Abstract
PURPOSE: The purpose of this study was to evaluate the reliability of angiography-based balloon test occlusion (BTO) criteria to decide whether to perform internal carotid artery (ICA) permanent occlusion.
METHODS: From March 1999 to August 2004, 60 patients underwent therapeutic ICA occlusion. Angiographic BTO was performed systematically in all patients under general anesthesia (GA). No clinical examination test was performed. After balloon inflation, contralateral carotid and vertebral arteries angiograms were obtained. The symmetry of the venous phases of each hemisphere was assessed. Occlusion was considered to be feasible when the delay between the venous drainage of the injected and the occluded hemisphere was not >2 seconds. Venous drainage delay >4 seconds was considered as contraindication to ICA permanent occlusion. In patients with venous drainage delay of 2–4 seconds, the occlusion was performed only in selected cases.
RESULTS: From a total of 60 patients, 44 had exact symmetry of the venous phase, 10 had delay of 1 second, and 3 other patients had 2-second delays. Clinical outcome for these 57 patients was uneventful. Three patients had venous drainage delay of 3 seconds. One of them had delayed watershed area infarction without clinical consequences at the time of hospital discharge. No periprocedural complications were observed.
CONCLUSION: Venous opacification symmetry in the tested and control vascular territories was a reliable predictor of a subject’s ability to tolerate carotid occlusion without developing neurologic deficit. Carotid sacrifice was found to be possible when the delay was <3 seconds.
Despite the development of new innovative technologies, endovascular occlusion of the internal carotid artery (ICA) remains an important procedural tool for the treatment of several head and neck diseases. ICA endovascular occlusion is used as a preoperative maneuver for extensive cervical and skull base tumors (1–3), an alternative approach for wide-necked inaccessible ICA aneurysms (4–7) and some direct arteriovenous fistulas.
Balloon test occlusion (BTO) of the ICA has been performed to identify patients who are at risk for ischemia and stroke following permanent ICA occlusion and to reduce neurologic ischemia complication rates. One review of 516 patients from the literature demonstrated a complication rate as high as 26% for abrupt ICA occlusion without any test compared with 13% when BTO was used (8). Although test occlusion in the waking patient can readily predict acute ischemia, no test protocol has been shown to predict delayed cerebral ischemia after permanent occlusion accurately (9–14), which can happen hours to days after the procedure.
Many other sophisticated blood flow measurements have been proposed in addition to the classic method (6, 15–20) to identify patients who will develop cerebral ischemia after having passed the classic occlusion clinical test. Most of these techniques require specialized equipment, thus increasing the complexity, costs, and theoretic complication rate of the procedure due to the extended inflation time of the balloon (21).
Since the first description of the clinical BTO by Serbinenko (22) in 1974, all the test protocols used to date evaluate the patient under local anesthesia. Clinical examination is then performed during a variable period of time.
Recently, 2 protocols for BTO of the ICA have been proposed on the basis of angiographic criteria. These protocols are based on synchronous venous filling parameters, very strict (BTO negative when the venous delay is <0.5 second) and still combined with clinical tests (3, 23). One of these protocols (23) suggests that, by using only on the angiographic parameters, the clinical test is probably unnecessary.
On the basis of personal experience, in this work we propose a simple and straightforward BTO for ICA that relies only on the angiographic analysis of the venous drainage symmetry between the occluded and the injected carotid or vertebral vascular territories. No clinical test is used, and the procedure is performed while the patient is under general anesthesia (GA).
Patients and Methods
Patients
From March 1999 to August 2004, 60 patients underwent BTO of the ICA followed immediately by therapeutic occlusion in this institute. Patients included 33 women and 27 men, their ages ranging from 12 to 84 years (mean age, 51 years). The diagnosis at the time of treatment included 35 ICA aneurysms, 23 cervical tumors, and 2 direct carotid-cavernous arteriovenous fistulas (Table 1). Among the 60 cases, 54 ICA occlusions were planned, whereas 6 others were performed immediately after failure of conservative strategy (4 ICA aneurysms and 2 direct carotid AV fistulas). Because this study describes our empiric experience, no control group is reported.
