Abstract
BACKGROUND AND PURPOSE: The incidence of blunt traumatic vertebral artery dissection/thrombosis varies widely in published trauma series and is associated with spinal trauma. The purpose of this study was to determine the frequency of traumatic vertebral artery thrombosis (VAT) in cervically injured patients by using routine MR angiography (MRA) and MR imaging and identify associations with the severity of neurologic injury.
METHODS: A retrospective review of 1283 patients with nonpenetrating cervical spine fractures with or without an associated spinal cord injury (SCI) was performed. Imaging consisted of routine cervical MR imaging and 2D time-of-flight MRA of the neck. The cervical injury level, neurologic level of injury, and American Spinal Injury Association (ASIA) grade were recorded.
RESULTS: In this study, 632 patients met the inclusion criteria, 83 (13%) of whom had VAT on the admission MR imaging/MRA. Fifty-nine percent (49/83) of VAT patients had an associated SCI. VAT was significantly more common in motor-complete patients (ASIA A and B, 20%) than in neurologically intact (ASIA E, 11%) cervical spine–injured patients (P = .019). VAT incidence was not significantly different between motor-incomplete (ASIA C and D, 10%) and neurologically intact (ASIA E, 11%) cervical spine–injured patients (P = .840).
CONCLUSION: The absence of neurologic symptoms in a patient with cervical spine fracture does not preclude VAT. VAT associated with cervical spinal injury occurs with similar frequency in both neurologically intact (ASIA E) and motor-incomplete patients (ASIA C and D) but is significantly more common in motor-complete SCI (ASIA A and B).
Vertebral artery thrombosis (VAT) is a complication of cervical spine injury with potentially fatal complications. VAT is a subset of vertebral artery injuries (VAI), which also include dissection and transection (rare). The incidence of VAIs overall following cervical spinal injury varies considerably in the published trauma literature, with incident rates ranging from 3% to 88% (1–5). Possible explanations for the variable incident rates include patient selection biases, small patient cohorts (usually <20 patients with VAT), variation in imaging technique, type of injury, and inconsistencies in patient evaluation. Posttraumatic VAT is often asymptomatic, and the impact of VAT on neurologic outcome is unknown. Moreover, unless this complication is screened for at the time of injury, it may remain undetected. The results of prior trauma series suggest that VAT has a high association with fractures or dislocations of the cervical spine or cervical spinal cord injury (SCI; 1–5). There are, however, no large reported series of spinal-injured patients with VAT to support this hypothesis. The purpose of this investigation was to approximate the overall incidence of VAT by using MR angiography (MRA) in a large series of spine trauma patients considered at “high risk” for VAI and to determine whether there are significant differences in the incidence of VAT relative to the severity of neurologic deficit.
Methods
Patient Cohort
Before performance, approval for this project was obtained through our local institutional review board. The cohort for this study was derived from a retrospective review of admissions to a regional SCI center for the period January 1995–February 2002. Eligible subjects sustained an osseous cervical spine injury and/or cervical SCI as a result of nonpenetrating trauma and had cervical MR imaging, including MRA, within 7 days of the initial injury. The MRA consisted of routine 2D time-of-flight acquisition of the neck by using a walking superior saturation pulse to suppress venous inflow.
During this period, 1283 patients were either referred or directly admitted to the Regional Spinal Cord Injury Center of the Delaware Valley with major cervical spinal trauma, defined as a cervical fracture or dislocation, or, in their absence, the presence of neurologic deficits. Fifty-five of the 1283 patients had injuries due to penetrating trauma and were excluded. Of the remaining 1228 patients, an additional 596 patients were excluded for the following reasons: MRA was omitted from the trauma protocol (n = 461), MR imaging/MRA study was not available for review (n = 7), MR imaging/MRA completed >7 days after the original injury (n = 81), indeterminate MR imaging/MRA because of the absence of 3D images and an insufficient number of axial images (n = 38), indeterminate MR imaging/MRA because of poor quality (n = 6), and undefined American Spinal Injury Association (ASIA) grade and/or neurologic level (n = 3). In all, 632 of the original 1228 patients (51%) underwent diagnostic cervical MR imaging studies and MRAs within 7 days of injury and were analyzed. For most of the 632 patients—533 (84%)—cervical MR imaging studies/MRAs were performed within 2 days of their injuries.
For the defined cohort of 632 subjects, the following clinical information was recorded: subject demographics, date of injury, cervical level of injury (fracture or dislocation), neurologic level of injury (defined as the lowest intact neurologic segment; 6), ASIA impairment grade (A–E; Table 1; 6), and etiology of injury.
