelination delay | 1 | 0 | 0 | 0 |
| White matter volume loss | 4 | 1 | 5 | 0 |
| Virchow-Robin spaces | 38 | 24 | 0 | 0 |
| Other abnormalities | ||||
| Cavum septi pellucidi or cavum vergae | 16 | 10 | 3 | 0 |
| Aqueduct stenosis | 0 | 0 | 0 | 0 |
| Arachnoid cysts | 2 | 0 | 1 | 0 |
| Porencephalic cysts | 0 | 0 | 1 | 0 |
| Cerebellar tonsil through occipital foramen* | 14 | 10 | 2 | 2 |
| Pachygyria | 0 | 0 | 0 | 0 |
| Polymicrogyria | 0 | 0 | 0 | 0 |
| Gray matter heterotopia | 1 | 1 | 0 | 0 |
| Schizencephaly | 0 | 0 | 0 | 0 |
| Enlarged cisterna magna | 20 | 6 | 2 | 0 |
| Brain stem anomalies | 1 | 0 | 0 | 0 |
| Skull shape anomalies | 1 | 0 | 0 | 0 |
As a guide for this item, “moderate” represents a displacement of 0–5 mm below the basio-opisthion line, and “marked” represents a displacement >5 mm.
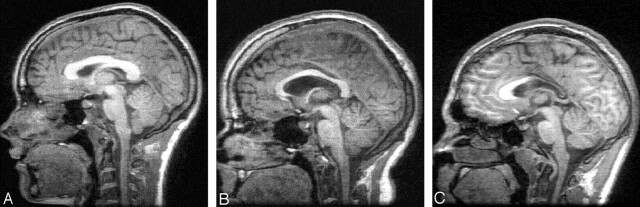
Fig 1.
Examples of absent (A), moderate (B), and marked (C) thinning of the corpus callosum on midsaggital MR images.
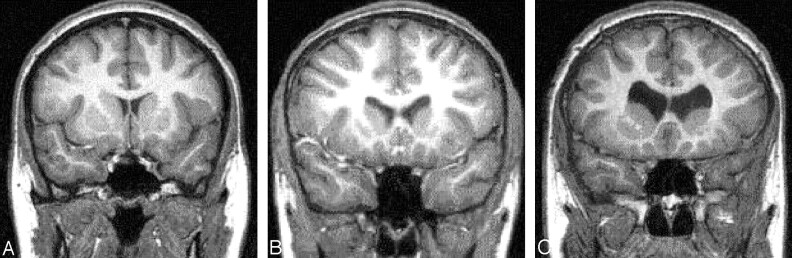
Fig 2.
Examples of absent (A), moderate (B), and marked (C) enlargement of the frontal horns of the lateral ventricles. These images also show absent (A), moderate (B), and marked (C) blunting of the lateral angles of the frontal horns of the lateral ventricles.
Reliability Analysis
For assessment of interobserver agreement, all 120 MR studies were presented to a second neuroradiologist (P.M.K.) experienced in pediatric neuroimaging, who was also blinded to any clinical or study group information. For assessment of intraobserver agreement, a randomly selected 10% sample of the 120 MR studies was presented on a second occasion to the first observer, who was blind to his initial ratings and the ratings of the second observer, as well as remaining blind to any clinical or study group information.
Statistical Analysis
Intraclass correlation (ICC) statistics were used to calculate interobserver and intraobserver agreement. Between-group comparisons of checklist scores were assessed by using the 2-tailed Mann-Whitney U test. Total abnormality scores were calculated as the sum of scores for all checklist items. The correlation between the total abnormality score and IQ was assessed with the Pearson correlation test. Post hoc between-group comparisons of the interactions between corpus callosum and lateral ventricle scores were assessed with the Kruskal-Wallis H test.
