Abstract
Summary: We present the case of an anomalous origin of the left anterior cerebral artery (ACA) from the supraclinoid segment of the right internal carotid artery. Because of improved imaging quality, anomalies of the ACA–anterior communicating artery (AComA) complex are increasingly recognized on transaxial images. Although most of these anomalies are incidental findings of little clinical significance, some ACA-AComA complex anomalies are clinically significant. Recognition of these anomalies may be instrumental in developing a differential diagnosis or for improved surgical planning.
A 2-year-old infant was admitted to the hospital for evaluation and management of new-onset seizures. She had a known history of Chiari II malformation and spina bifida with related hydrocephalus, for which she was treated with a ventriculoperitoneal shunt. A head CT was obtained to evaluate for possible shunt dysfunction. The head CT demonstrated diffuse hypoattenuation of the left cerebral hemisphere. Therefore, MR imaging of the brain was performed to verify left cerebral ischemia.
Case Report
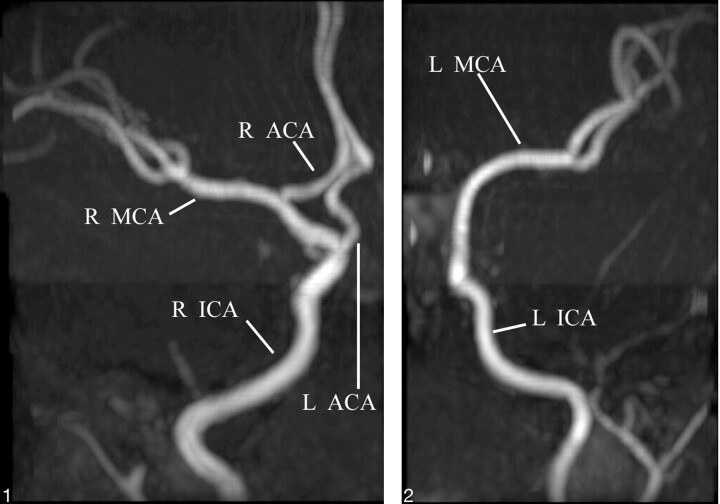
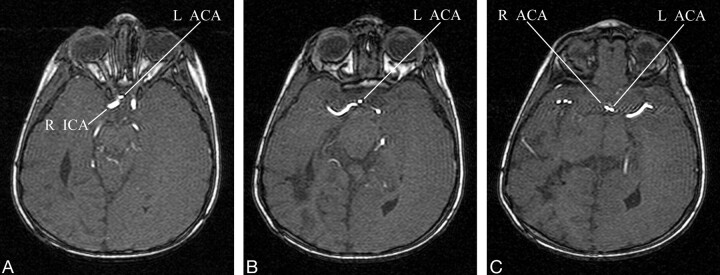
Diffusion-weighted MR images showed decreased perfusion to the entire left cerebral hemisphere consistent with infarction. In light of this distribution, cerebral MR angiography (MRA) was also performed to visualize the anterior and posterior cerebral vasculature. The MRA demonstrated an anomalous origin of the left anterior cerebral artery (ACA) from the supraclinoid segment of the right internal carotid artery (ICA; Figs 1 and 2). More specifically, the origin of the left A1 segment was adjacent to, but separate from, the origin of the right ophthalmic artery. The anomalous segment coursed anterior to the optic chiasm before running close to the A1 segment of the right ACA to form the anterior communicating artery (AComA; Fig 3). In view of the anomalous origin of the left A1 segment and the normal perfusion pattern of the territories supplied by the right ICA (including the left ACA territory), a vascular etiology for the left hemispheric infarct was much less likely.
Fig 1.
Anteroposterior 3D time-of-flight MRA of the right ICA distribution demonstrates the origin of the left A1 segment (L ACA) near the origin of the ophthalmic artery. R ACA, right anterior cerebral artery; R MCA, right middle cerebral artery.
Fig 2.
Anteroposterior 3D time-of-flight MRA of the left ICA distribution demonstrates the absence of the left ACA from the left ICA. L MCA, left middle cerebral artery.
Fig 3.
A–C, Selected axial images of the 3D time-of-flight MRA demonstrates the paraophthalmic origin of the left A1 segment (L ACA) from the right ICA. The anomalous vessel loops anterior to the optic chiasm before ascending to join the AComA.
