Abstract
BACKGROUND AND PURPOSE: Primary central nervous system lymphomas (PCNSLs) are usually high-grade and are rarely low-grade non-Hodgkin lymphomas (NHLs). On MR imaging, PCNSLs typically present as contrast-enhancing lesions in contact with the subarachnoid space without evidence of necrosis. We evaluated the radiologic morphology and clinical characteristics of low-grade PCNSLs, hypothesizing that they may differ from high-grade PCNSLs.
METHODS: Records were reviewed from 332 patients screened for inclusion in 3 multicenter prospective trials. MR imaging scans were obtained from all patients and were centrally reviewed by 2 consultant neuroradiologists.
RESULTS: Ten patients (3%) with low-grade PCNSLs (7 men and 3 women; median age, 59 years; age range, 19–61 years) were identified. Four patients had one lesion, 2 patients 2 lesions, and 4 patients had multiple lesions. The following radiologic features infrequently seen in high-grade PCNSLs were found in a substantial proportion of patients: location in deep structures or spine (n=6); lack of periventricular location (n=5); hyperintensity on T2-weighted images (n=10); moderate or absent contrast enhancement (n=6); and heterogeneous contrast enhancement (n=5). In 8 patients, >2 of these features were present in at least one lesion, and, thus, the radiologic appearance was assessed atypical of high-grade PCNSLs. The atypical radiologic appearance in combination with atypical or mild symptoms resulted in a false or delayed diagnosis.
CONCLUSION: Low-grade PCNSLs may have a variable and atypical radiologic morphology compared with high-grade PCNSLs with the risk of false or delayed diagnosis.
Primary central nervous system lymphoma (PCNSL) is defined as a non-Hodgkin lymphoma (NHL) that arises within the central nervous system and is confined to it at the time of diagnosis (1). It is a rare tumor, accounting for approximately 1% of malignant central nervous system tumors (2), but its incidence seems to be increasing (3). Almost all PCNSLs are high-grade B-cell lymphomas with a predominance of immature, blastic cells with a high growth fraction—ie, the proportion of proliferating neoplastic cells is >50% (2). PCNSL is generally an aggressive disease with a median survival of only a few months after resection alone and as long as 30 months with high-dose methotrexate-based chemotherapy. In contrast to classical high-grade NHL, low-grade NHL is defined by the predominance of small, mature lymphocytes (>90% of neoplastic cells) and demonstrates a growth fraction of ≤20%. Low-grade PCNSLs are very rare, and their exact incidence is not known. Analogous to extracerebral lymphoma, low-grade PCNSLs could represent a less aggressive disease than high-grade PCNSLs and may thus require less aggressive treatment limited to the tumor region (ie, surgery or local radiation therapy) with the advantage of a reduced risk of late neurotoxicity.
Here, we report a first series of 10 patients with low-grade PCNSLs. The aims of this retrospective study were to characterize the radiologic and clinical features of these patients, hypothesizing that they differ from classic high-grade PCNSLs.
Methods
We reviewed records of 332 patients screened for inclusion in 3 prospective multicenter German trials, all of which included only immunocompetent patients with newly diagnosed and histologically or cytologically (CSF) confirmed PCNSLs (4–6). Informed consent was obtained from all patients, and the 3 studies were approved by the institutional ethics committees. All patients had a thoracic and abdominal CT scan and underwent a cytologic and histologic bone marrow examination to exclude systemic lymphoma. Patients with low-grade PCNSLs were selected. The diagnosis of low-grade PCNSL was made by a local pathologist on the basis of morphologic and immunohistologic criteria. In all selected patients, the pathologic diagnosis was confirmed by a consultant hematopathologist (H.S.). Analogous to systemic extracerebral lymphomas, low-grade PCNSL was defined as a tumor predominantly (>90%) consisting of small, mature lymphocytes with a growth fraction of ≤20% (as evidenced by staining with Ki-67 or MIB-1 antibody). Immunocytologic staining was performed with antibodies against CD3, CD4, CD5, CD7, CD8, CD19, CD20, CD45, CD79a, lambda/kappa light chains, and Ki-67/MIB-1 for diagnosis establishment and against CD23, BCL-2, and BCL-6 for further lymphoma characterization. B-cell lymphoma was identified by a predominance of B-cell markers (CD19, CD20, CD 79a). In addition, in some patients an exclusive expression of either the kappa or the lambda light chain could be detected. T-cell lymphoma was identified by a predominance of T-cell markers (CD3, CD4, CD5, CD7, CD8).
