Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to evaluate preliminarily the efficacy and safety of intravenous tirofiban combined with intra-arterial pharmacologic and mechanical thrombolysis in patients with stroke.
METHODS: Twenty-one consecutive patients with an acute ischemic stroke due to major cerebral arteries occlusion and a National Institutes of Health Stroke Scale [NIHSS] score ≥18 were treated with an intravenous bolus of tirofiban and heparin followed by intra-arterial administration of urokinase coupled with mechanical thrombolysis.
RESULTS: Thirteen patients had an anterior circulation stroke (T-siphon internal carotid artery [ICA]=7; middle cerebral artery [MCA]=6), 6 patients a posterior circulation stroke, and 2 patients an anterior plus posterior circulation stroke (left ICA or M1 tract of MCA plus basilar artery occlusions). Mean NIHSS score on admission was 21 (range, 18–27). Immediate recanalization was successful (thrombolysis in myocardial infarction [TIMI] 2–3) in 17 of 21 patients. The following day, 14 of 19 patients improved substantially and complete vessel patency (TIMI 3–4) was confirmed by digital subtraction angiography. Intracranial bleeding occurred in 5 of 21 patients (3 symptomatic cerebral hemorrhages and 2 subarachnoid hemorrhages) and was fatal in the case of 3 patients. At discharge, the mean NIHSS was 5.4 (range, 0–25). Overall, at 3-month follow-up the functional outcome was favorable (modified Rankin Scale score = 0–2) in 13 of 21 (62%) patients. Death (including all causes) at 90 days occurred in 6 of 21 (28%) cases.
CONCLUSIONS: The combination of intravenous tirofiban with intra-arterial urokinase and mechanical thrombolysis may be successful in reestablishing vessel patency and result in a good functional outcome in patients with major cerebral arteries occlusions.
After intravenous thrombolysis, partial or complete recanalization is obtained in only 10% of internal carotid artery (ICA) and in 25% of middle cerebral artery (MCA) occlusions (1). Symptomatic cerebral hemorrhage (SICH) in 4%–6% and vessel reocclusion are the major limitations to this treatment (2, 3). In the Prolyse in Acute Cerebral Thromboembolism (PROACT) studies (4, 5) intra-arterial thrombolysis (IAT) in patients with MCA occlusion was at least as effective as intravenous thrombolysis in improving the outcome and more effective in reopening the occluded artery even if performed in a larger time window. The idea of combining the advantages of intravenous fibrinolysis with those of IAT was explored in several trials and case series (6, 7, 8). Inhibitors of the glycoprotein (GP) IIb/IIIa receptor are being used alone or in combination with fibrinolytics to treat patients with acute myocardial or cerebral ischemia (8–12). The goal of this study was to assess the feasibility, efficacy, and safety of the intravenous administration of tirofiban (Aggrastat, Merck, Whitehouse Station, NJ) followed by intra-arterial pharmacologic and mechanical thrombolysis in a consecutive series of patients with stroke.
Patients and Techniques
Between April 2003 and June 2004, we treated 21 consecutive patients (12 men and 9 women; age range, 34–85 years; mean age, 66.9 years). The therapeutic window for anterior circulation stroke was <6 hours. There was no exclusionary time period for posterior circulation stroke.
All patients were evaluated at arrival by a stroke team expert; the evaluation excluded intravenous recombinant tissue plasminogen activator (rtPA) therapy. Inclusion criteria were based on the prediction of a poor outcome, the demonstration of major cerebral artery(ies) occlusion(s), and no or subtle early ischemic signs on admission CT (Alberta Stroke Program Early CT [ASPECT] score >7) (13). Neurologic assessment was performed by an independent neurologist by using the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale (mRS) scores.
The cause of the ictus was cardioembolic in 15 patients (chronic atrial fibrillation in 12, combined aortic valve endocarditis and acute atrial fibrillation in 2, and patent foramen ovalis in one). In the remaining 6 patients, despite the presence of one or more important risk factors for thromboembolism (smoking, use of oestroprogestinics, diabetes, ischemic heart disease), the source of embolism remained unidentified.