Results
Table 1 shows the observed frequencies for the anomalies assessed within this study. Table 2 shows the ICC values for interobserver and intraobserver agreement. The interobserver agreement for evaluating the MR studies is substantial for the entire checklist (ICC = 0.75), and excellent for the total abnormality score (ICC = 0.93). Intraobserver agreement is excellent for the entire checklist (ICC = 0.85) and for the total abnormality score (ICC = 0.97).
TABLE 2:
Item-by-item intraclass correlation (ICC) values for interobserver and intraobserver agreement
| Feature | Interobserver Agreement | Intraobserver Agreement |
|---|---|---|
| Lateral ventricle | ||
| Blunting of lateral angles of midpoint of body of lateral ventricles | 0.95 | 0.90 |
| Blunting of lateral angles of frontal horns of lateral ventricles | 0.91 | 0.85 |
| Enlargement of frontal horns of lateral ventricles | 0.79 | 0.62 |
| Enlargement of body of lateral ventricles | 0.80 | 0.62 |
| Enlargement of occipital horns of lateral ventricles | 0.57 | 0.82 |
| Asymmetry of lateral ventricle | 0.75 | 0.91 |
| Abnormal shape of lateral ventricle | 0.47 | 0.80 |
| Third ventricle | ||
| Widening of anterior portion of third ventricle | 0.74 | 0.52 |
| Widening throughout third ventricle | 0.77 | 0.52 |
| Cortex | ||
| Enlargement of cortical sulci | 0.58 | 0.75 |
| Enlargement of subarachnoid spaces | 0.64 | 0.83 |
| Enlargement of interhemispheric fissure | 0.51 | 1 |
| Enlargement of subarachnoid cisterns | 0.54 | 0.73 |
| Temporal lobe | ||
| Abnormal shape of hippocampus | 0.82 | 1 |
| Abnormal gyral pattern of hippocampus | 0.72 | 1 |
| Reduced size of hippocampus | 0.65 | 1 |
| Enlargement of temporal horns | 0.61 | 0.90 |
| White matter abnormalities | ||
| Corpus callosal thinning | 0.75 | 0.90 |
| Corpus callosal notching | 0.72 | 0.89 |
| Focal high-intensity white matter lesions | 0.51 | 0.94 |
| White matter volume loss | 0.68 | 1 |
| Virchow-Robin spaces | 0.55 | 0.73 |
| Other abnormalities | ||
| Cavum septi pellucidi or cavum vergae | 0.70 | —* |
| Arachnoid cysts | 0.94 | 1 |
| Cerebellar tonsil through occipital foramen | 0.87 | 0.85 |
| Enlarged cisterna magna | 0.69 | 0.58 |
| Total abnormality score | 0.93 | 0.97 |
Note.—This table excludes 10 items for which the observed frequency was <2% in 120 scans.
*ICC could not be calculated due to 0 variance within one set of observations.
Compared with controls, individuals receiving learning support have a significantly greater total abnormality score (P = .005) and a significantly greater score on 8 items—namely, thinning of the corpus callosum (P = .002), enlargement of the body of the lateral ventricles (P = .002), blunting of the lateral angles of the frontal horns of the lateral ventricles (P = .009), blunting of the lateral angles at the midpoint of the body of the lateral ventricles (P = .012), abnormal shape of the lateral ventricles (P = .012), enlargement of the frontal horns of the lateral ventricles (P = .020), enlargement of the occipital horns of the lateral ventricles (P = .030), and widening of the anterior part of the third ventricle (P = .049).
Mean total abnormality scores for the study groups were as follows: (1) Subjects receiving learning support (n = 80): 9.1 (range, 0–38), 95% confidence interval (CI) of mean 7.4–10.8; (2) controls (n = 40): 5.2 (range, 0–14), 95% CI of mean 3.9–6.4. A total abnormality score of one or more is found in 75 of 80 subjects receiving learning support and in 39 of 40 controls.