Brain MR images were obtained from all patients before steroid medication and centrally reviewed by 2 consultant neuroradiologists. All examinations included T1- and T2-weighted sequences as well as contrast-enhanced studies. T1-weighted spin-echo sequences were applied in all patients. T2-weighted imaging included turbo spin-echo sequences in all and fluid-attenuated inversion recovery (FLAIR) sequences in 2 (9 and 10) patients. Typical parameters used for T2-weighted turbo spin-echo sequences were a turbo factor of 15, a repetition time of 4,500 msec, and an echo time of 100–120 msec. Cerebral imaging was performed on various MR scanners with field strengths of 1–1.5T. For the T1-weighted scans, all patients received 0.1 mmol gadolinium-diethylenetriaminepenta-acetic acid per kilogram body weight. The scans were evaluated for the number of lesions, location, contrast enhancement, T2 signal intensity, necrosis, edema, and proximity to the subarachnoid space, ventricles, calvaria, and meninges. Necrosis was defined as an area without enhancement inside a contrast-enhancing lesion. Edema was rated as extensive if it exceeded the contrast-enhancing lesion in size and as moderate if this was not the case, as reported elsewhere (7).
Results
Ten patients (3%) with low-grade PCNSLs were identified. The group included 7 men and 3 women, with a median age of 59 years (range, 19–61 years). The diagnosis was made by biopsy in 7 patients and by tumor resection in 3. The pathologists at the centers from where the patients were recruited correctly diagnosed all but one patient (6), in whom encephalitis had originally been diagnosed. In this patient, the correct diagnosis was established by the consultant hematopathologist by PCR detection of rearranged immunoglobulin heavy-chain genes, which indicated monoclonality of the lymphatic cells. Seven patients had a B-cell lymphoma. In 3 patients, the tumor could be further classified as lymphoplasmacellular lymphoma (characterized by the presence of small, mature lymphocytes with eccentric and attenuated nuclei; Fig 1) in 2 patients and as follicular lymphoma grade 1 in another. In the latter patient, biopsy revealed a predominance of small cells with cleaved nuclei (centrocytes) and ≤5 large blastic cells per high-power field of 0.159 mm2 (centroblasts), positivity for CD10 and BCL-2, and absence of CD5 and CD23. Four B-cell lymphomas could not be further specified because of the small amount of biopsy material. Three patients had low-grade T-cell lymphomas, which were not further specified.
Fig 1.

Histologic preparations of low-grade PCNSLs (patient 9; lymphoplasmacellular lymphoma), opposed to high-grade PCNSLs. A, Infiltrates of small, mature lymphocytes surrounded by amorphous substance (immunohistologic demonstration of light-chain deposits) and absence of blastic, immature cells (hematoxylin and eosin; original magnification ×400). The lymphocytes are positive for the B-cell antigen CD20 and show a predominant expression of the immunoglobulin light chain lambda, indicating monoclonality (not shown). B, Typical appearance of high-grade PCNSL, composed of immature blasts with large and partly bean-shaped nuclei and prominent nucleoli (hematoxylin and eosin; original magnification ×400). C, Lymphoma cells of low-grade PCNSL demonstrate a low growth fraction of only 2% (Ki-67 antibody; original magnification ×200). D, High-grade PCNSL with a typical high growth fraction of 95% (MIB-1 antibody; original magnification ×400). Note.—Reproduced from K. Jahnke et al, Br J Haematol 2005;128:616–624 (© British Society for Haematology).
The tumor growth fraction was specified with a median of 4% (range, 1%–20%) in 7 patients but was estimated as being <5% in 3 specimens. Lymphoma cells were found in the CSF of one patient. The median time from the onset of symptoms to the histologic diagnosis was 1.75 months (0.5–32), and in 5 patients ≥2 months. Patients’ clinical and pathologic characteristics are summarized in Table 1. Three patients underwent complete tumor resection, one with additional chemotherapy and another with chemotherapy and local radiation therapy. As for the other patients, 4 received chemotherapy and 3 chemotherapy plus whole-brain irradiation, resulting in 4 complete remissions, 2 cases without change, and one case of progressive disease. The overall survival was 2–58+ months. The 1-year progression-free survival was 80%, and the 2-year overall survival was 67%.