Five patients with chronic atrial fibrillation received warfarin, with international normalized ratio (INR) values ranging from 2.0 to 2.5. For 6 patients, CT angiography (CTA) was obtained. In all patients, tirofiban (0.4 μg/kg/min bolus for 3 minutes followed by infusion of 0.1 mcg/kg/min) was started together with heparin (2000–3000 IU bolus followed by 1000 IU/hour infusion throughout the procedure) immediately after the plain CT study performed on admission and as soon as occlusion of a major cerebral artery was demonstrated by either CTA (6 cases) or digital subtraction angiography (DSA). In patients with anterior circulation stroke, the endovascular approach was homolateral to the occluded vessel in 19 patients and controlateral in 2 patients whose occlusion sites were reached by a controlateral approach through the anterior communicating artery because of anatomic vessel constraints (kinking and looping of ipsilateral cervical ICA). Recanalization was assessed following the thrombolysis in myocardial infarction (TIMI) classification.
An immediate postprocedural CT study was performed for all patients, and they were monitored with transcranial Doppler for ≥72 hours. Follow-up CT and DSA were obtained 24 hours later in 19 patients. The infusion of tirofiban (0.1 μg /kg/min) and heparin (500 IU/hour) was maintained for 24 hours in 8 patients and for 48 hours in another. In 12 patients, tirofiban was suspended earlier on the presumption of an increased risk of cerebral (parenchymal contrast enhancement or extravasation) or extracerebral bleeding (groin hematoma, hematuria, nasal bleeding).
Endovascular Techniques
All procedures were performed in a neuroangiography suite equipped with DSA and road-mapping capabilities. Six patients who were agitated and uncooperative required general anesthesia.
A 6F sheath was placed in the femoral artery, and a 6F or 5F guiding catheter was advanced in the ICA or the vertebral artery. Thrombus aspiration was performed with a 60-mL syringe through the guiding catheter whose tip was placed proximal to the petrous bone in 6 cases of ICA occlusion and the C2–C3 turn in a case of basilar artery (BA) occlusion. In all cases, a microcatheter was advanced into the occluding thrombus under road mapping. Attempts of microcathether-microguidewire (Terumo 0.016-inch, SilverSpeed 0.010-inch or 0.014-inch, X-Pedion 10)–aided thrombus disruption and/or vessel angioplasty (percutaneous transarterial angioplasty [PTA]) were performed before the local delivery of the fibrinolytic agent (urokinase, ukidan). The latter was locally administered up to a maximum total dose of 500,000 IU for anterior circulation (mean, 400,000 IU; range, 300,000–500,000 IU) and 1,000,000 IU for posterior circulation (mean, 837,000 IU; range, 300,000–1,000,000 IU) stroke. In all cases but 2, PTA of the occluding clot was also performed. PTA was performed with an over-the-wire (X-Pedion 10), soft, compliant 4 × 15 mm (Hyperglide, MTI Micro Therapeutics Inch Goodyear, Irvine, CA) microballoon that was slowly (>2 minutes) and gently inflated once or twice for a few seconds under road-mapping visual control by using a hand-held device (Cadence MTI, Micro Therapeutics).
Tirofiban and heparin infusion were continued throughout the interventional procedure and for 24–48 hours thereafter whenever there was neither intraprocedural bleeding nor suspicion of increased risk of bleeding. In one case, they were suspended because of hematuria, initial groin hematoma, and nasal bleeding that required neither hemotransfusion nor surgical intervention. In 11 patients, tirofiban and heparin infusions were stopped because of demonstration of a marked enhancement of the ischemic core (basal ganglia in 10 patients and brain stem in another) on the immediate postprocedural CT.
In all but 2 patients, this enhancement disappeared 24 hours later and was consistent with contrast medium extravasation through a disrupted blood-brain barrier. Femoral artery hemostasis was obtained with a vascular closure device (AngioSeal, St. Jude Medical, Minnetonka, MN) 24 hours later. After the interventional procedure, all the patients were moved to the neurointensive care unit for 24–48 hours and then to the stroke unit, or directly to the stroke unit.
The study was approved by the local ethics committee, and informed consent was obtained from each patient.