TABLE 1:
The ASIA classification of neurologic impairment
| ASIA Grade | Description |
|---|---|
| A | No motor or sensory function preserved in the sacral segments |
| B | Sensory but not motor function preserved in at least the sacral segments |
| C | Motor function preserved below the neurologic level and most key muscles have motor score <3 |
| D | Motor function preserved below the neurologic level and most key muscles have a motor score of ≥3 |
| E | Motor and sensory function is normal. |
Note.—ASIA indicates American Spinal Injury Association. Adapted from Ditunno (6).
MRA Evaluation
In all 632 patients, MRA was performed in conjunction with a cervical MR imaging study on a 1.5T scanner (General Electric, Milwaukee, WI) by using a receive-only anterior neck coil or a transmit-receive posterior multiarray coil; when a halo device was present, 2 circular 5-inch receive-only coils were used over the front and back of the neck. The MRA evaluation of the extracranial circulation consisted of a single 2D time-of-flight acquisition with a walking superior saturation pulse to suppress venous inflow (TR/TE, 40/8.7). The typical acquisition span was sufficient to evaluate the V2 (C6–C2) and V3 segments (extradural segment between C2 and skull base) of the vertebral arteries, with variable evaluation of the V1 (proximal) vertebral segment or vertebral origin and V4 (intradural segment). The “black-blood” MRA technique (superior and inferior spatial saturation with active fat saturation) was not routinely used in the cervical spine trauma MR imaging protocol and was not included in this study.
The remainder of the cervical trauma MR protocol consisted of a sagittal T1 sequence (TR/TE/section/gap/NEX: 350/8.0/3 mm/1 mm/4), a sagittal spin-attenuation sequence (TR/TE/echo train length/section/gap/NEX: 1800/15/4/3 mm/1 mm/2), a late-echo T2-weighted sequence (TR/TE/echo train length/section/gap/NEX: 2800/90/8/3 mm/1 mm/4), a sagittal gradient echo (GRE) sequence (TR/TE/flip/section/gap/NEX: 400/13/30/3 mm/1 mm/2), and axial 3D GRE images (TR/TE/flip: 37/minimum/15).
All 632 studies were initially screened by evaluating the radiologic report. All normal MRA studies of the vertebral arteries were reviewed by at least one board-certified neuroradiologist. Those studies in which the official report specifically indicated a normal appearance of the vertebral arteries were not secondarily reviewed and were considered normal (n = 381). Secondary review was performed on studies in which the official report described an abnormality in one or both of the vertebral arteries (n = 91) or when the report failed to address the integrity of the vertebral arteries (n = 160).
All studies with equivocal findings, unreported MRA evaluation, or definite abnormalities were given secondary review by a second board-certified neuroradiologist (A.E.F.).
For the secondary review, the minimum imaging requirements for evaluation included the maximum intensity projection (MIP) images and the MRA source images in addition to the cross-sectional GRE images (TR/TE/flip: 37/minimum/15) from the standard MR study of the cervical spine.
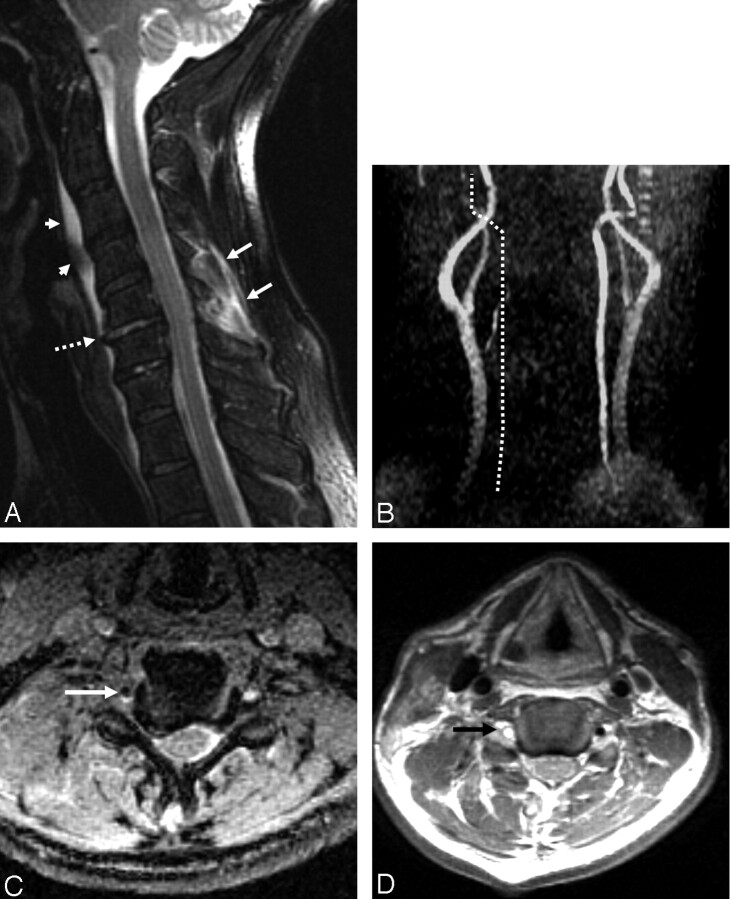
All selected MRA studies were reviewed only for the presence or absence of VAT. Images were considered positive for VAT if there was absence of flow-related enhancement on the MRA images in the expected course of the vertebral artery (Fig 1B) and identification of an acute thrombus in the foramen transversarium on the corresponding side on the GRE (hypointense clot), (Fig 1C).