TABLE 1:
Patient characteristics, therapy, and outcome
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex/age (y) | M/58 | M/58 | M/60 | F/61 | F/61 | M/58 | M/60 | F/60 | M/19 | M/45 |
| Clinical symptoms | Focal motor/sensory deficits | Hemiparesis | Ataxia, diplopia, neuropsychologic deficits, somnolence | Cerebellar syndrome, neuropsychologic deficits | Focal sensory deficits | Epilepsy (grand-mal) | Epilepsy (grand-mal), ataxia, nmestic deficits, incontinence | None | Epilepsy (grand-mal status) | Neuropsychologic deficits |
| KPS (initially/at last follow-up), % | 40/30 | 80/90 | 80/50 | 80/80 | 80.60 | 70/90 | 60/60 | 70/100 | 20/100 | 20/100 |
| Median time to diagnosis (months) | 1 | 1 | 1.5 | 2.5 | 0.5 | 32 | 2 | 0.5 | 3.5 | 6.5 |
| Biopsy site | Cervicothoracic spinal cord | Right parietal | Left basal ganglia | Left parietal | Right thalamus | Left temporal | Right frontal | Left occipital | Right temporal | Left frontal |
| Histopathology, growth fraction (Ki67/MIB-1) | T-cell (perivascular infiltrates; small, mature lymphocytes); Ki-67/MIB-1: 20% | T-cell (perivascular infiltrates; small, mature lymphocytes); Ki-67/MIB-1: 1% | B-cell, not specified (perivascular infiltrates; small, mature lymphocytes with round nuclei); Ki-67/MIB-1: 1% | B-cell, not specified (perivascular infiltrates; small, mature lymphocytes with round nuclei); Ki-67/MIB-1: 5% | T-cell (perivascular infiltrates; small, mature lymphocytes with irregularly shaped nuclei); Ki-67/MIB-1: <5% | B-cell, lymphoplasmacellular (small, mature lymphocytes with round, eccentric, nuclei); Ki-67/MIB-1: <5% | Follicular lymphoma (diffuse variant), grade 1 (small cells with cleaved nuclei (centrocytes), 3 large cells per hpf with scant, basophillic cytoplasm, round to oval nuclei, 1–2 nucleoli (centroblasts); Ki-67/MIB-1: <5% | B-cell, not specified (infiltrates with small, mature lymphocytes with round nuclei); Ki-67/MIB-1: 15% | B-cell lymphoplasmacellular (small, mature lymphocytes with round, eccentric, nuclei); Ki-67/MIB-1; 2% | B-cell, not specified (perivascular, small, mature lymphocytes with round nuclei); Ki-67/MIB-1: 4% |
| Therapy | Total resection, HDMTX, local RT | HDMTX, WBI | HDMTX | HDMTX | BMPD, MTX i.th. | none | BMPD, MTX i.th., CHOP, WBI | HDMTX | HDMTX | HDMTX, WBI |
| PFS/OAS (months) | 3/5.5 | 27+/27+ | 44.5+/44.5+ | 22.5/22.5 | 2/3 | 14.5+/14.5+ | 54+/54+ | 58+/58+ | 33.5+/33.5+ | 10+/10+ |
| Last status | Died due to sepsis | CR | CR | Died due to acute renal failure | Died due to lymphoma | CR | CR | CR | NC | CR |
Note.—KPS indicates Karnofsky performance score; Ki-67/MIB-1, growth fraction of neoplastic cells as evidenced by staining with Ki-67 or MIB-1 antibody; hpf, high-power field of 0.159 mm2; (HD)MTX, (high-dose) methotrexate; RT, radiotherapy; WBI, whole-brain irradiation; i.th., intrathecally; BMPD, BCNU, methotrexaste, procarbazine, dexamethasone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; PFS, progression-free survival; OAS, overall survival; CR, complete response; NC, no change.
MR imaging scans were abnormal in all patients. The lesions were localized superficially in the hemispheres in 7 patients, in the basal ganglia or thalamus in 4, and in the cerebellum and spinal cord in one patient each. A detailed analysis of the MR imaging data of all patients is presented in Table 2 and Figs 2–6, and a summary is given in Table 3. The following radiologic features infrequently seen in high-grade PCNSLs were found in a substantial proportion of the patients: location in deep-brain structures (ie, basal ganglia, corpus callosum, brain stem, cerebellum) or spine (n=7); lack of periventricular localization (n=5); hyperintensity on T2-weighted images (n=10); moderate or absent contrast enhancement (n=6); and heterogeneous contrast enhancement (n=5). All five features were present in 2 patients (3 and 6), 4 in one patient (8), and 3 in 5 patients (1, 4, 5, 9, and 10). Two patients (2 and 7) demonstrated only one of these features (hyperintensity on T2-weighted images). In 8 patients, >2 of these features were present in at least one lesion, and, thus, the radiologic appearance was assessed atypical of high-grade PCNSL. The atypical radiologic appearance in combination with atypical or mild symptoms resulted in a false or delayed diagnosis.