Results
The timing and the most important neurologic and outcome features are summarized in the Table. Exclusion criteria from intravenous rtPA therapy for the 6 patients who arrived within the 3-hour frame were as follows: ongoing anticoagulant (warfarin) therapy within therapeutic range (INR, 2.0–2.5) for chronic atrial fibrillation for 5 patients and age >80 years for one patient. After the intravenous administration of tirofiban, the time elapsed to local administration of urokinase ranged from 30 to 80 minutes (mean, 53 minutes), which corresponds to the time necessary to prepare the angiographic suite and/or reach the occlusion site with the microcatheter. On admission, 13 patients presented with anterior circulation and 6 with posterior circulation stroke, and 2 patients presented with anterior plus posterior circulation stroke, the severity of which ranged from 18 to 27 (mean, 21.5) points on the NIHSS. Pretreatment plain CT scan of the head showed a spontaneous relative high attenuation of the MCA in the case of 5 patients. Subtle early CT signs of parenchymal ischemia were detected in 12 patients. In 9 patients, the pretreatment plain CT scan did not show any abnormalities. CTA performed in of 6 patients showed occlusion of a major cerebral vessel consistent with the clinical picture in all of them. DSA revealed that collateral circulation through cortical leptomeningeal anastomoses between anterior cerebral artery (ACA) and MCA and/or posterior cerebral artery (PCA) and MCA territories was present in 9 patients with T-siphon (n=4) or MCA (n=5) occlusions, and it was poor or absent in all the remaining cases. At the end of the procedure, the recanalization was absent or insufficient (TIMI 0–1) in 4 patients. Successful, albeit partial, recanalization (TIMI 2) was observed in 6 patients. Near-complete or complete immediate reperfusion (TIMI 3–4) was obtained in 11 patients. Intraperiprocedural neurologic improvement was noted in 15 patients (5 left T-siphon, 4 left M1, one right M1, 5 BA) despite an unsuccessful (TIMI 1 in one patient) or partial (TIMI 2 in 3 patients) recanalization after the procedure. The clinical picture remained unchanged in 2 patients with TIMI 2 recanalization and worsened in 4 patients (one TIMI 2 and 3 TIMI 1, respectively). In one patient with anterior plus posterior circulation stroke, an intraprocedural rupture of the left MCA during PTA occurred. Postprocedural CT showed single or multiple foci of marked contrast enhancement in 8 patients (basal ganglia, 7; brain stem, one). In 6 of these patients, the enhancement occurred in the same areas where the preprocedural CT scan had showed subtle early ischemic changes, and it was associated with mass effect in one patient. In 2 patients, the immediate postprocedural CT showed a subarachnoid hemorrhage (SAH) (Fisher grade 1 in one patient and Fisher grade 4 in the patient with intraprocedural MCA rupture). In the 24 hours that followed, 2 patients died: one from a devastating intracerebral bleeding with ventricular inundation and the other as a consequence of the intraprocedural MCA rupture. The clinical picture progressively improved in 14 of 19 patients, and it remained stable or worsened in the remaining 5 patients. On the control CT (24 hours later), all foci of parenchymal enhancement but one disappeared and were replaced by an area of low attenuation without mass effect. In one patient, enhancement was replaced by a hemorrhagic transformation of the infarct and the patient died 3 weeks later. In another patient, the clinical picture that had initially improved suddenly deteriorated 2 days later and a CT scan of the head showed hemorrhagic transformation of a cortical left frontal lobe infarction. Overall, 5 of 21 patients had intracranial bleeding (symptomatic intracerebral hemorrhage [SICH]; 3; SAH, 2), which resulted in a fatal outcome for 3 of them. No systemic bleeding requiring transfusion or surgery occurred. The control DSA at 24 hours was performed in 19 of 21 patients and showed vessel recanalization with good or complete reperfusion of its peripheral territories (TIMI 3–4) in 14 of 19 patients, recanalization without reperfusion (TIMI 2) in 3 patients, and insufficient recanalization (TIMI 1) in 2 patients. On the CT scan obtained before discharge, small ischemic lesions in the basal ganglia or at cortical-subcortical level were visible in 10 of 19 patients. Two patients had a large brain stem infarct associated with cerebellar infarcts, and 2 patients had a small infarction in the temporo-occipital lobes associated with scattered ischemic foci in the cerebellum and brain stem. In 3 patients, the CT scan did not show any abnormality, and in 2 patients a partial left MCA territory infarct was detected. At discharge, the mean NIHSS score was 5.9. At 3-month follow-up, the functional outcome was favorable (mRS score 0–2) in 13 of 21 patients. Six patients died, 3 as a consequence of the intracranial bleeding, one as a consequence of a large brain stem and cerebellar infarctions, and 2 of causes not directly related to the procedure or the cerebral ischemic damage (pulmonary embolism and myocardial infarct). Of the 2 patients with unfavorable outcomes at 90 days (mRS score >2), one was in a persistent vegetative state and the other had a mRS score of 3.