TABLE 1:
Medical diagnosis in 60 patients undergoing balloon test occlusion of the internal carotid artery followed immediately by therapeutic occlusion, according to gender
| Aneurysms | Tumors | Fistulae | Total | |
|---|---|---|---|---|
| Female | 28 | 4 | 1 | 33 |
| Male | 7 | 19 | 1 | 27 |
| Total | 35 | 23 | 2 | 60 |
Digital Subtraction Angiography
All procedures were performed under GA as follows. A 6F or 8F femoral introducer was inserted on the right common femoral artery, depending on the strategy for the ICA occlusion, and a 5F introducer was inserted on the left side to perform the 4-vessel digital subtraction angiography (DSA).
After the femoral punctures, 5000 U of heparin were systematically administrated intravenously by bolus, followed by a continuous intravenous infusion of 2500–3000 IU per hour to maintain an activated clotting time (ACT) between 200 and 300 seconds. In accordance with our anticoagulation protocol, a 250-mg intravenous bolus of aspirin was also administered at the same time.
Complete DSA study was performed for each patient, including common carotid arterial bifurcations, internal carotid arteries, and vertebrobasilar system. Additional rotational 3D imaging was acquired in case of ICA aneurysm to better study its anatomy. After the DSA, the 5F diagnostic catheter was kept in the contralateral ICA and a 6- or 8F guiding catheter (Envoy; Cordis, Miami Lakes, FL) was positioned in the tested ICA.
Test Occlusion
When permanent occlusion was planned to be performed by using a detachable balloon, a BAL3 X Ray (Balt Extrusion, Montmorency, France) mounted on a Mabdpe microcatheter (Balt Extrusion) and secured with a handmade ligature was directly positioned in the chosen occlusion site. Detachment was then performed once the BTO was negative. When occlusion was planned to be performed by using coils, a nondetachable balloon was used—either an Endeavor (Boston Scientifics/Target, Fremont, CA) or a Hyperglide balloon (Micro Therapeutics, Irvine, CA).
A digital road map of the ICA was obtained to control balloon inflation, which was performed under fluoroscopic view. Occlusion was confirmed by an angiographic series through the guiding catheter. Two angiogram series—contralateral ICA followed by dominant vertebral artery (VA)—were performed after balloon inflation.
A total of 12 mL of iodinate contrast was injected at 5 mL/s for the ICA, and 10 mL at 5 mL/s was used for the VA; the same protocol used for our standard angiograms. Angiographic series were obtained systematically by using a program of one image per second to allow the correct analysis of the symmetry of the venous drainage between the occluded and injected territories.
Occlusion Criteria
The symmetry of the cortical veins filling between the occluded carotid artery territory and examined artery (contralateral ICA or dominant VA) was the main focus of attention. If collateral filling was mainly via anterior communicating artery (Acom), the cortical venous filling of the injected hemisphere would be compared with the occluded one (Figs 1 and 2). Nevertheless, if collateral filling was mainly via posterior communicating artery (Pcom), the symmetry of the venous filling would then be compared between the occluded hemisphere and the posterior circulation territory on the vertebral angiogram (Fig 3).
Fig 1.
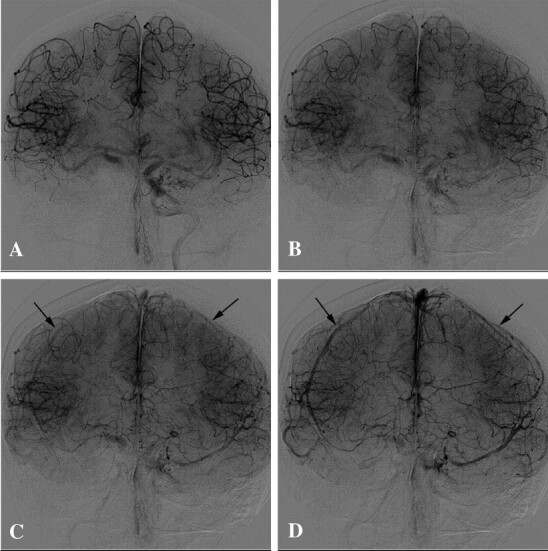
Angiogram AP view demonstrates a right carotid occlusion test in a 42-year-old woman with large ICA aneurysm. A and B, Late arterial phase demonstrates excellent cross-filling by Acom. C, Early venous phase—1 second after second image—shows the beginning of venous phase in both sides (black arrows). D, One second later, the venous phase synchronous becomes more evident in both hemispheres (black arrows). This patient represents an example of perfect symmetry of venous phase delay during BTO.
Fig 2.