TABLE 2:
MR imaging features of the lesions for each patient
| Patient No. | No. of Lesions | Location of Lesions | Enhancement | T2-Weighted Image |
|---|---|---|---|---|
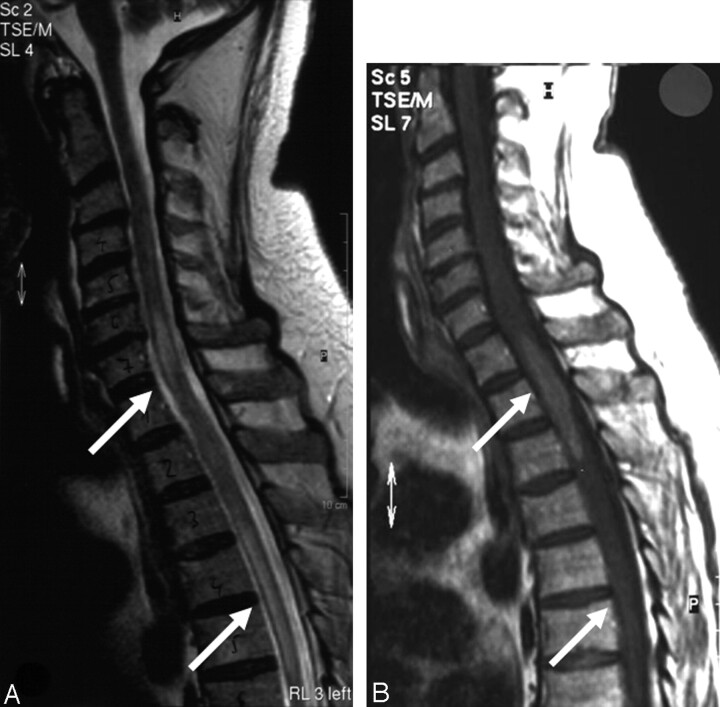
| 1 (Fig 4) | 2 | Spinal cord (cervico-thoracal) | Strong, homogeneous | Hyperintense, marked edema |
| Spinal cord (Th 4–5 level) | Moderate, homogeneous | |||
| 2 (Fig 5) | Multiple | Right parietal (periventricular), cerebellar | Strong, homogeneous | Hyperintense, moderate edema |
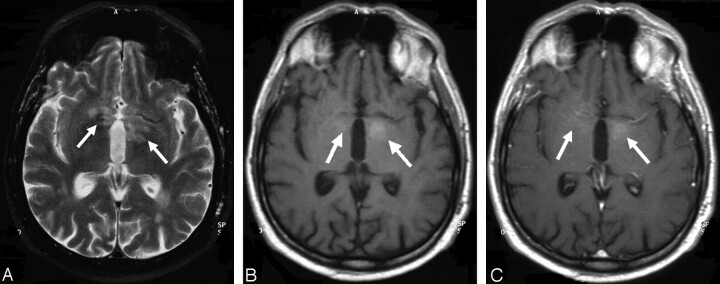
| 3 (Fig 2) | Multiple | Basal ganglia bilaterally | Moderate, heterogeneous | Hyperintense, marked edema |
| 4 | 2 | Basal ganglia (superior to sella) | Strong, homogeneous | Hyperintense, moderate edema |
| Left parietal | Isointense, edema absent | |||
| 5 | 1 | Right thalamus (periventricular) | Strong, heterogeneous | Hyperintense, moderate edema |
| 6 | Multiple | Left temporal, left basal ganglia | Moderate, heterogeneous | Hyperintense, marked edema |
| 7 | 1 | Right frontal (periventricular) | Strong, homogeneous | Hyperintense, moderate edema |
| 8 | 1 | Left occipital | Moderate, heterogeneous | Hyperintense, moderate edema |
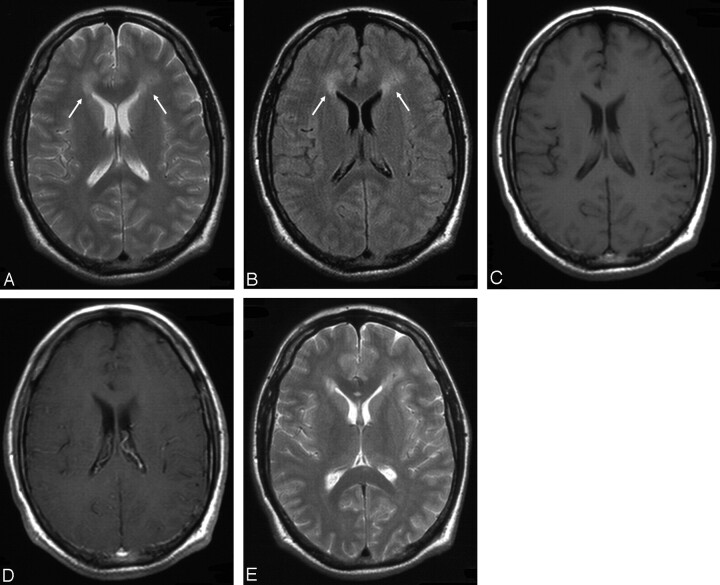
| 9 (Fig 3) | Multiple | Right temporal/occipital (periventricular) | Moderate, heterogeneous | Hyperintense, marked edema |
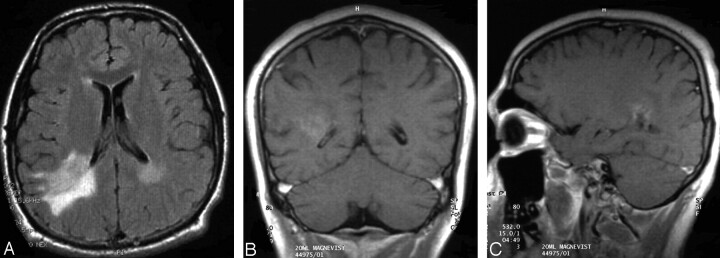
| 10 (Fig 6) | 1 | Bifrontal (periventricular) + caput corporis callosi | None | Hyperintense, moderate edema |
Fig 2.
Case 3, 60-year-old man with low-grade B-cell lymphoma (no further histologic specification). A, T2-weighted turbo spin-echo sequence. B, Precontrast T1-weighted spin-echo sequence. C, Postcontrast T1-weighted spin-echo sequence. On the precontrast T1-weighted image, the lymphoma (arrows) already demonstrates mild hyperintense spots, and only minor contrast enhancement is noted on the postcontrast T1-weighted sequence. In addition, on T2-weighted imaging, periventricular and basal ganglia edema is noted. Note.—Reproduced from K. Jahnke et al, Br J Haematol 2005;128:616–624 (© British Society for Haematology).