Preprocedural NIHSS score, occlusion location, timings, immediate and late recanalization scores, and treatment features with NIHSS and mRS evaluation at discharge and at 3-month follow-up
| Patient No./Age (y)/Sex | NIHSS (pre) | Location | Time to Tirofiban (min) | TIMI, Immediate | TIMI at 24 Hours | NIHSS at Discharge | mRS at 3 Months | Notes |
|---|---|---|---|---|---|---|---|---|
| 1/65/F | 25 | T-siph L | 220 | 3 | 4 | 2 | 1 | |
| 2/69/F | 18 | BA | 110 | 3 | 4 | 2 | 0 | |
| 3/73/M | 28 | M1 L | 110 | 3 | 3 | 2 | 2 | SAH |
| 4/73/M | 18 | T-siph L | 120 | 3 | 3 | 2 | 2 | 48-hour tirofiban infusion |
| 5/42/F | 20 | BA | 600 | 3 | 4 | 8 | 1 | |
| 6/49/M | 22 | T-siph L | 180 | 1 | 2 | 12 | Death | Pulmonary embolism |
| 7/73/M | 25 | BA | 300 | 2 | 3 | 10 | 1 | |
| 8/66/M | 18 | BA | 240 | 3 | 4 | 5 | 1 | Bleeding from frontal basalioma |
| 9/81/F | 18 | M1 L | 180 | 2 | 4 | 0 | 0 | |
| 10/72/F | 18 | M1 L | 150 | 2 | 4 | 2 | 1 | |
| 11/79/M | 20 | T-siph L | 240 | 3 | 4 | 0 | 0 | |
| 12/70/F | 22 | T-siph L | 300 | 1 | 2 | 15 | Death | SICH, septic shock |
| 13/73/M | 18 | M1–M2 L | 265 | 2 | 2 | 12 | 3 | Groin hematoma, hematuria |
| 14/85/M | 25 | BA | 280 | 3 | 4 | 5 | Death | Myocardial infarct |
| 15/52/F | 18 | M1 R | 240 | 3 | 32 | 2 | Nasal bleeding | |
| 16/66/M | 27 | BA + M1 L | 240 | 2 | N/A | Death | N/A | SAH from L MCA rupture during PTA |
| 17/34/M | 25 | BA | 300 | 1 | 1 | 25 | 5 | Brain stem infarct |
| 18/64/F | 27 | BA + T-siph L | 290 | 1 | 1 | Death | N/A | Brain stem infarct |
| 19/71/M | 19 | T-siph R | 150 | 2 | N/A | Death | N/A | SICH |
| 20/68/M | 20 | T-siph L | 290 | 3 | 3 | 3 | 2 | SICH |
| 21/80/F | 18 | M1 L | 250 | 4 | 4 | 0 | 0 |
Note.—NIHSS indicates National Institutes of Health Stroke Scale; TIMI, thrombolysis in myocardial infarction grade flow; mRS, modified Rankin Scale; T-siph, T-siphon occlusion of internal carotid artery; BA, basilar artery; M1, M1 tract of middle cerebral artery; M2, M2 tract of middle cerebral artery; R, right; L, left, SAH, subarachnoid hemorrhage; SICH, symptomatic intracerebral hemorrhage; MCA, middle cerebral artery; PTA, percutaneous transarterial angioplasty.