Fig 1.
A 58-year-old man who sustained a unilateral interfacetal dislocation at C5–C6 without SCI.
A, Sagittal T2-weighted fast spin-echo (FSE) image (TR/TE/ETL: 2000/80/8) shows an injured disk at C5–C6 with increased signal intensity in the disk and probably avulsion of the anterior longitudinal ligament (dashed arrow). Prevertebral edema (arrowheads) and edema in the posterior paraspinal musculature (white arrows) are present.
B, Nonvisualization of the right vertebral artery. MIP image (anterior view) from a 2D time-of-flight acquisition (TR/TE/flip: 40/8.7/30) shows absence of signal intensity in the expected course of the right vertebral artery (dotted line).
C, Thrombus in the right foramen transversarium. Axial image from a 3D GRE acquisition (TR/TE/flip: 37/min/15) shows an oval area of low signal intensity in the right foramen transversarium corresponding to thrombus in the right vertebral artery. Note the normal flow-related enhancement in the left foramen transversarium.
D, Thombus in the right vertebral artery. Axial FSE image (TR/TE/ETL: 3000/28/4) obtained at a similar level to image in panel C shows a high-signal-intensity thrombus (arrow) in the right foramen transversarium indicative of a thrombosed vertebral artery. Note the normal flow void of the left vertebral artery in the left foramen transversarium.
Absence of flow in a vertebral artery alone (from congenital absence or chronic occlusion) did not constitute an acute thrombosis. The date of imaging and quality and completeness of the imaging study were recorded.
Vertebral arteries were classified as patent, acutely thrombosed, or occluded/hypoplastic (indeterminate age). There was no attempt to subclassify arterial abnormalities in the setting of a patent vessel (eg, intimal injury, narrowing, psuedoaneurysm, or intimal flap). Arterial irregularity, distortion at the level of subluxation, or narrowing were noted but were not designated as abnormal as long as the parent vessel proved patent. An acutely thrombosed vertebral artery was distinguished from a chronic thrombosis or aplasia by the presence of acute thrombus on the corresponding GRE images.
Statistical Analysis
χ2 analysis was used with a P value <.05 being significant. Subjects were grouped categorically by the presence or absence of vertebral thrombosis versus the degree of neurologic impairment (ASIA impairment grade). χ2 analysis was used to compare subjects with and without VAT relative to their ASIA classification (ASIA A–E), motor-complete and -incomplete patients (ASIA A and B vs ASIA C and D), all neurologic deficits and neurologically normal subjects (ASIA A–D and ASIA E), and the presence or absence of fracture. The mechanism of injury and classification of bony injury were inconsistently recorded in subject records and could not be accurately subclassified.
A substantial portion (n = 461) of the original 1283 patients were excluded because of failure to perform an MRA examination (a variance from our standard protocol). As a result, approximately half (n = 648) of the original patient population did not meet the inclusion criteria. A comparative analysis of the included and excluded cohorts was performed to determine whether there was a significant difference in neurologic injury and demographics between the patients included in the study and those who were excluded. A comparison of patient demographics and distribution of neurologic abnormalities was performed (by using χ2 and Student t test) to determine whether the excluded subset of patients differed significantly from the study group.