Fig 6.
Case 10, 45-year-old man with low-grade B-cell lymphoma (no further histologic specification; arrows) with bifrontal hyperintense periventricular white matter lesions on A, T2-weighted, and B, FLAIR images. C, The lesions are not visible on T1-weighted precontrast imaging. D, No contrast enhancement is noted on the T1-weighted postcontrast section. E, This T2-weighted image additionally demonstrates a small area of hyperintensity located in the head of the corpus callosum.
TABLE 3:
MR imaging features of the lesions: summary
| Characteristics | Number of Patients |
|---|---|
| Number of lesions | |
| 1 | 4 |
| 2 | 2 |
| >2 | 4 |
| Localization | |
| Supratentorial/supra- and infratentorial | 8/1 |
| Spinal | 1 |
| Deep/superficial/both | 2/3/4 |
| Necrosis | 1 |
| T2-weighted signal* | |
| Hyperintense/isointense | 10/1 |
| Contrast enhancement* | |
| Strong/moderate/none | 5/5/1 |
| Homogeneous/heterogeneous | 5/5 |
| Edema* | |
| Marked/moderate/absent | 4/6/1 |
| Ventricular ependymal involvement | 5 |
Some patients had multiple sites and appearances of involvement.
Discussion
Our findings seem to support the hypothesis that low-grade PCNSLs could represent a distinct and perhaps less-aggressive PCNSL subentity, which implies some important clinical consequences. Low-grade PCNSL depicts a special challenge for neuroradiologists because it may display a radiologic morphology different from that of classic high-grade PCNSLs. Making the tentative diagnosis of PCNSL is desirable to avoid steroid medication, which, because of its antiproliferative effect on lymphocytes, may hamper histologic evaluation. The performance of a stereotactic biopsy should be facilitated rather than resection, which does not improve the prognosis, at least in classic PCNSLs. Low-grade PCNSLs may require less aggressive treatment limited to the area of the tumor (ie, surgery or local radiation therapy only, abandonment of whole brain irradiation) as compared with high-grade PCNSL, which is regarded and treated as a whole-brain disease.