Discussion
In comparison with the PROACT II study, our group of patients was characterized by a high NIHSS score on admission (mean NIHSS of 21.5 vs 17). In our series, 71% of patients had an angiographically demonstrated T-siphon, BA, or ICA/MCA plus vertebrobasilar embolism, involving the nondominant cerebral hemisphere in only 2 patients. This group of patients is usually bound to a dismal natural history. In our series, successful revascularization occurred in most patients at the end of the procedure (80% with TIMI 2 or higher), and it progressed throughout the following 24 hours even in those patients whose vessel(s) appeared to be only partially reopened. The following day, DSA showed that an improvement of recanalization and reperfusion occurred in 43% of patients. The fact that vessel recanalization, together with improvement of the clinical conditions, continued to progress in the following 24 hours suggests that tirofiban may play a role in continuing the thrombolytic process and preventing reocclusion. Overall, despite the small number of patients that limits comparisons, our recanalization rate (80%) was better than in any reported series of stroke patients with a major cerebral artery occlusion treated through an intra-arterial approach and similar to the recently reported experience of Deshmukh et al (14). Compared with the poor prognosis of patients with this type of stroke (controls) and with the results of series of patients treated with IAT reported in a meta-analysis of Lisboa et al (15), both the clinical and the functional outcomes may be good. Favorable outcomes at 3 months (mRS 0–2) were more frequent in our series than in the control or the IAT group (66.6% vs 23% and 41.5%) with a mortality rate (28.5%) only slightly higher than the IAT group (27.2%) and lower than the control group (40.0%). The outcome is especially favorable in posterior circulation stroke (66.6% or 83%, if we include a patient who did very well after the procedure and died a month later of myocardial infarction). The bleeding risk related to the association of intravenous GPIIb/IIIa inhibitors with intra-arterial urokinase in acute stroke patients is yet unknown. The overall incidence of intracerebral hemorrhage in the Abciximab in Emergent Stroke Treatment Trial study (16) was 15.9% for the abciximab-treated group versus 1% for the placebo group. Seitz et al (17) reported no higher incidence of hemorrhagic conversion in patients who received intravenous rtPA plus tirofiban compared with intravenous rtPA alone. By contrast, Cheung and Ho (18) reported devastating hemorrhagic transformation of an ischemic cerebral infarction in a patient receiving intravenous abciximab together with aspirin and tiklid. Qureshi et al (19) reported 7 patients who developed fatal intracerebral hemorrhages after receiving abciximab during neurointerventional procedures, all patients receiving concomitantly heparin and clopidogrel. Tirofiban and low-dose rtPA—both given intravenously in a small group of ischemic stroke patients—did not produce any symptomatic hemorrhage (10). No difference was observed (8) comparing the bleeding risk after the intra-arterial use of urokinase with or without intravenous abciximab. Eckert et al (11) treated 3 patients with vertebrobasilar occlusion with local intra-arterial rtPA in combination with abciximab. Recanalization with clinical improvement occurred in 2 of the 3 patients, and there were no hemorrhagic complications. At variance with the reported incidence of SICH in controls and IAT group (3% and 9%), in our series we had 3 SICHs (14%), 2 of which proved fatal. Deshmukh et al (14) treated 21 patients with a combination of intravenous rtPA, intra-arterial rtPA, mechanical revascularization, and intravenous and/or intra-arterial IIb/IIIa agents reporting a global incidence of intracerebral hemorrhages similar to ours (14%), though none symptomatic. In the series of Deshmukh et al the mean NIHSS score on admission was 15 (range, 4–27) and only one of their patients had an ICA occlusion, most being MCA (12 patients) or BA (8 patients) occlusions. The increased risk of SICH in our series compared with controls and IAT did not translate into a correspondingly increased mortality; however, because of the small series of patients, the heterogeneous treatment and the uncontrolled study design, caution is mandatory in generalizing from these conclusions. We performed direct PTA of the thrombus employing a very low invasive technique to crush the clot (20). In one patient, repeated PTA of the left MCA caused the vessel’s rupture with a fatal SAH. Although the procedure was being performed by an experienced operator well accustomed to angioplasty and balloon-assisted aneurysm coiling, inflation at the level of the distal M1 tract with an early bifurcation resulted in vessel dissection and rupture. Particular care should be taken when performing multiple PTA of occluded vessels without knowledge of distal anatomy. We used tirofiban in combination with endovascular mechanical clot disruption supported by the idea that it could sustain the microcirculation before, during, and after the interventional procedures. In the small vessels, GP IIb/IIIa inhibitors may prevent fibrin and platelet aggregates deposition triggered by the endothelial procoagulant response to flow reduction and leading to the collapse of microcirculation (the so-called no-reflow phenomenon; 21, 22) In contrast, the marked procoagulant activity observed in stroke patients treated with intravenous alteplase (23) suggests that fibrinolytics may activate the coagulation cascade resulting in thrombin formation. Thrombin increases fibrin deposition favoring incorporation of platelets into the thrombus in the downstream vascular territories (24) and may obscure heparin-binding sites impeding heparin to prevent reocclusion (25). According to these observations GP IIb/IIIa inhibitors might enhance the fibrinolytic response. The interaction between hypoperfusion and thromboembolism may explain the reduced chance of late recanalization after intravenous or intra-arterial treatment by using fibrinolytics only or the reocclusion of partially disobstructed vessels (accompanied by clinical deterioration) in a fairly high proportion of patients in the TIMI 1 or 2 classes at the end of interventional procedures. Compared with abciximab, the nonpeptide antagonist tirofiban has better steering properties, in light of its short half-life (2 hours), and no allergenic properties, which results in fewer side effects and allows its repeated use (26). Finally, if proven effective, combining intravenous/IAT may be logistically challenging. Intravenous treatment may start at a community hospital and be continued after transfer to a referral tertiary care center for intra-arterial lysis; even in our series the mean time interval between intravenous tirofiban and intra-arterial urokinase was 53 minutes.