Results
A summary of the study cohort is provided in Table 2. The mean age of the study group was 49 years (range, 12–102 years), and 71% (n = 449) were men or boys. Three hundred thirty-four (53%) of the 632 patients sustained a SCI. The most severe neurologic deficit (ASIA A) was also the most common (34% [n = 115]) followed by ASIA D (28% [n = 94]), ASIA C (25% [n = 83]), and ASIA B (13% [n = 42]). The remaining 298 (47%) patients were neurologically intact (ASIA E). The distribution of the neurologic level of injury was as follows: C4, 22% (n = 141); C5, 16% (n = 103); C6, 8% (n = 49); C3, 3% (n = 16); C7, 2% (n = 11); and C2, 2% (n = 11). Eighty-three (13%) of the 632 patients had confirmed acute VAT. Left VAT occurred in 42 (48%) patients, right VAT in 37 (43%) patients, and VAT was bilateral in 4 (9%) patients. All 4 bilateral VAT patients sustained an ASIA A–type neurologic deficit. Of the 438 patients with documented fractures, 16% (n = 68) sustained VAT.
TABLE 2:
Demographic summary of study population, including ASIA impairment grade, etiology, level of bony injury, and neurologic level of injury
| (N = 63) | % (n) |
|---|---|
| Patients | |
| Male | 71 (449) |
| Female | 29 (183) |
| Mean age (y) | 48.6 (12–102) |
| ASIA impairment grade | |
| A | 34 (115) |
| B | 13 (42) |
| C | 25 (83) |
| D | 28 (94) |
| E | 47 (298) |
| Etiology | |
| Fall | 43 (270) |
| Automobile crash | 34 (213) |
| Not specified | 7 (46) |
| Diving accident | 6 (39) |
| Pedestrian accident | 3 (17) |
| Assault | 2 (13) |
| Sports injury | 2 (13) |
| Motorcycle crash | 2 (12) |
| Projectile | 1 (9) |
| Level of bony injury | |
| C1 | 3 (32) |
| C2 | 5 (52) |
| C3 | 9 (82) |
| C4 | 18 (168) |
| C5 | 26 (246) |
| C6 | 26 (247) |
| C7 | 13 (120) |
| Neurologic level of injury | |
| C2 | 2 (11) |
| C3 | 3 (16) |
| C4 | 22 (141) |
| C5 | 16 (103) |
| C6 | 8 (49) |
| C7 | 2 (11) |
| C8 | 0.5 (3) |
| No level (ASIA E) | 47 (298) |
Note.—ASIA indicates American Spinal Injury Association.
Multiple fractures were possible in individual patients.
The distribution of cervical fracture levels associated with VAT in descending order of frequency was C5 (34%), C6 (24%), C4 (21%), C7 (9%), C3 (6%), C2 (4%), and C1 (3%). The incidence of VAT was significantly more common in patients with confirmed cervical fractures of all types compared with patients without (P < .01). Of the 334 SCI patients, 15% (n = 49) sustained VAT (Table 3). ASIA impairment grades for the SCI patients with VAT were as follows: ASIA A, 30% (n = 25); ASIA B, 7% (n = 6); ASIA C, 11% (n = 9); and ASIA D, 11% (n = 9). Thirty-four patients (41%) with VAT were neurologically intact (ASIA E). Most VAT patients in all ASIA classifications also had cervical fractures: 88% (22/25) of ASIA A, 83% (5/6) of ASIA B, 56% (5/9) of ASIA C, 67% (6/9) of ASIA D, and 88% (30/34) of ASIA E patients with VAT had an acute cervical fracture on admission.
TABLE 3:
Demographic data of patients with proved vertebral artery thrombosis (VAT), including ASIA impairment grade, etiology, level of bone injury, and neurologic level of injury
| (N = 83) | % (n) |
|---|---|
| Patients with VAT | |
| Male | 76 (63) |
| Female | 24 (20) |
| Mean age (y) | 44 (14–88) |
| Vertebral artery affected | |
| Left | 48 (42) |
| Right | 43 (37) |
| Both | 9 (4) |
| ASIA impairment grade | |
| A | 30 (25) |
| B | 7 (6) |
| C | 11 (9) |
| D | 11 (9) |
| E | 41 (34) |
| Etiology | |
| Automobile crash | 45 (37) |
| Fall | 28 (23) |
| Sports injury | 12 (10) |
| Other | 15 (13) |
| Level of bony injury | |
| C1 | 3 (5) |
| C2 | 4 (6) |
| C3 | 6 (10) |
| C4 | 21 (34) |
| C5 | 33 (55) |
| C6 | 24 (39) |
| C7 | 9 (14) |
| Neurologic level of injury | |
| C3 | 5 (3) |
| C4 | 28 (23) |
| C5 | 18 (15) |
| C6 | 6 (5) |
| C7 | 1 (2) |
| C8 | 1 (2) |
| No neurologic level | 41 (34) |
Note.—ASIA indicates American Spinal Injury Association.