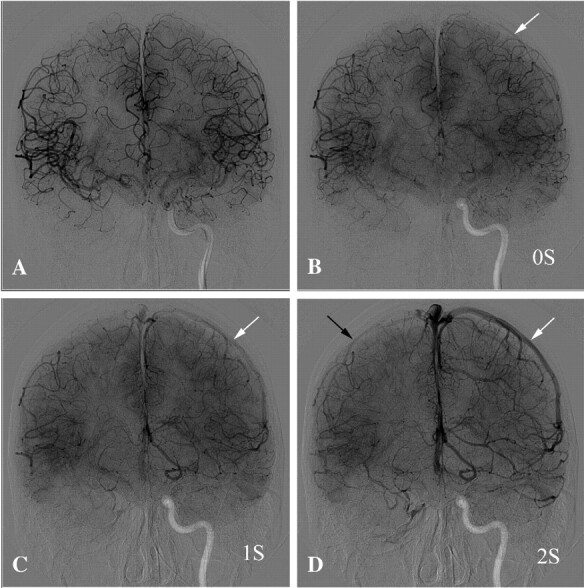
Angiographic AP view demonstrates a right carotid occlusion test in a 45-year-old man with a large cervical tumor. A, Late arterial phase demonstrates excellent cross-filling through Acom. B, One second after the previous image, beginning of venous phase on the left side (white arrow) and still some cortical arteries in the right hemisphere (indicated as 0 seconds). C, One second later, there are still cortical arteries on right side and veins on the left side (white arrow). D, One second later (ie, venous phase on 2 seconds) shows the veins filled in both hemispheres (black arrow on the right side and white arrow on the left side). This patient had a 2-second venous drainage delay, and permanent occlusion was performed at the same time.
Fig 3.
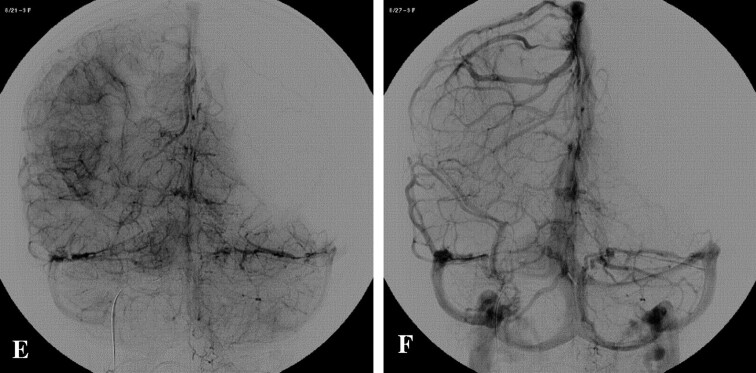
A 15-year-old girl test occlusion for a right giant ICA aneurysm. A, Angiogram AP view, injected by left ICA shows late arterial phase. B and C, Early and late venous phases, respectively, demonstrate perfect synchronous venous filling of left hemisphere and territory of right anterior cerebral artery through Acom. D, Arterial phase of the injection through left vertebral artery, AP view, demonstrates excellent cross-filling through Pcom. E and F, Early and late venous phases, respectively, show symmetry of venous filling of vertebrobasilar territory and right MCA territory through the right Pcom. This patient presented a supply of the right internal carotid artery territory via both Acom and Pcom, without venous delay. ICA permanent occlusion was performed at the same time.
We considered the appearance of the first cortical veins to determine the beginning of the venous phase.
Because the patients were under GA, no clinical test was performed during the angiographic evaluation. The balloon inflation time was limited to the strictly necessary period needed for contralateral ICA and VA angiograms, thus considerably reducing the time of temporary ICA occlusion.
The test occlusion was considered to be negative when the delay of the venous drainage between the territories of the injected artery (ICA or dominant VA) and the occluded hemisphere was not >2 seconds. Between 2 and 4 seconds, permanent occlusion was performed only in selected cases. A delay of >4 seconds was always considered to be a formal contraindication for permanent occlusion (angiographic test considered to be positive).
After negative angiographic BTO for ICA, the balloon was detached and a second one was positioned just below for security. When occlusion was performed by using coils after BTO, the balloon was retrieved and a microcatheter Excelsior 10–18 (Boston Scientifics/Target) or Nautica (Micro Therapeutics) was positioned at the level of the planned occlusion. Occlusion was performed by using coils until no contrast could pass through the ICA.