Conclusion
Our experience suggests that the combination of intravenous tirofiban with intra-arterial urokinase and mechanical thrombolysis may be successful in reopening an occluded major cerebral artery and preventing its reocclusion; however, because of the small series of patients, the heterogeneous treatment, and the uncontrolled study design, caution is mandatory in generalizing from these conclusions.
Fig 1.
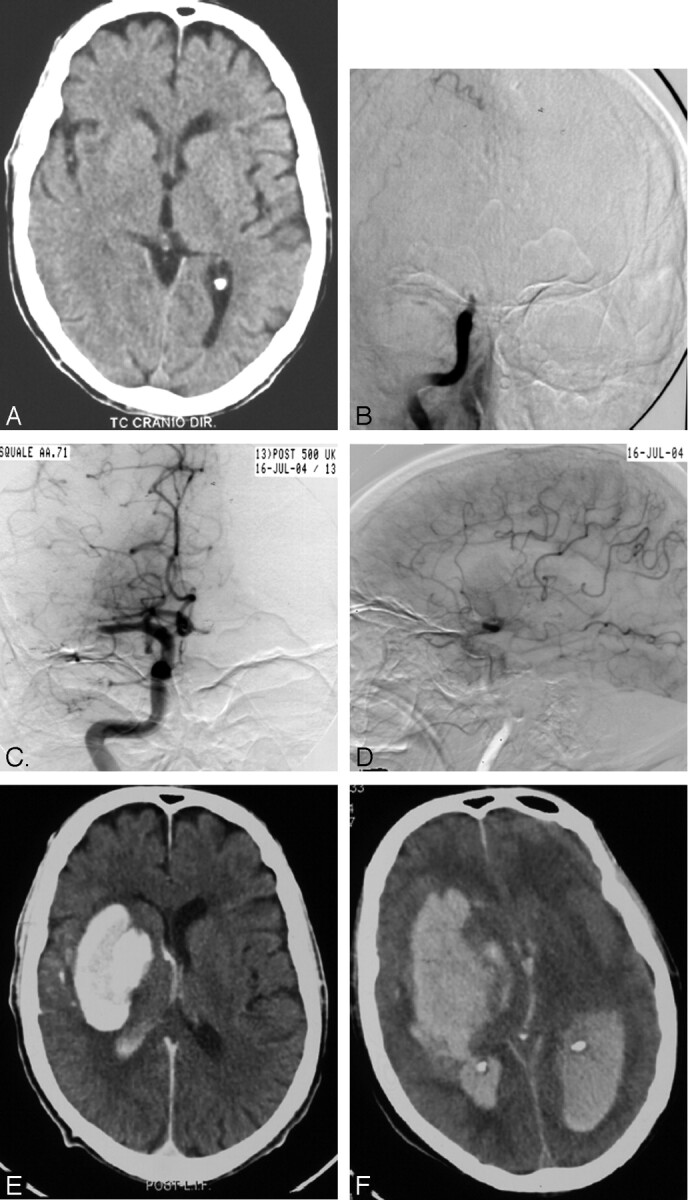
Patient 19 with chronic atrial fibrillation on warfarin (INR = 2.5) and sudden onset of left hemiplegia and mental confusion (NIHSS score=19).
Subtle early signs of cerebral ischemia were seen in the right basal ganglia region on the pretreatment plain CT (A). The subsequent DSA with selective right common carotid injection in the AP view (B) showed a thromboembolic right siphon occlusion in the absence of collateral circulation (not shown). Clot aspiration, PTA, and local administration of as much as 500,000 IU of urokinase resulted in reopening of the siphon with good filling of the proximal M1 tract, anterior cerebral artery, posterior cerebral artery, and ophthalmic artery, the latter originating directly from the siphon through the posterior communicating artery (C). The late arterial phase of selective right internal carotid injection (LL view) showed retrograde filling of distal MCA branches through leptomeningeal anastomosis as well as a deep avascular area (D). The immediate postprocedural CT scan showed marked (>90 HU) enhancement of right basal ganglia with mass effect consistent with contrast extravasation (E). After a transitory clinical improvement (>4 points in the NIHSS score) the patient worsened dramatically 6 hours later and on the control CT scan a devastating cerebral bleeding with intraventricular inundation was observed (F). The patient died the following day.