Multiple fractures were possible in individual patients.
The incidence of VAT for patients presenting with complete motor paralysis (ASIA A and B) was 20%, with motor-incomplete (ASIA C and D) injuries was 10%, and with neurologically intact (ASIA E) patients was 11%. There was no significant difference in the incidence of VAT between neither each ASIA grade (P < .1) nor neurologically impaired (ASIA A–D) and ASIA E (P < .1) patients. There were, however, significant differences in the incidence of VAT when patients were grouped on the basis of motor deficit (ie, motor-complete, motor-incomplete, and normal) (P < .025). VAT was more common in motor-complete patients (ASIA A and B) than in neurologically intact (ASIA E) SCI patients (P = .019). There was a trend toward significance when motor-complete (ASIA A and B) patients were compared with motor-incomplete (ASIA C and D) patients (P = .057). Vertebral artery thrombosis was not significantly different between motor-incomplete (ASIA C and D) and neurologically intact (ASIA E) SCI patients (P = .840).
Comparison of the demographic and neurologic impairment data for the patients who met the study criteria, and the patients who were excluded are listed in Table 4. The 2 populations differed statistically with respect to gender distribution (P < .01) and neurologic level (P < .001), but not for age (P < 1) or neurologic deficit (ASIA A–D; P = .939).
TABLE 4:
Comparison of patient demographics, ASIA neurologic impairment score, and neurologic level of injury for patients included and excluded from analysis
| Included | Excluded | Total | |
|---|---|---|---|
| Number | 635 | 648 | 1280* |
| Average age (y) | 49 | 53 | 102 |
| Male | 452 | 408 | 860 |
| Female | 183 | 240 | 423 |
| ASIA impairment grade | |||
| A | 115 | 92 | 207 |
| B | 42 | 29 | 71 |
| C | 83 | 57 | 140 |
| D | 94 | 49 | 143 |
| E | 298 | 417 | 715 |
| Level of injury | |||
| C1 | 0 | 6 | 6 |
| C2 | 11 | 18 | 29 |
| C3 | 16 | 19 | 35 |
| C4 | 141 | 81 | 222 |
| C5 | 104 | 61 | 165 |
| C6 | 50 | 21 | 71 |
| C7 | 11 | 17 | 28 |
| C8 | 3 | 6 | 9 |
Note.—ASIA indicates American Spinal Injury Association. Populations differed statistically with respect to sexual distribution (P < .01) and neurologic level (P < .001), but not for age (P < 1) or neurologic deficit (ASIA impairment grades A–D, P <.939).
Three patients were excluded because of unavailable clinical data.
Discussion
Many published studies on vascular injury to the neck collectively refer to traumatic blunt injury to either the carotid or vertebral arteries (BCVI, or blunt carotid and vertebral injury). Multicenter trauma reviews report an incidence of blunt carotid injury ranging from 0.08% to 1.1% and isolated vertebral artery injury as less frequent (2, 7–11). Several investigators have suggested that blunt carotid injury is underdiagnosed because of either inadequate screening criteria or the delayed presentation of clinical symptoms (7, 9). In addition to these 2 factors, the wide variation in reported incidence of vertebral artery injury is also attributable to patient selection biases, varying injury mechanisms (eg, rapid head turning, chiropractic manipulation, seizure), different imaging techniques (eg, arteriography vs MRA), and the type of injury (eg, intimal injury/dissection vs thrombosis). These differences preclude critical comparison between studies. In this context, our cohort has a selection bias because they represent a subselection of patients admitted to a level I trauma center and an SCI center. This selection bias is tempered by selecting only for vertebral artery thrombosis instead of all types of vertebral injuries (including dissections), which are likely to be more frequent in this population. Despite the inherent selection bias for patients with a relative high risk for VAT, the overall incidence of VAT in our cohort remains relatively low (13%) compared with other reports of the incidence of posttraumatic VAT, some as high as 88% (1, 2, 4, 5).
Early recognition of vertebral artery injury remains important because of its potential to produce significant neurologic comorbidity and permanent neurologic damage. Moreover, the secondary injury is potentially preventable with early institution of therapy (eg, anticoagulation, embolization, or surgical ligation) (7). The incidence of isolated vertebral artery injury in the setting of cervical spine trauma is not well known because the patient is frequently asymptomatic at the time of injury (1).
In a prospective study of 47 cervical spine trauma patients, Parbhoo et al (1) reported that 26% (n = 12) of the patients showed vertebral artery damage on MR imaging/MRA; in 9 patients (19%), the vertebral artery was thrombosed. Most of the patients with vertebral artery injury (n = 10) had an associated unilateral facet dislocation (1).