Postocclusion Protocol
The femoral punctures were closed with an arterial system closure (Angioseal; Daig, Saint Jude Medical, St. Paul, MN) immediately after the procedure.
After permanent occlusion, each patient was kept at the intensive care unit (ICU) for at least 48 hours for close monitoring of blood pressure (BP), fluid balance, and neurologic status. After 48 hours, neurologic status was assessed daily until the patient was discharged. Imaging was performed when any new neurologic event was noted. The BP was closely monitored and, if necessary, kept pharmacologically elevated for these 48 hours to prevent any possible episode of hypotension, which could lead to stroke. Baseline BP was measured before the procedures during the anesthetic consultation. It was then checked during GA and finally at the ICU during the first 48 hours after embolization. For each patient, the measurement of BP was recorded as mean BP, corresponding to an average of systolic and diastolic BP.
Heparin (to maintain ACT of 200–300 seconds) and oral aspirin (250 mg per day) were continued for 48 hours.
Results
Sixty ICA occlusion tests followed by permanent occlusion were performed. Forty-four patients (73.3%) had exact venous phase symmetry, 10 patients (16.7%) had 1-second delays, 3 patients (5%) had 2-second delays, and 3 others (5%) had 3-second delays (Table 2). No periprocedural complications were observed.
TABLE 2:
Venous drainage delay assessed by angiography during balloon test occlusion in 60 patients undergoing permanent internal carotid artery occlusion
| Venous Drainage Delay* | 0 Seconds | 1 Second | 2 Seconds | 3 Seconds | Total |
|---|---|---|---|---|---|
| Aneurysms | 26 | 6 | 2 | 1 | 35 |
| Tumors | 16 | 4 | 1 | 2 | 23 |
| Direct fistulae | 2 | 0 | 0 | 0 | 2 |
| Total | 44 | 10 | 3 | 3 | 60 |
Beginning of venous phase is determined by appearance of first cortical veins at the angiogram. Venous drainage delay corresponds to cortical vein–filling delay of the occluded territory compared to the injected artery (contralateral internal carotid artery or dominant vertebral artery).
For the 57 patients who had venous drainage delay as long as 2 seconds in the BTO, no ischemic events after permanent ICA occlusion were observed. Three patients had venous drainage delay of 2–4 seconds. One of those patients developed watershed area ischemia 5 days after the occlusion procedure because of low cerebral blood flow (CBF) status following an infectious disease.
Fifty-four of 60 ICA occlusions performed were planned. Of these subjects, 40 had exact venous phase drainage symmetry (Figs 1 and 3), 10 patients had 1-second delays, 2 had 2-second delays (Fig 2), and 2 others had 3-second delays. The 2 patients in this group who presented with a venous phase drainage delay of 3 seconds had extensive cervical tumors, and occlusion was mandatory before the surgical resection. No clinical complications at the time of the discharge were observed for this group of patients who had planned ICA occlusion.
Among our patients, 6 unplanned ICA occlusions were performed as a rescue maneuver immediately after failure of conservative treatment of their aneurysm or direct carotid cavernous fistula. For this group, 4 patients had exact symmetry of venous drainage, one patient had a 2-second delay, and another presented with a 3-second delay of the venous drainage. Clinical complication occurred in a patient presenting with a large, right paraophthalmic artery aneurysm in which the initial therapeutic strategy consisted of intracranial stent placement (INX 4 × 18; Medtronic Vascular, Santa Rosa, CA) of the carotid at the level of the neck and posterior filling of the aneurysm with Onyx (Micro Therapeutics). After stent placement, thrombus formation was noted at its level. The occlusion of the ICA was then mandatory due to the impossibility to control the progression of the thrombus. This patient had 3 seconds of venous drainage delay during the BTO performed prior to treatment, but the ICA occlusion with coils at the level of the aneurysm had to be performed as an emergency. The patient had no neurologic deficit until the fifth day postprocedure, when he developed septicemia following a urinary tract infection. This complication resulted in low CBF status, reducing the mean BP of 100–70 mm Hg during a 12-hour period. At this time, the patient presented with transitory hemiparesis of the left side and the CT scan revealed a watershed area infarction. The neurologic deficit was rapidly reverted with management of the BP and the patient was discharged at the fifteenth day with no clinical deficit.
ICA occlusion was performed preferentially at the C2 segment when possible, ideally just below the origin of the ophthalmic artery. This occlusion site was achieved in 14 cases (23.3%), for the most part patients with cervical tumors. In the remaining 46 patients, largely those with an aneurysm, occlusion was achieved in other sites. For these patients, the parent vessel was occluded at the level of the aneurysm.