On the basis of the imaging findings, we proposed that the cause of the left-sided cerebral infarct was prolonged seizure activity that was confined to that hemisphere by lack of the corpus callosum. Evaluation for other etiologies by echocardiogram, carotid duplex examination, ventriculoperitoneal shunt evaluation, and laboratory tests for infection and coagulopathy was unrevealing. Medical management of the seizure activity was optimized, and the patient began to receive baclofen to reduce right-sided spasticity. At the time of discharge, there was a high likelihood of left-sided cortical blindness, but the patient’s overall status continued to improve with rehabilitation.
Discussion
Padget described 8 stages in the development of the cerebral arteries, including the adult configuration (1). The first 7 stages occur during embryogenesis in the first trimester. When the embryo is approximately 40 mm in length (estimated ovulation age 52 days), cerebrovascular development corresponds with the seventh stage of development. At this stage, the circle of Willis is recognizable and the stems of all the cerebral arteries attain their adult configuration (1). In the final embryonic period of cerebrovascular development, the vessels branch in accordance with the developing parenchyma (1). This pattern of development has been termed “cerebrovascular ontogenic plasticity” (2). It is postulated that anomalous arteries and vascular malformations develop during this period (2).
In the adult configuration, the A1 segment usually arises from the ICA bifurcation. It then extends anteromedially toward the interhemispheric fissure, following a supraoptic course above the ipsilateral optic nerve or chiasm to join the AComA. Variation within the ACA-AComA complex is extremely common: some degree of asymmetry between the 2 A1 segments is identified in approximately 80% of patients (3). The most frequently reported anomalies include aplasia or hypoplasia of the A1 segment, fenestration or duplication of the AComA, and presence of 3 distal ACAs (4).
An anomalous origin of the ACA from the contralateral ICA is very rare. In addition to this case report, there has been only one other report of an anomalous origin of the ACA from the contralateral ICA (5). These cases differ in the course of the anomalous vessel as it ascends to its normal position.
Though still quite rare, an anomalous origin of the ACA from the paraclinoidal segment of the ipsilateral ICA is more common. There have been approximately 30 case reports of an infraoptic course of the A1 segment, which is associated with a low bifurcation of the ICA at or near the origin of the ophthalmic artery (4). One case of an anomalous A1 originating proximal to the ophthalmic artery has been reported (6). Anomalies with an infraoptic course are often associated with agenesis or hypoplasia of the contralateral A1. There are numerous variations in the ascent of the infraoptic A1 segment to the optic apparatus; most frequently, the infraoptic ACA ascends anterior to the chiasm between the optic nerves or pierces the ipsilateral optic nerve chiasm (3). A cavernous origin of the A1 segment has also been described (2).
Although these anomalies are frequently normal variants found incidentally during diagnostic studies, the incidence of coexistent abnormalities is higher in these patients. Specifically, the prevalence of cerebral aneurysms and arteriovenous malformations is increased in this patient population (2, 6). Cerebrovascular ontogenic plasticity theory suggests altered blood flow dynamics during embryogenesis may account for these coexistent anomalies. Other reported associated vascular anomalies include agenesis of the ICA, anomalous origin of the ipsilateral ophthalmic artery from the external circulation, fused pericallosal arteries, Moya-Moya disease, and symptoms related to compression of the optic apparatus.
Recognition of these anomalies is crucial when planning surgical or endovascular treatment of aneurysms. Characterization of these anomalies can be pivotal when developing a differential diagnosis, as shown in this case.
References
- 1.Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol 1948;32:207–262 [Google Scholar]
- 2.Singer RJ, Abe T, Taylor WH, et al. Intracavernous anterior cerebral artery origin with associated arteriovenous malformations: a developmental analysis: case report. Neurosurgery 1997;40:829–831 [DOI] [PubMed] [Google Scholar]
- 3.Maurer J, Maurer E, Perneczky A. Surgically verified variations in the A1 segment of the anterior cerebral artery; report of two cases. J Neurosurg 1991;75:950–953 [DOI] [PubMed] [Google Scholar]
- 4.Spinnato S, Pasqualin A, Chioffi F, Da Pian R. Infraoptic course of the anterior cerebral artery associated with an anterior communicating artery aneurysm: anatomic case report and embryological considerations. Neurosurgery 1999;44:1315–1319 [PubMed] [Google Scholar]
- 5.Sincoff EH, Abdulrauf SI. Anomalous origin of the left A1 segment from the right internal carotid artery. J Neurosurg 2003;99:1101. [DOI] [PubMed] [Google Scholar]
- 6.Given CA, Morris PP. Recognition and importance of an infraoptic anterior cerebral artery: case report. AJNR Am J Neuroradiol 2002;23:452–454 [PMC free article] [PubMed] [Google Scholar]