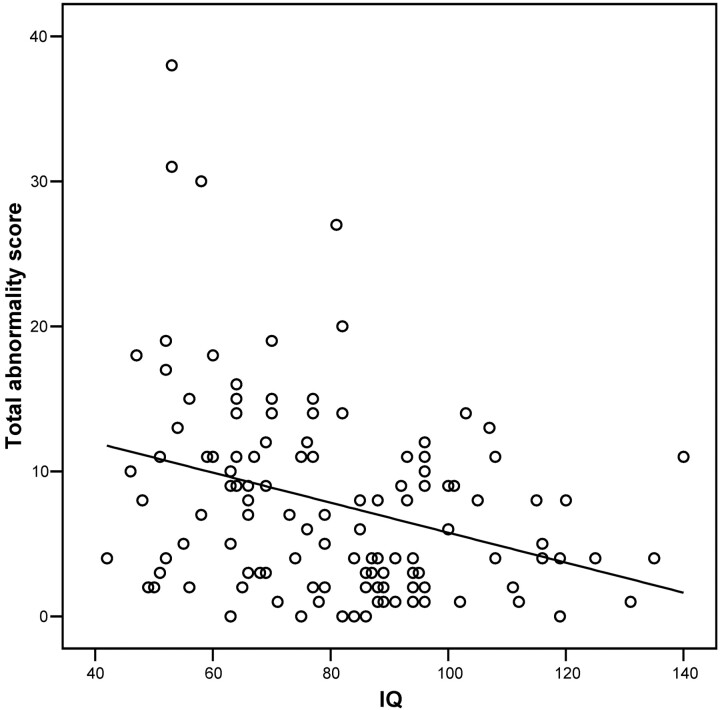
IQ is significantly negatively correlated with the total abnormality scores (Pearson r = −0.320; P < .001) (Fig 3). Compared with subjects with IQs ≥ 70, subjects with IQs <70 have a significantly greater total abnormality score (P = .003) and a significantly greater score on 12 items from the checklist—namely, thinning of the corpus callosum (P = .001), abnormal shape of the lateral ventricles (P = .002), enlargement of the frontal horns of the lateral ventricles (P = .005), enlargement of the body of the lateral ventricles (P = .007), blunting of the lateral angles of the midpoint of the body of the lateral ventricles (P = .009), blunting of the lateral angles of the frontal horns of the lateral ventricles (P = .012), increased sulcal widening (P = .012), loss of white matter (P = .017), arachnoid cysts (P = .017), enlargement of subarachnoid spaces (P = .020), enlargement of the interhemispheric fissure (P = .038), and widening of the anterior part of the third ventricle (P = .048).
Fig 3.
Scatterplot of total abnormality score against IQ for all 120 subjects. There is a significant negative correlation for total abnormality score versus IQ (r = −0.320; P < .001).
Mean total abnormality scores for subjects were as follows: (1) IQ < 70 (n = 42): 10.4 (range, 0–38), 95% CI of mean 7.9–13.0; (2) IQ ≥ 70 (n = 78): 6.3 (range, 0–27), 95% CI of mean 5.1–7.6. A total abnormality score of one or more is found in 41 of 42 subjects with IQ <70 and in 73 of 78 subjects with IQs ≥70.
Post hoc comparison of corpus callosal thinning and lateral ventricle scores shows that thinning of the corpus callosum is significantly associated with scores on all checklist items relating to the lateral ventricles—namely, blunting of the lateral angles of the midpoint of the body of the lateral ventricles (P < .001), blunting of the lateral angles of the frontal horns of the lateral ventricles (P = .001), enlargement of the frontal horns of the lateral ventricles (P < .001), enlargement of the body of the lateral ventricles (P < .001), enlargement of the occipital horns of the lateral ventricles (P < .001), asymmetry of the lateral ventricles (P = .004), and abnormal shape of the lateral ventricles (P < .001).