Data on the MR imaging/CT appearance of PCNSLs are derived from a few series composed almost exclusively of high-grade tumors. MR imaging discloses contrast-enhancing lesions in contact with the subarachnoid space, a minimal mass effect, and no necrosis as characteristic features of classic PCNSLs in immunocompetent patients (7–10). An atypical PCNSL appearance has already been reported (11, 12) but has not been attributed to a particular subentity. In a series of 100 patients enrolled in 2 of the studies from which our patients were recruited (4, 6), 65 patients had one lesion and 35 had ≥2 lesions. The location of the lesions was usually supratentorial (n=82) and rarely infratentorial (n=16). Necrosis was very rarely seen (n=6). Extensive edema was present in 5 and moderate edema in 85 patients, whereas 6 patients with cerebral lesions had no edema. These data are comparable to our patients. In contrast to our patients, location in deep brain structures such as brain stem (n=3) and thalamus (n=13) and in the spine (n=2) was infrequent. In addition, contrast enhancement was present in all but 5 patients—among them 4 with isolated ocular involvement—and was predominantly strong and homogeneous (n=85). Moderate enhancement was seen in 10 patients only. Almost all (95 of 96) patients with cerebral or spinal lymphoma had lesions adjacent to the CSF. The exact histopathology of the lymphomas was not indicated, but, in light of the very low prevalence of low-grade PCNSLs, the features are representative for high-grade PCNSLs (10). When these data are compared with those of our patients, the following features infrequently seen in high-grade PCNSLs were observed in a substantial proportion of our patients: location in deep brain structures or spine, lack of periventricular localization, and moderate or absent and heterogeneous contrast enhancement. In addition, in light of the percentage of PCNSL lesions with hyperintensity on T2-weighted images varying between 15% and 42% in the literature (7, 13, 14), this feature seems very frequent in low-grade PCNSLs (Table 4). In summary, imaging findings may differ in low- and high-grade PCNSLs, even though significant overlap was observed. The radiologic appearance of some lesions was suggestive of other brain tumors such as meningioma (patient 4) or glioma (patient 9) rather than PCNSLs or even was atypical for any kind of brain tumor. In patients 3, 6, and 10, radiologic appearance was suggestive of edema, encephalitis, and gliosis, respectively.
TABLE 4.
Comparison of radiologic and clinical characteristics between low-grade and high-grade PCNSL in immunocompetent patients
| Low-Grade PCNSL | High-Grade PCNSL |
|---|---|
| Radiological morphology | |
| Moderate and inhomogeneous or absent contrast enhancement frequent | Usually strong and homogemeous contrast enhancement |
| Localization of lesions often without contact to subarachnoid space | Lesions typically in contact with the subarachnoid space |
| Hyperintensity of T2-weighted images often present | Hyperintensity on T2-weighted images possible |
| Location in deep brain structures and spine common | Location in deep brain structures and spine possible but infrequent |
| Clinical characteristics | |
| Indolent clinical course possible | Aggressive clinical course almost invariably seen |
| Delays in diagnosis establishment possible due to paucity of symptoms and variable radiologic appearance | Diagnosis establishment usually rapid due to severe symptoms and typical radiological appearance |
| Long survival with absence of complete tumor remission possible | Survival without complete remission usually short |
| Long survival after local treatment (surgery, local radiotherapy) possible | Survival with local treatment short, whole brain treatment required |
Note.—PCNSL indicates primary central nervous system lymphoma.
The MR imaging appearance of low-grade PCNSLs has infrequently been described. In the report by Braks et al (15) the lesion was located in the semioval center, presented hyperintense on T2-weighted images, and showed faint contrast enhancement. The 2 patients reported by Johnson et al (13) had 3 and 5 lesions, respectively, with no necrosis and moderate edema; the absence or presence of ventricular ependymal involvement and the intensity of contrast enhancement were not reported. The patient reported by Roman-Goldstein et al (8), as well as another case described by Mikol et al (16), had solitary lesions with radiologic appearance typical of high-grade PCNSLs.
Diagnosis confirmation and further histologic specification may be difficult in PCNSLs, because of the small amount of biopsy material that, however, is a general problem in brain tumors diagnosed by stereotactic biopsy. Moreover, none of the histologic classification systems is generally accepted or considered clinically useful for PCNSLs. Most PCNSLs are B-cell lymphomas; T-cell lymphomas are infrequently observed. Applying the most recent lymphoma classification systems, the Revised European-American and the World Health Organization classification (17, 18), >90% of PCNSLs can be classified as 2 entities: diffuse large B-cell lymphoma and high-grade B-cell lymphoma, Burkitt-like. Lymphoplasmacellular lymphoma, diagnosed in 2 of our 3 patients in whom further histologic specification was possible, is the most common low-grade PCNSL subtype reported. It was found in 2 patients described in case reports (15, 16) and in low-grade PCNSL cases in pathologic series (19–21). Low-grade histology seems to be more common in T-cell PCNSLs than in PCNSLs of B-cell origin (22). Three of our patients had a T-cell lymphoma. According to our findings and those reported by others (23, 24), there appears to be no correlation of lymphoma histology to imaging data in PCNSLs. In neither the series of Jack et al (23) nor in that of Lanfermann et al (24), a histologic characterization based on neuroradiologic appearance proved possible. Both series were almost exclusively composed of high-grade PCNSL cases. One of the series, however, included HIV-positive patients in whom a neuroradiologic appearance of PCNSL distinct from the classic high-grade type is well recognized (24). Furthermore, cerebral MR imaging studies were performed in none (23) and only the minority (24) of the patients, respectively.