Hospitalization time ranged from 2 to 15 days (mean, 4.5 days). Nine patients were transferred to the referring hospital within 2 days after the procedure for surgical resection of head and neck tumors.
Average mean BP assessed in all patients during the anesthetic consultation before the procedure was 106.6 mmHg. GA induced a 26.1% reduction in mean BP. Average mean BP measured during GA was 78.7 mmHg. Average mean BP during the first 48 hours after the procedure in the ICU was 110.4 mmHg.
Discussion
Therapeutic occlusion of the ICA remains an important procedure in interventional neuroradiology (1–7, 13). Temporary BTO is imperative to evaluate ischemic risks before definitive occlusion is performed (7, 9, 12–15). The introduction of clinical BTO of the ICA was associated with significant reduction in postocclusion morbidity. A review of the literature comprising 516 patients demonstrated that the use of BTO of the ICA reduced the morbidity of permanent ICA occlusion from 26% to ∼13% (8). Many variations of the original method have been proposed to reduce the residual ischemic rate that persisted in cases despite negative clinical tests. These variations usually consist of measurement of the CBF reorganization after abrupt occlusion of one ICA. These procedures are of increased complexity and costs and, probably, add risks for the patients.
Many BTO protocols have been designed to increase the sensitivity of the clinical occlusion test and reduce the remaining risk of delayed ischemic events. Most of the methods proposed so far require prolonged balloon inflation time, ranging from 15 to 120 minutes (24–26), with patients locally anesthetized. The extended duration of occlusion in these protocols may increase the risk of thromboembolic complications, even when full anticoagulation with heparin is performed.
Neurologic monitoring is then associated to one or more additional tests, which try to estimate (directly or indirectly) the tolerance of the ICA occlusion during the BTO. Techniques such as stable Xe CT (6, 15), 99mTc hexamethylpropylene amine oxamine single-photon emission CT (27, 28), positron-emission tomography (17), electroencephalography (16, 29), transcranial Doppler sonography (18), perfusion MR imaging (12), or others have been used combined with clinical neurologic monitoring.
The definition of the threshold of tolerance, which directly affects the predictive value of the test, is a main problem in these semiquantitative tests. Moreover, they increase the complexity, costs, and time of evaluation of the procedure and, yet, are not widely available.
In this work, we present a simple angiographic BTO criterion, without any clinical test, performed under GA. The procedure is fast and straightforward and thus reduces the risks of thrombus formation associated with prolonged balloon inflation time in the ICA.
Our results demonstrate that the angiographic evaluation of the venous phase symmetry between the occluded and the injected territories can predict the safety of permanent ICA occlusion. Angiographic analysis during BTO is ultimately one simple method to measure the redistribution of blood flow.
Even if the angiographic evaluation of the venous drainage delay in BTO of the ICA has been recently reported (3, 23), the parameters proposed are very strict, requiring exact venous drainage symmetry between the occluded and injected territories. This exact symmetry of the venous filling was achieved in only 73.3% of our patients.
Our findings suggest that patients having a delay of the venous drainage of not >2 seconds in the BTO can safely undergo ICA occlusion, corresponding to a negative CBF test. Patients with venous drainage delay of 2–4 seconds probably correspond to those with borderline CBF evaluation. In these cases, it is difficult to assure the incidence of complications. Finally, >4 seconds of venous drainage delay is probably equivalent to a poor CBF test. These patients are at higher risk to develop complications after permanent ICA occlusion.
It is important to point out that GA induced significant reduction in mean BP in our patients (26.1% on average compared with baseline preanesthetic mean BP). This per operative hypotension can be compared with a hypotensive challenge test (30), which is known to sensitize BTO. It is possible that the hypotension following GA may have sensitized the results in our series. It is also interesting to note that there were no complications associated with GA in our patients, especially if one considers the great number of head and neck tumors in our sample (23), with especially difficult anesthetic conditions.
Monitoring of the patient in the ICU after ICA occlusion is mandatory to prevent ischemic events. Preventing low CBF status during at least 48 hours after the abrupt reorganization of CBF due to ICA occlusion is crucial for the outcome. Low debit status can lead to watershed area infarctions even if the BTO allows for permanent occlusion. This is especially a concern for patients with venous drainage delay of 2–4 seconds. In these patients, BP management after ICA occlusion must be very strict. Patients undergoing tumor resection surgeries a few days after ICA occlusion also have to be closely observed. Special care during GA must be taken to prevent severe hypotension during the surgery that could lead to ischemic events (31).