Discussion
We found a high degree of interobserver and intraobserver agreement in the evaluation of brain anomalies by using the checklist described in this study. The fact that the range and mean of the abnormality scores is greater in subjects with learning disability than controls, and greater in subjects with IQs <70 than among those with IQs ≥70, supports the reliability and validity of the findings. The validity of this instrument in the assessment of brain anomalies in mental retardation is further supported by the finding that the total abnormality score is significantly correlated in a negative manner with IQ. Our findings support the use of this checklist as a reliable and valid way to assess qualitative brain anomalies on MR imaging in subjects with mental retardation and controls. A further refinement to the checklist would be to exclude the 10 items with a frequency of <2%.
Our basis for recruitment of subjects and controls was being in receipt or not, respectively, of learning support, rather than on the basis of IQ. We recruited controls from among the siblings of subjects and the age- and sex-matched members of the same social networks and communities as the subjects. We consider this degree of matching and these attempts to reduce potential bias to be important strengths of the study. As noted earlier, 5 subjects had IQs >100 and 2 controls had IQs <70. Although IQ is clearly related to the provision of learning support services by the educational authorities, an IQ cutoff of 70 is evidently not the sole determinant. It is for this reason that, within this study, we made 2 separate sets of comparisons. One set of comparisons was made on the basis of the recruitment group (ie, being in receipt, or not, of learning support), and the second set of comparisons was made on the basis of IQ (ie, an IQ of <70 compared with an IQ ≥70).
Mental retardation is a common condition causing significant disability, but its neural basis is poorly understood. This study has identified qualitative brain anomalies that are significantly associated with low IQ, particularly thinning of the corpus callosum and abnormalities in the size and shape of the lateral ventricles. Reduced corpus callosal size has been reported in mentally retarded children (11, 15) and developmentally retarded infants (16) and has been suggested to be a marker of abnormal cerebral development (11). A positive association has been reported between IQ and midsagittal posterior (17) and total (18) corpus callosal areas. In the present study, we found marked thinning of the corpus callosum in 7.5% of intellectually disabled subjects (and found none in controls) and moderate thinning in 21.3% of subjects (compared with 5.0% in controls). The corpus callosum is the largest axonal pathway in the brain and is an important route for the interhemispheric transfer of information. Consequently, our finding of greater corpus callosal thinning in individuals with mental retardation suggests that impaired cortical connectivity may have a role in the pathogenesis of mental retardation.
The corpus callosum and its precursors form between 8 and 20 weeks of gestation (19), and there is evidence that maturation continues into the third decade of life (20, 21). Hypogenesis and agenesis of the corpus callosum often occur in combination with other structural brain abnormalities such as interhemispheric cysts, ventriculomegaly, cavum septi pellucidi, gray matter heterotopias, and cortical dysplasia and may occur as component of syndrome complexes, including Aicardi syndrome, the Dandy-Walker malformation, and fetal alcohol syndrome (22). Thinning of the corpus callosum and ventricular dilation have also been described in individuals born very preterm, where they have been suggested to be associated with hypoxic-ischemic damage (23, 24).
The corpus callosum physically constrains the size and shape of the lateral ventricles. In hypogenesis of the corpus callosum, the lateral ventricle expands, particularly in its occipital horns, resulting in colpocephaly. Indeed, we found that thinning of the corpus callosum was significantly associated with all checklist scores relating to abnormalities of the size and shape of the lateral ventricles. Ventricular abnormalities are also commonly reported in psychotic disorders, such as schizophrenia (13), where they may reflect relatively specific interactions between genetic and environmental factors (25) or a more general reduction in cortical gray matter. In the premature neonate, ventricular enlargement can occur as a result of volume loss in the periventricular white matter secondary to periventricular leukomalacia, which leads to irregular expansion of the ventricles to occupy these areas (26). Abnormalities of size and shape of the lateral ventricles, together with thinning of the corpus callosum, were found in the present study, and this raises the possibility that neonatal brain injury may account for some of our findings. Further work is therefore indicated in exploring possible associations between the qualitative brain anomalies reported in this study and adverse events in the pre- and perinatal periods.