Fig 2.
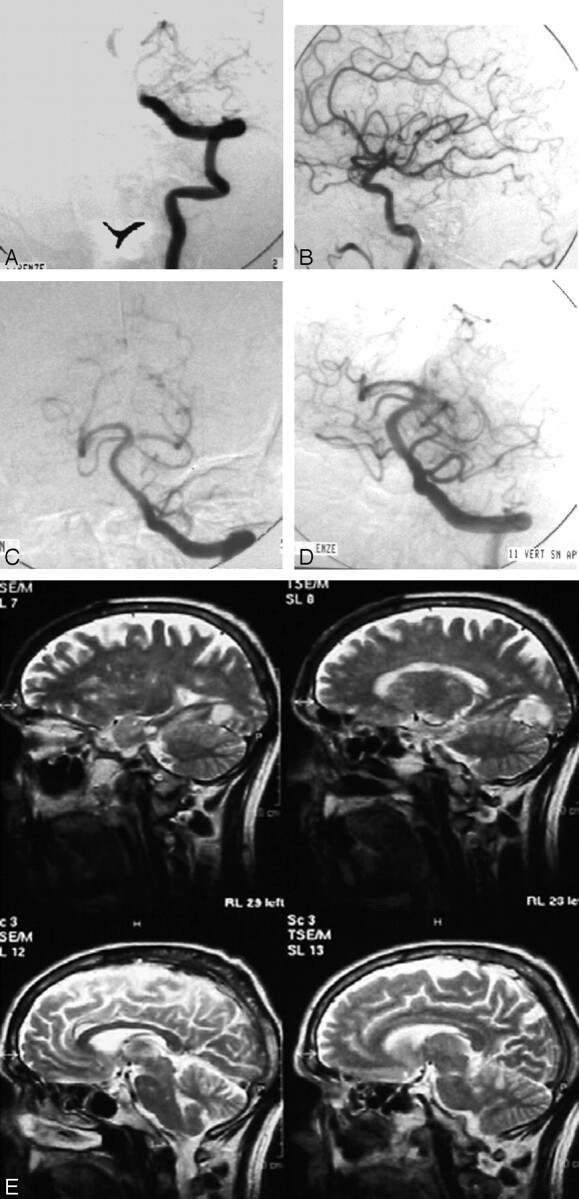
Patient 8 with parossistic atrial fibrillation and acute onset of dysarthria, right hemiplegia and third cranial nerve palsy followed by loss of consciousness (NIHSS score=18).
The AP view of selective left vertebral artery injection (A) showed complete occlusion of the basilar artery. The left PCA completely filled through the posterior communicating artery during selective left internal carotid artery injection as observed in the LL view (B). Mechanical clot disruption (PTA) and as much as 1,000,000 IU of locally administered urokinase resulted in a complete recanalization of the basilar artery and its collaterals (C). Persistent embolic occlusion of the right P2–P3 segments of the PCA is also seen. The AP view of selective left vertebral artery injection obtained at the control DSA 24 hours later showed complete revascularization of the right PCA (D). Turbo spin-echo T2-weighted MR images in the sagittal plane 3 months later demonstrated a small cortical infarct in the left posterior and basal aspect of the temporal lobe with scattered ischemic spots in the brain stem and cerebellar hemispheres (E). The patient was discharged with a NIHSS score of 5 and at 3-month follow-up was able to conduct a completely independent lifestyle (mRS score=1).