PCNSL is usually a very aggressive tumor, and survival generally does not exceed a few months after local therapy alone (eg, surgery or local radiation therapy). With respect to the distribution of the most firmly established risk factors in PCNSLs such as age and performance status, our patients did not differ significantly from other PCNSL patients reported in the literature or from the patients included in the 3 studies from which our cases were recruited (4–6). The 2-year overall survival rates in these studies were 33.9% and 51%, and the 3-year survival rates were 24.8% and 35%, respectively (6; U. Herrlinger, unpublished data). The third study (5) is still ongoing. The 2-year survival of 67% in our collective seems favorable; however, the small number of patients prevents definite conclusions from being drawn. Although therapy in our patients was rather limited, no patient relapsed after a median follow-up of 33.5 months. Moreover, one patient was disease-free 14.5 months after tumor resection alone, which suggests that local treatment modalities may play a role in this PCNSL subentity. Another patient with no change in the visible tumor after high-dose methotrexate was alive without disease progression after 33.5 months. Such disease courses have not been reported in patients with classical PCNSLs. Thus, local and symptom-oriented therapy may be the best approach at least for some patients with low-grade PCNSLs, considering the high risk of late neurotoxicity after intensive PCNSL treatment (Table 4).
A major limitation of this study is the small number of cases. In view of the rarity of this condition, however, such a retrospective review constitutes the best available approach for characterizing low-grade PCNSLs. Collecting even 10 cases could be accomplished only by nationwide cooperation of many centers.
Conclusion
Low-grade PCNSLs should be considered a radiologic differential diagnosis of brain lesions to avoid steroid medication, which hampers neuropathologic diagnosis confirmation by its antiproliferative effect on lymphocytes, and to facilitate the performance of a stereotactic biopsy rather than a resection, which does not improve the prognosis, at least of classic PCNSLs. The radiologic morphology of low-grade PCNSLs may be variable and may overlap with high-grade PCNSLs. Location in deep brain structures or spine, absence of periventricular location, hyperintensity on T2-weighted images, and moderate or absent and heterogeneous contrast enhancement seem more frequent in low-grade PCNSLs as compared with high-grade PCNSLs. The clinical course may be more indolent in low-grade PCNSLs than in classic PCNSLs, and less aggressive treatment may thus be required.
Fig 3.
Case 9, 19-year-old man with low-grade B-cell lymphoma (subtype lymphoplasmacellular lymphoma). A, Extensive hyperintense appearance of the lesion adjacent to the posterior aspect of the right lateral ventricle in FLAIR sequence. B and C, Surprisingly low contrast enhancement on T1-weighted imaging. Moderate edema is noted on T2-weighted imaging (not shown).
Fig 4.
Case 1, 58-year-old man with an intramedullary low-grade T-cell lymphoma. A, T2-weighted turbo spin-echo sequence. B, Postcontrast T1-weighted spin-echo sequence with 2 lymphoma manifestations, situated at the level of the cervicothoracic junction and the thoracic vertebrae 4 and 5 (arrows). Both lesions demonstrate pronounced local edema. The upper lesion shows a marked, homogeneous contrast enhancement, whereas the lower lesion demonstrates only mild contrast enhancement. This patient did not have cerebral lymphoma manifestations. Note.—Reproduced from K. Jahnke et al, Br J Haematol 2005;128:616–624 (© British Society for Haematology).
Fig 5.
Case 2, 58-year-old man with T-cell lymphoma. A right periventricular lesion with strong and homogeneous contrast enhancement is noted on T1-weighted postcontrast spin-echo sequence. Despite radiologic features typical of high-grade PCNSLs, histopathologic investigation revealed a low-grade PCNSL with a growth fraction of 1%.