Willis et al (5) prospectively selected 26 patients with bony injuries associated with vertebral artery injury and found VAT in 9 patients (35%), normal VA in 14 patients (54%), and dissection in 3 patients (11%) by using angiography; SCI was present in half of the patients, and no neurologic sequelae were attributed to the arterial injuries.
In another prospective MR imaging/MRA study, Friedman et al (12) identified VAT in 9 (24%) of 37 consecutive cervical SCI patients. VAT was significantly more common in the motor-complete SCI patients than in the motor-incomplete patients.
By using cerebral arteriography, Biffl et al (2) found 47 vertebral artery injuries in 38 patients in a selected cohort derived from 7205 consecutive patients (0.53%) admitted with blunt trauma to a single level I trauma center; 350 patients were selected for cerebral arteriography on the basis of mechanism of injury or the existence of acute cerebrovascular symptoms. The most frequent related injury associated with vertebral injury included spine fracture/dislocation (71%), followed by chest and extremity injury (45%). The actual frequency of VAT in the subgroup evaluated with angiography was 2.6%. These authors found no relationship between grade of vertebral artery injury, neurologic deficit, or neurologic outcome (2). In a recently published retrospective study that used identical selection criteria, Cothren et al (13) reported vertebral artery injuries in 92 patients during a 6-year period. Associated injuries included cervical spine fractures in 69 patients. As with the prior study, because the patient cohort was preselected, there was substantial selection bias. At another level I trauma center, 48 patients were preselected from 1941 blunt trauma patients during an 18-month period by using screening criteria that selected for potential high risk of vascular injury. Ten of the 48 high-risk patients were found to have blunt vascular injury to the vertebral arteries (0.5% of all blunt trauma patients). The selection criteria that were most predictive of a vascular injury included severe cervical spine fracture, cerebrovascular accident, or transient ischemic attack (14).
By using cerebral angiography, Miller et al (15) identified 64 vertebral artery injuries in 50 patients in a 4-year retrospective review of blunt trauma admissions, an overall incidence of 0.4%. Twenty-two percent of the vertebral artery injuries were occlusions, and 12% of the vertebral artery injuries were predicted by symptoms of posterior circulation ischemia, whereas most were suspected on the basis of injury pattern alone (15).
Other investigators have shown a higher incidence of vertebral artery thrombosis on cerebral arteriography when selecting for patients with specific types of cervical injuries, such as facet joint dislocations (75%; unilateral or bilateral) and foramen transversarium fractures (88%; 3, 4). The frequency of VAT was lower overall for similar studies that used MRA as the imaging technique: 33% for patients with foramen transversarium fractures and as high as 24% for multiple contiguous cervical spinal injuries (4, 12, 16).
There is some disparity in the literature whether the severity of neurologic deficit has a relationship to the frequency of VAT. Giacobetti et al (16) prospectively reviewed MRA examinations of 61 consecutive cervical spine injuries, finding VAT in 12 (19.7%). Twenty-eight of the 61 patients sustained some form of SCI. The frequency of VAT stratified by ASIA impairment scale included ASIA A (n = 3), ASIA B (n = 3), ASIA D (n = 2), and ASIA E (n = 4). This suggested that the severity of neurologic injury was not predictive of VAT. No permanent neurologic deficits related to the VAT were identified (16). Six of the patients were reimaged by MRA 12–26 months after injury. The vertebral arteries in 5 patients remained thrombosed on the subsequent MRA study (17).
Blunt injuries to the vertebral arteries have the potential to present with devastating strokes. Miller et al (15) reported a stroke rate of 54% for untreated blunt vertebral artery injury (BVAI), whereas Biffl et al (2) reported a posterior circulation stroke rate of 25% with documented BVAI. It is of interest that no other investigators have reported such a large proportion of cerebrovascular accidents directly attributed to BVAI.
The use of systemic anticoagulation with blunt carotid and vertebral dissections or occlusions has not definitively been shown to be protective against cerebral thromboembolism (11, 18). In one series of 29 blunt carotid and vertebral injuries, systemic anticoagulation provided no clinical benefit and 16% of the patients experienced complications related to anticoagulation (11). Miller et al (15) found that the rate of posterior circulation stroke decreased from 54% in untreated patients with vertebral artery injury to 2.6% when either antiplatelet or systemic anticoagulation was instituted. The generally held empirical method of treatment of spontaneous dissection of the carotid or vertebral arteries is a prolonged course of anticoagulation (approximately 6 months) to prevent thromboembolic events and ischemia while the artery repairs itself (11, 15, 19). In cases where anticoagulation is contraindicated or not effective, the parent vessel may be deliberately occluded surgically or via an endovascular approach.