The site of occlusion for the ICA should be ideally at the C2 segment, immediately below the origin of the ophthalmic artery. This site reduces the column of thrombus that is formed and thus minimizes the risk of thromboembolic events after the occlusion. Anticoagulation was kept for at least 48 hours after the procedure. These measures were taken because, in our experience, the circle of Willis may create a vacuum effect, aspirating thrombus from the occluded ICA to distal territories, especially to the ophthalmic artery resulting in acute visual loss (32, 33).
Conclusion
Our results demonstrate that BTO of the ICA based on the analysis of the symmetry of the venous phase is a safe, reliable, and simple procedure to be adopted for patients undergoing permanent ICA occlusion. The procedure reduces the need for prolonged balloon inflation periods and is consequently associated with lower thromboembolic risk. Clinical examination proved to be unnecessary to evaluate the feasibility of the occlusion, because all BTOs were performed under GA.
GA is not mandatory, but we believe that the present protocol gives the parameters to test the feasibility of the ICA occlusion, whether under local anesthesia or GA.
Our series suggest that permanent ICA occlusion will be safe when venous drainage delay in the BTO is not >2 seconds. In patients with venous drainage delay of 2–4 seconds, the ICA occlusion is recommended only in selected cases, because of the impossibility to predict ischemic events.
References
- 1.Adams GL, Madison M, Remley K, Gapany M. Preoperative permanent balloon occlusion of internal carotid artery in patients with advanced head and neck squamous cell carcinoma. Laryngoscope 1999;109:460–466 [DOI] [PubMed] [Google Scholar]
- 2.Nayak UK, Donald PJ, Stevens D. Internal carotid artery resection for invasion of malignant tumors. Arch Otolaryngol Head Neck Surg 1995;121:1029–1033 [DOI] [PubMed] [Google Scholar]
- 3.Sanna M, Piazza P, Ditrapani G, Agarwal M. Management of the internal carotid artery in tumors of the lateral skull base: preoperative permanent balloon occlusion without reconstruction. Otol Neurotol 2004;25:998–1005 [DOI] [PubMed] [Google Scholar]
- 4.Larson JJ, Tew JM Jr, Tomsick TA, van Loveren HR. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery 1995;36:26–30 [PubMed] [Google Scholar]
- 5.Fox AJ, Vinuela F, Pelz DM, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg 1987;66:40–46 [DOI] [PubMed] [Google Scholar]
- 6.Field M, Jungreis CA, Chengelis N, et al. Symptomatic cavernous sinus aneurysms: management and outcome after carotid occlusion and selective cerebral revascularization. AJNR Am J Neuroradiol 2003;24:1200–1207 [PMC free article] [PubMed] [Google Scholar]
- 7.Lubicz B, Gauvrit JY, Leclerc X, et al. Giant aneurysms of the internal carotid artery: endovascular treatment and long-term follow-up. Neuroradiology 2003;45:650–655 [DOI] [PubMed] [Google Scholar]
- 8.Linskey ME, Jungreis CA, Yonas H, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol 1994;15:829–843 [PMC free article] [PubMed] [Google Scholar]
- 9.Brunberg JA, Frey KA, Horton JA, et al. [15O]H2O positron emission tomography determination of cerebral blood flow during balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol 1994;15:725–732 [PMC free article] [PubMed] [Google Scholar]
- 10.Dare AO, Chaloupka JC, Putman CM, et al. Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayed hemodynamic ischemia after therapeutic carotid occlusion. Surg Neurol 1998;50:147–155 [DOI] [PubMed] [Google Scholar]
- 11.Eskridge JM. Xenon-enhanced CT: past and present. AJNR Am J Neuroradiol 1994;15:845–846 [PMC free article] [PubMed] [Google Scholar]
- 12.Michel E, Liu H, Remley KB, et al. Perfusion MR neuroimaging in patients undergoing balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol 2001;22:1590–1596 [PMC free article] [PubMed] [Google Scholar]
- 13.van der Schaaf IC, Brilstra EH, Buskens E, Rinkel GJ. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke 2002;33:313–318 [DOI] [PubMed] [Google Scholar]