References
- 1.Plasswilm L, Herrlinger U, Korfel A, et al. Primary central nervous system (CNS) lymphoma in immunocompetent patients. Ann Hematol 2002;81:415–423 [DOI] [PubMed] [Google Scholar]
- 2.Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med 1993;119:1093–1104 [DOI] [PubMed] [Google Scholar]
- 3.Eby NL, Gruffermann S, Flannelly CM, et al. Increasing incidence of primary brain lymphoma in the US. Cancer 1988;62:2461–2465 [DOI] [PubMed] [Google Scholar]
- 4.Herrlinger U, Schabet M, Brugger W, et al. German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma. Ann Neurol 2002;51:247–252 [DOI] [PubMed] [Google Scholar]
- 5.Jahnke K, Korfel A, Martus P, et al. High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol 2005;16:445–449 [DOI] [PubMed] [Google Scholar]
- 6.Korfel A, Martus P, Nowrousian MR, et al. The German Primary Central Nervous System Lymphoma Study Group (G-PCNSL-SG): response to chemotherapy and treating institution predict survival in primary central nervous system lymphoma. Br J Haematol 2005;128:177–183 [DOI] [PubMed] [Google Scholar]
- 7.Bühring U, Herrlinger U, Krings T, et al. MRI features of primary central nervous system lymphomas at presentation. Neurology 2001;57:393–396 [DOI] [PubMed] [Google Scholar]
- 8.Roman-Goldstein SM, Goldman DL, Howieson J, et al. MR of primary CNS lymphoma in immunologically normal patients. AJNR Am J Neuroradiol 1992;13:1207–1213 [PMC free article] [PubMed] [Google Scholar]
- 9.Schwaighofer BW, Hesselink JR, Press GA, et al. Primary intracranial CNS lymphoma: MR manifestations. AJNR Am J Neuroradiol 1989;10:725–729 [PMC free article] [PubMed] [Google Scholar]
- 10.Küker W, Nägele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol 2005;72:169–177 [DOI] [PubMed] [Google Scholar]
- 11.Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol 2002;12:329–340 [DOI] [PubMed] [Google Scholar]
- 12.Gutmann J, Kendall B. Unusual appearances of primary central nervous system non-Hodgkin’s lymphoma. Clin Radiol 1994;49:696–702 [DOI] [PubMed] [Google Scholar]
- 13.Johnson BA, Fram EK, Johnson PC, Jacobowitz R. The variable MR appearance of primary lymphoma of the central nervous system: comparison with histopathologic features. AJNR Am J Neuroradiol 1997;18:563–572 [PMC free article] [PubMed] [Google Scholar]
- 14.Gliemroth J, Kehler U, Gaebel C, et al. Neuoradiological findings in primary cerebral lymphomas of non-AIDS patients. Clin Neurol Neurosurg 2003;105:78–86 [DOI] [PubMed] [Google Scholar]
- 15.Braks E, Urbach H, Pels H, et al. Primary central nervous system immunocytoma: MRI and spectroscopy. Neuroradiology 2000;42:738–741 [DOI] [PubMed] [Google Scholar]
- 16.Mikol J, Wassef M, Galian A, et al. Primary malignant non-Hodgkin’s lymphomas of the central nervous system: report of two cases with a predominant callosal localisation. In: Chatel M, Darcel F, Pecker J, eds. Brain oncology. Dordrecht: Martinus Nijhoff;1987. :257–266
- 17.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994;84:1361–1392 [PubMed] [Google Scholar]
- 18.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press;2001
- 19.Jellinger K, Radaskiewicz TH, Slowik F. Primary malignant lymphomas of the central nervous system in man. Acta Neuropathol Suppl (Berl) 1975;suppl 6:95–102 [DOI] [PubMed] [Google Scholar]
- 20.Bergmann M, Kuchelmeister K, Edel G, Heinecke A. Primary non-Hodgkin lymphomas of the CNS: classification, tissue reaction and proliferative activity. Zentralbl Pathol 1993;139:37–44 [PubMed] [Google Scholar]
- 21.Bogdahn U, Bogdahn S, Mertens HG, et al. Primary non-Hodgkin’s lymphomas of the CNS. Acta Neurol Scand 1986;73:602–614 [DOI] [PubMed] [Google Scholar]
- 22.Ferracini R, Bergmann M, Pileri S, et al. Primary T-cell lymphoma of the central nervous system. Clin Neuropathol 1995;14:125–129 [PubMed] [Google Scholar]
- 23.Jack CR Jr, O’Neill BP, Banks PM, Reese DF. Central nervous system lymphoma: histologic types and CT appearance. Radiology 1988;167:211–215 [DOI] [PubMed] [Google Scholar]
- 24.Lanfermann H, Heindel W, Schaper J, et al. CT and MR imaging in primary cerebral non-Hodgkin’s lymphoma. Acta Radiol 1997;38:259–267 [DOI] [PubMed] [Google Scholar]