With traditional qualitative assessment techniques, previous studies of structural brain images in mental retardation have estimated the prevalence of abnormalities to be 35–40% of patients with mental retardation (7). The reliability of such estimates is, however, unknown. In our study, in contrast, almost all subjects and controls were found to have one or more anomalies present, and these findings were reliable. Very few (3 of 40) of the controls, however, had anomalies present to a marked degree. The prevalence of detected anomalies among our controls is comparable to that among controls within other qualitative controlled studies, which have reported overall abnormality rates of 43% (12) and 23.7% (27), ventricular dilation rates of 20% (8) and 19% (14), cavum septi pellucidi rates of 58.8% (28) and 68 –80% (29), a cortical atrophy rate of 41% (14), and a focal white-matter high-signal-intensity rate of 14% (13). The inclusion of a large number of anomalies in the checklist—including a number of anomalies not routinely considered to be clinically important, such as cavum septi pellucidi and enlarged Virchow-Robin spaces—and the provision for moderate as well as marked ratings probably contributed to the high anomaly yield we obtained. It is difficult to assess the clinical significance of a single anomaly, considered in isolation, and indeed it is likely that a number of the anomalies detected in this study would be traditionally viewed as incidental findings; however, a comprehensive qualitative assessment, as facilitated by this checklist, yields far broader results, which are of direct clinical relevance, as is evident from the significant negative correlation between total scores and IQ in our study.
Conclusion
A novel checklist of qualitative brain anomalies was developed with a high degree of interobserver and intraobserver agreement. We found that low IQ was associated with qualitative brain anomalies, particularly thinning of the corpus callosum and ventricular abnormalities, and that the overall degree of abnormality was negatively correlated with IQ.
Acknowledgments
We gratefully acknowledge the participation of the individuals and families involved in the study.
Footnotes
This research was funded by a program grant (to E.C.J.) from the United Kingdom Medical Research Council.
References
- 1.American Association on Mental Retardation. Mental retardation: definition, classification, and systems of supports. 10th ed. Washington, DC: American Association on Mental Retardation;2002
- 2.Roeleveld N, Zielhuis GA, Gabreels F. The prevalence of mental retardation: a critical review of recent literature. Dev Med Child Neurol 1997;39:123–132 [DOI] [PubMed] [Google Scholar]
- 3.Curry CJ, Stevenson RE, Aughton D, et al. Evaluation of mental retardation: recommendations of a Consensus Conference: American College of Medical Genetics. Am J Med Genet 1997;72:468–477 [DOI] [PubMed] [Google Scholar]
- 4.Achenbach TM. Manual for the child behaviour checklist 4–18 and 1991 profile. Burlington: Department of Psychiatry, University of Vermont;1991
- 5.Wechsler D. Wechsler Intelligence Scale for Children,3rd U.K. edition. London: Psychological Corporation;1992
- 6.Wechsler D. Wechsler Adult Intelligence Scale. New York: Psychological Corporation;1981
- 7.Schaefer GB, Bodensteiner JB. Developmental anomalies of the brain in mental retardation. Int Rev Psychiatry 1999;11:47–55 [Google Scholar]