- 14.Vazquez Anon V, Aymard A, Gobin YP, et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring, and results. Neuroradiology 1992;34:245–251 [DOI] [PubMed] [Google Scholar]
- 15.Barr JD, Lemley TJ, McCann RM. Carotid artery balloon test occlusion: combined clinical evaluation and xenon-enhanced computed tomographic cerebral blood flow evaluation without patient transfer or balloon reinflation: technical note. Neurosurgery 1998;43:634–637 [DOI] [PubMed] [Google Scholar]
- 16.Frampas E, Desal HA, Lenoir V, et al. Endovascular carotid occlusion: a retrospective study of complications in 33 cases. J Neuroradiol 2000;27:238–246 [PubMed] [Google Scholar]
- 17.Grubb RL Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055–1060 [DOI] [PubMed] [Google Scholar]
- 18.Hetzel A, von Reutern G, Wernz MG, et al. The carotid compression test for therapeutic occlusion of the internal carotid artery: comparison of angiography with transcranial Doppler sonography. Cerebrovasc Dis 2000;10:194–199 [DOI] [PubMed] [Google Scholar]
- 19.Morishima H, Kurata A, Miyasaka Y, et al. Efficacy of the stump pressure ratio as a guide to the safety of permanent occlusion of the internal carotid artery. Neurol Res 1998;20:732–736 [DOI] [PubMed] [Google Scholar]
- 20.Sugawara Y, Kikuchi T, Ueda T, et al. Usefulness of brain SPECT to evaluate brain tolerance and hemodynamic changes during temporary balloon occlusion test and after permanent carotid occlusion. J Nucl Med 2002;43:1616–1623 [PubMed] [Google Scholar]
- 21.Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol 1995;16:749–754 [PMC free article] [PubMed] [Google Scholar]
- 22.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurgery 1974;41:125–145 [DOI] [PubMed] [Google Scholar]
- 23.van Rooij WJ, Sluzewski M, Slob MJ, Rinkel GJ. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol 2005;26:175–178 [PMC free article] [PubMed] [Google Scholar]
- 24.Monsein LH, Jeffery PJ, van Heerden BB, et al. Assessing adequacy of collateral circulation during balloon test occlusion of the internal carotid artery with 99mTc-HMPAO SPECT. AJNR Am J Neuroradiol 1991;12:1045–1051 [PMC free article] [PubMed] [Google Scholar]
- 25.Tarr RW JC, Horton JA, Pentheny S, et al. Complications of preoperative balloon test occlusion of internal carotid arteries: experience with 300 cases. Skull Base Surg 1991;1:240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterman SB, Taylor A Jr, Hoffman JC Jr. Improved detection of cerebral hypoperfusion with internal carotid balloon test occlusion and 99mTc-HMPAO cerebral perfusion SPECT imaging. AJNR Am J Neuroradiol 1991;12:1035–1041 [PMC free article] [PubMed] [Google Scholar]
- 27.Ryu YH, Chung TS, Lee JD, et al. HMPAO SPECT to assess neurologic deficits during balloon test occlusion. J Nucl Med 1996;37:551–554 [PubMed] [Google Scholar]
- 28.Yamamoto Y, Nishiyama Y, Toyama Y, et al. Preliminary results of Tc-99m ECD SPECT to evaluate cerebral collateral circulation during balloon test occlusion. Clin Nucl Med 2002;27:633–637 [DOI] [PubMed] [Google Scholar]
- 29.Cloughesy TF, Nuwer MR, Hoch D, et al. Monitoring carotid test occlusions with continuous EEG and clinical examination. J Clin Neurophysiol 1993;10:363–369 [DOI] [PubMed] [Google Scholar]
- 30.Standard SC, Ahuja A, Guterman LR, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol 1995;16:1453–1458 [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez CF, Moret J. Balloon occlusion of the carotid artery prior to surgery for neck tumors. AJNR Am J Neuroradiol 1990;11:649–652 [PMC free article] [PubMed] [Google Scholar]
- 32.Russell EJ, Goldberg K, Oskin J, et al. Ocular ischemic syndrome during carotid balloon occlusion testing. AJNR Am J Neuroradiol 1994;15:258–262 [PMC free article] [PubMed] [Google Scholar]
- 33.Hurst RW, Goldberg HI. Transient monocular blindness in carotid occlusion testing. AJNR Am J Neuroradiol 1994;15:255–257 [PMC free article] [PubMed] [Google Scholar]