There is some literature that supports aggressive management of VAI with anticoagulation to prevent stroke. Miller et al (15) found that significantly fewer asymptomatic VAI patients developed stroke following heparin or aspirin therapy and that VAI patients treated with heparin had significantly higher Glasgow Outcome Scores on discharge. Biffl et al (2) also reported improved neurologic outcomes in a small number of patients with BVAI. The latter study did not use any standard measures of neurologic impairment and did not reliably demonstrate any statistical difference between treated and untreated cohorts.
Although this report is the largest single series of VAT to date, there are epidemiologic and diagnostic limitations. Because our institution is both a level I trauma center and a model SCI center, there is a selection bias in favor of all types of vertebral injury compared with the general referral population. Although we are critical of the obvious selection biases in previously published reports, we have, by virtue of our own referral patterns, preselected for high-risk spinal injuries and not trauma of all types as has been reported by others. Because prior authors have emphasized the strong correlation between cervical spine fractures/subluxations and vertebral artery injury, it might be inferred that our 13% overall incidence of thrombosis represents a sample with a high probability of vertebral artery injuries. Our relatively low incidence of VAT is confounded by the fact that we have deliberately chosen to eliminate the more common vertebral artery injuries and focus on vertebral artery thrombosis, a finding that can be established unequivocally with MRA/MR imaging without angiographic confirmation (20, 21). Therefore, the overall incidence of VAI in our cohort was undoubtedly >13%.
There are further limitations in this study that should be addressed. First, this is a retrospective analysis, and therefore it is subject to the innate inconsistencies inherent to such a review. The most significant of these was the surprising variability in the use of the MRA sequence, which is normally part of our routine cervical trauma protocol. This deficiency alone excluded a substantial portion of patients from our review and analysis. Although the patient cohort and excluded patients showed a similar distribution in neurologic impairment and gender, there were significant differences in neurologic level of injury and the proportion of neurologically normal (ASIA E) patients that would have had some effect in determining an overall incidence of VAT in our population. In addition, because of the inherent limitations of the MRA technique, this study was designed only to identify the incidence of incontrovertible posttraumatic vertebral artery thrombosis, excluding other types of injury such as vertebral dissection. Whereas routine MRA has been reported to provide a high degree of sensitivity to arterial thrombosis, it is less sensitive to intimal injuries (21, 22). Therefore, the incidence of vertebral artery injuries overall (thrombosis, dissection, and transection) is likely greater than indicated in this report.
Because no “black-blood” techniques were employed in the imaging protocol, there is a theoretical possibility that some patients who were imaged late (ie, after 3 or 4 days) would exhibit sufficient T1 shortening of a methemoglobin thrombus to lead to a false-negative MRA. Three-quarters of our patient cohort received their MR imaging/MRA evaluation within the first 48 hours after injury, so the risk of this is small. Moreover, in our experience, usually entire segments of the vertebral artery are absent above (or below) the level of injury to alert the radiologist that a thrombosis exists.
To have a full understanding of VAT, its neurologic consequences, and potential benefits of treatment, a reproducible method to assess the end-organ (ie, tissues supplied by the posterior circulation) is required. Unfortunately, most patients in our cohort did not undergo routine MR evaluation of the brain, and the routine admission CT study of the brain was insufficient to assess either the brain stem or cerebellum acutely. It is important to recognize that a spinal cord–injured patient may harbor a substantial, yet clinically occult cerebellar infarction that is masked clinically by the deficits produced by the SCI. Therefore, it was not possible to determine prospectively or retrospectively whether the patients in our cohort were at a greater risk for cerebral thromboembolism than patients without VAT. Therefore, although we were able to demonstrate a correlation between VAT and the severity of neurologic injury and that VAT occurs in a substantial portion of neurologically intact patients with spinal fractures, we were not able to objectively determine the neurologic consequences of VAT.
Conclusion
Vertebral artery thrombosis associated with cervical spine injury occurs with similar frequency in both neurologically intact (ASIA E) and motor-incomplete (ASIA C and D) patients and is significantly more frequent in motor-complete cervical spinal cord–injured patients (ASIA A and B). The absence of neurologic symptoms in patients with cervical fracture does not, however, preclude VAT. MRA should be performed in conjunction with cervical MR imaging for all cervical spine–injured patients to improve sensitivity in identifying thrombosis to the vertebral arteries. The occurrence of a vertebral thrombosis after a spinal injury may alter the surgical treatment plan such as to limit possible bilateral vertebral injuries. Therefore, MRA should be performed in conjunction with cervical MR imaging as part of a surgical evaluation.