- 8.Gabrielli O, Coppa GV, Manzoni M, et al. Minor cerebral alterations observed by magnetic resonance imaging in syndromic children with mental retardation. Eur J Radiol 1998;27:139–144 [DOI] [PubMed] [Google Scholar]
- 9.Soto-Ares G, Joyes B, Lemaitre MP, Vallee L, Pruvo JP. MRI in children with mental retardation. Pediatr Radiol 2003;33:334–345 [DOI] [PubMed] [Google Scholar]
- 10.Deb S. Structural neuroimaging in learning disability. Br J Psychiatry 1997;171:417–419 [DOI] [PubMed] [Google Scholar]
- 11.Schaefer GB, Bodensteiner JB, Thompson JN Jr, Wilson DA. Clinical and morphometric analysis of the hypoplastic corpus callosum. Arch Neurol 1991;48:933–936 [DOI] [PubMed] [Google Scholar]
- 12.Lieberman J, Bogerts B, Degreef G, et al. Qualitative assessment of brain morphology in acute and chronic schizophrenia. Am J Psychiatry 1992;149:784–794 [DOI] [PubMed] [Google Scholar]
- 13.Lawrie SM, Abukmeil SS, Chiswick A, et al. Qualitative cerebral morphology in schizophrenia: a magnetic resonance imaging study and systematic literature review. Schizophr Res 1997;25:155–166 [DOI] [PubMed] [Google Scholar]
- 14.Galderisi S, Vita A, Rossi A, et al. Qualitative MRI findings in patients with schizophrenia: a controlled study. Psychiatry Res 2000;98:117–126 [DOI] [PubMed] [Google Scholar]
- 15.Njiokiktjien C, de Sonneville L, Vaal J. Callosal size in children with learning disabilities. Behav Brain Res 1994;64:213–218 [DOI] [PubMed] [Google Scholar]
- 16.Fujii Y, Konishi Y, Kuriyama M, et al. Corpus callosum in developmentally retarded infants. Pediatr Neurol 1994;11:219–223 [DOI] [PubMed] [Google Scholar]
- 17.Strauss E, Wada J, Hunter M. Callosal morphology and performance on intelligence tests. J Clin Exp Neuropsychol 1994;16:79–83 [DOI] [PubMed] [Google Scholar]
- 18.Atkinson DS Jr, Abou-Khalil B, Charles PD, Welch L. Midsagittal corpus callosum area, intelligence, and language dominance in epilepsy. J Neuroimaging 1996;6:235–239 [DOI] [PubMed] [Google Scholar]
- 19.Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. J Comp Neurol 1968;132:45–72 [DOI] [PubMed] [Google Scholar]
- 20.Cowell PE, Allen LS, Zalatimo NS, Denenberg VH. A developmental study of sex and age interactions in the human corpus callosum. Brain Res Dev Brain Res 1992;66:187–192 [DOI] [PubMed] [Google Scholar]
- 21.Pujol J, Vendrell P, Junque C, et al. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol 1993;34:71–75 [DOI] [PubMed] [Google Scholar]
- 22.Barkovich AJ. Pediatric neuroimaging. Philadelphia: Lippincott Williams and Wilkins;1999. :251–381
- 23.Stewart AL, Rifkin L, Amess PN, et al. Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet 1999;353:1653–1657 [DOI] [PubMed] [Google Scholar]
- 24.Nosarti C, Rushe TM, Woodruff PW, et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 2004;127:2080–2089 [DOI] [PubMed] [Google Scholar]
- 25.McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry 2000;157:203–212 [DOI] [PubMed] [Google Scholar]
- 26.Barkovich AJ. Pediatric neuroimaging. Philadelphia: Lippincott Williams and Wilkins;1999. :157–249
- 27.Lubman DI, Velakoulis D, McGorry PD, et al. Incidental radiological findings on brain magnetic resonance imaging in first-episode psychosis and chronic schizophrenia. Acta Psychiatr Scand 2002;106:331–336 [DOI] [PubMed] [Google Scholar]
- 28.Nopoulos P, Swayze V, Flaum M, et al. Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biol Psychiatry 1997;41:1102–1108 [DOI] [PubMed] [Google Scholar]
- 29.Born CM, Meisenzahl EM, Frodl T, et al. The septum pellucidum and its variants: an MRI study. Eur Arch Psychiatry Clin Neurosci 2004;254:295–302 [DOI] [PubMed] [Google Scholar]