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.Bourekas EC, Slivka AP, Shah R, et al. Intraarterial thrombolytic therapy within 3 hours of the onset of stroke. Neurosurgery 2004;54:39–46 [DOI] [PubMed] [Google Scholar]
- 3.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 2002;59:862–867 [DOI] [PubMed] [Google Scholar]
- 4.Del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomised trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke: PROACT Investigators: Prolyse in Acute Cerebral Thromboembolism. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 5.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–2011 [DOI] [PubMed] [Google Scholar]
- 6.Lewandowsky CA, Frankel M, Tomsick TA, et al. Combined intra-venous and intra-arterial rtPA versus intra-arterial therapy of acute ischemic stroke : Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598–2605 [DOI] [PubMed] [Google Scholar]
- 7.IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–912 [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Jo KD, Kim HG, et al. Local intraarterial urokinase thrombolysis of acute ischemic stroke with or without intravenous abciximab: a pilot study. J Vasc Interv Radiol 2002;13:769–723 [DOI] [PubMed] [Google Scholar]
- 9.Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. N Engl J Med 1998;338:1488–1497 [DOI] [PubMed] [Google Scholar]
- 10.Straub S, Junghans U, Jovanovic V, et al. Systemic thrombolysis with recombinant tissue plasminogen activator and tirofiban in acute middle cerebral artery occlusion. Stroke 2004;35:705–709 [DOI] [PubMed] [Google Scholar]
- 11.Eckert B, Koch C, Thomalla G, et al. Acute basilar artery occlusion treated with combined intravenous abciximab and intra-arterial tissue plasminogen activator: report of 3 cases. Stroke 2002;33:1424–1427 [DOI] [PubMed] [Google Scholar]
- 12.Cloft HJ, Samuels OB, Tong FC, Dion JE. Use of abciximab for mediation of thromboembolic complications of endovascular therapy. AJNR Am J Neuroradiol 2001;22:1764–1767 [PMC free article] [PubMed] [Google Scholar]
- 13.Barber PA, Demehuk AM, Zhang J, Buchan AM, for the ASPECT Study Group. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: Alberta Stroke Programme early CT score. Lancet 2000;355:1670–1674 [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh VR, Fiorella DJ, Albuquerque FC, et al. Intra-arterial thrombolysis for acute ischemic stroke: preliminary experience with platelet glycoprotein IIb/Iia inhibitors as adjunctive therapy. Neurosurgery 2005;56:46–55 [DOI] [PubMed] [Google Scholar]
- 15.RC Lisboa, BD Jovanovic, MJ Alberts. Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke 2002;33:2866–2871 [DOI] [PubMed] [Google Scholar]
- 16.Adams H. Effect of abciximab for acute ischemic stroke: final results of Abciximab in Emergent Stroke Treatment Trial (AbESTT) presented at the 28th International Stroke Conference February 2003. Stroke 2003;34:252 [Google Scholar]
- 17.Seitz RJ, Hamzavi M, Junghans U, et al. Thrombolysis with recombinant tissue plasminogen activator and tirofiban in stroke: preliminary observations. Stroke 2003;34:1932–1935 [DOI] [PubMed] [Google Scholar]
- 18.Cheung RT, Ho DS. Fatal hemorrhagic transformation of acute cerebral infarction after the use of abciximab. Stroke 2000;31:2526–2527 [DOI] [PubMed] [Google Scholar]
- 19.Qureshi AL, Saad M, Zaidat OO, et al. Intracerebral hemorrhages associated with neurointerventional procedures using a comination of antithrombotic agents including abciximab. Stroke 2002;33:1916–1919 [DOI] [PubMed] [Google Scholar]
- 20.Ueda T, Sakaki S, Nochide I, et al Angioplasty after intra-arterial thrombolysis for acute occlusion of intracranial arteries. Stroke 1998;29:2568–2574 [DOI] [PubMed] [Google Scholar]
- 21.Abumiya T, Fitridge R, Mazur C, et al. Integrin alfa(IIb)beta(3) inhibitor preserves microvascular patency in experimental acute focal cerebral schemia. Stroke 2000. :31 ;1402–1410 [DOI] [PubMed] [Google Scholar]
- 22.Fassbender K, Dempfle CE, Mielke O, et al. Changes in coagulation and fibrinolysis markers in acute ischemic stroke treated with recombinant tissue plasminogen activator. Stroke 1999;30:2101–2104 [PubMed] [Google Scholar]
- 23.Siess W. Molecular mechanisms of platelet activation. Physiol Rev 1989;69:58–178 [DOI] [PubMed] [Google Scholar]
- 25.Weitz JI, Leslie B, Hudoba M. Thrombin binds to soluble fibrin degradation products where it is protected from inhibition by heparin-antithrombin but susceptible to inactivation by antithrombin-independent inhibitors. Circulation 1998;97:544–552 [DOI] [PubMed] [Google Scholar]
- 26.Junghans U, Seitz RJ, Aulich A, et al. Bleeding risk of tirofiban, a nonpeptide GPIIb/IIIa platelet receptor antagonist in progressive stroke: an open pilot study. Cerebrovasc Dis 2001;12:308–312 [DOI] [PubMed] [Google Scholar]
- 27.Tcheng JE. Clinical challenges of platelet glycoprotein IIb/IIIa receptor inhibitor therapy: bleeding, reversal, thrombocytopenia, and retreatment. Am Heart J 2000;139:S38–S45 [DOI] [PubMed] [Google Scholar]