Footnotes
This project was supported in part by grant H133N-000023 from the National Institute on Disability and Rehabilitation Research, Office of Special Education and Rehabilitative Services, U.S. Department of Education, Washington, DC.
Parts of this article were presented at the 88th annual meeting of the Radiological Society of North America, December 1–6, 2002, Chicago, IL.
References
- 1.Parbhoo AH, Govender S, Corr P. Vertebral artery injury in cervical spine trauma. Injury Int J Care Injured 2001;32:565–568 [DOI] [PubMed] [Google Scholar]
- 2.Biffl WL, Moore EE, Elliott JP, et al. The devasting potential of blunt vertebral arterial injuries. Annals Surgery 2000;23:672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louw JA, Mafoyane NA, Small B, Neser CP. Occlusion of the vertebral artery in cervical spine dislocations. J Bone Joint Surg [Br]1990;72:679–681 [DOI] [PubMed] [Google Scholar]
- 4.Weller SJ, Rosstich E, Malek AM. Detection of vertebral artery injury after cervical spine trauma using magnetic resonance angiography. J Trauma 1999;46:660–666 [DOI] [PubMed] [Google Scholar]
- 5.Willis BK, Greiner F, Orrison WW, Benzel EC. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery 1994;34:435–442 [DOI] [PubMed] [Google Scholar]
- 6.Ditunno J, ed. Standards for neurological and functional classification of spinal cord injury. 4th ed. Chicago: American Spinal Injury Association;1992
- 7.Biffl WL, Moore EE, Offner PJ, Burch JM. Blunt carotid and vertebral arterial injuries. World J Surg 2001;25:1036–1043 [DOI] [PubMed] [Google Scholar]
- 8.Ramadan F, Rutledge R, Oller D, et al. Carotid artery trauma: a review of contemporary trauma center experiences. J Vasc Surg 1995;21:4655; discussion 55–56 [DOI] [PubMed] [Google Scholar]
- 9.Cogbill T, Moore E, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma 1994;37:473–479 [DOI] [PubMed] [Google Scholar]
- 10.Davis JW, Holbrook TL, Hoyt DB, et al. Blunt carotid artery dissection: incidence, associated injuries, screening and treatment. J Trauma 1990;30:1514–1517 [PubMed] [Google Scholar]
- 11.Eachempati SR, Vaslef SN, Sebastian MW, Reed RL. Blunt vascular injuries of the head and neck: is heparinization necessary? J Trauma 1998;45:997–1004 [DOI] [PubMed] [Google Scholar]
- 12.Friedman DP, Flanders AE, Thomas C, Millar W. Vertebral artery injury after acute cervical spine trauma: rate of occurrence as detected by MR angiography and assessment of clinical consequences. AJR Am J Roentgenol 1995;164:443–447 [DOI] [PubMed] [Google Scholar]
- 13.Cothren CC, Moore EE, Biffl WL, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma 2003;55:811–813 [DOI] [PubMed] [Google Scholar]
- 14.Kerwin AJ, Bynoe RP, Murray J, et al. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma 2001;51:308–314 [DOI] [PubMed] [Google Scholar]
- 15.Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma 2001;51:279–286 [DOI] [PubMed] [Google Scholar]
- 16.Giacobetti FB, Vaccaro AR, Bos-Giacobetti MA, et al. Vertebral artery occlusion associated with cervical spine trauma: a prospective analysis. Spine 1997;22:188–192 [DOI] [PubMed] [Google Scholar]
- 17.Vaccaro AR, Klein GR, Flanders AE, et al. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine 1998;23:789–794 [DOI] [PubMed] [Google Scholar]
- 18.Colella JJ, Diamond DL. Blunt carotid injury: reassessing the role of anticoagulation. Am Surg 1996;62:212–217 [PubMed] [Google Scholar]
- 19.Fabian TC, Patton JH, Croce MA. Blunt carotid injury: importance of early diagnosis and anticoagulant therapy. Ann Surg 1996;223:513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen BC, Quencer RM, Margosian P, Paltany PM. MR angiography of occlusive disease of the arteries in the head and neck: current concepts. AJR Am J Roentgenol 1994;162:9–18 [DOI] [PubMed] [Google Scholar]
- 21.Levy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: three dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology 1004;190:97–103 [DOI] [PubMed] [Google Scholar]