Abstract
BACKGROUND AND PURPOSE: Despite the widespread use of angioplasty, adjunct chemical therapy is often needed to treat patients with cerebral vasospasm. In this study, we examined the safety of intraarterial administration of verapamil to patients with cerebral vasospasm. We herein summarize our 2-year experience with this treatment.
METHODS: We retrospectively reviewed the procedure reports, anesthesia records, clinical charts, and brain images of 29 patients who received intraarterially administered verapamil in 34 procedures for the treatment of vasospasm after subarachnoid hemorrhage from July 1998 to June 2000. The average changes in mean arterial pressure and heart rate were used to measure cardiovascular side effects. The neurologic effects were assessed by angiographic findings, the results of neurologic examinations performed before and after the procedure, and findings of CT of the head.
RESULTS: The average dose of verapamil per patient was 3 ± 0 mg or 44 ± 5 mcg/kg. The average changes in mean arterial pressure at 10 and 20 minutes were −5 ± 1 mm Hg and −2 ± 1 mm Hg or −3.8 ± 1.0% and −1.7 ± 1.1%, respectively. No significant change of heart rate was observed at 10 minutes. The patients showed no sign of increased intracranial pressure by hemodynamic parameters, neurologic examination, or CT of the head. On 10 occasions, when the effect of verapamil infusion was assessed angiographically, there was 44 ± 9% increase of vessel diameter in the spastic segment. Neurologic improvement was noted after five of 17 procedures when verapamil was used as the sole treatment.
CONCLUSION: Low dose verapamil is safe when administered intraarterially to patients with cerebral vasospasm. Beneficial effects are achieved in some patients, prompting further study of its efficacy.
Delayed cerebral ischemia due to vasospasm is a major cause of morbidity and mortality for patients with subarachnoid hemorrhage (1). Although recent studies have shown that balloon angioplasty can alleviate the stenosis of proximal cerebral arteries and significantly improve the clinical outcome of patients with cerebral vasospasm (2–5), some vessels are not reachable with a balloon catheter because of the tortuosity of the aorta and carotid arteries. The distal pial arteries and the penetrating arteries can also have vasospasm and are too small to undergo angioplasty (6–9). Intraarterially administered papaverine has been widely used as an adjunct therapy with mixed results (10–14). Some case series showed temporary dilatation of the spastic vessels (11, 13, 15), whereas other groups reported paradoxical vasospasm, worsening ischemia, and profound neurologic deterioration (16–18). Cerebral blood flow studies have shown that intraarterially administered papaverine could temporarily increase cerebral blood flow but had detrimental or no effect on clinical outcome (19–21). Another intraarterially administered vasodilator, nitroglycerin, has been shown to increase intracranial pressure, presumably by dilating the veins and thereby decreasing cerebral perfusion pressure (22, 23). Other classes of intraarterially administered vasodilators need to be evaluated for their safety and efficacy in the treatment of cerebral vasospasm.
Several lines of evidence suggest that intraarterially administered verapamil, a calcium channel blocker, may be beneficial for patients with cerebral vasospasm. Randomized, placebo-controlled clinical trials have shown that an orally administered calcium channel blocker, nimodipine, can improve the clinical outcome of vasospasm (24, 25). As a result, oral nimodipine has become a standard medical therapy for patients with subarachnoid hemorrhage. Verapamil, an injectable calcium channel blocker, has been widely used to treat coronary vasospasm (26–28). The cerebral arteries are similar in size to coronary arteries and may have a similar response to intraarterially administered verapamil. A study of patients undergoing balloon test occlusion found that these patients tolerated ≥7.5 mg of verapamil administered into the carotid arteries without significant systemic effects (29). Increased cerebral blood flow was observed, showing the potential of verapamil to alleviate cerebral ischemia. Further study with superselective intraarterial infusion of verapamil into normal arteries adjacent to cerebral arteriovenous malformations supported its safety and vasodilatory effects (30). Finally, an animal study showed that verapamil caused little rise of intracranial pressure compared with nitroprusside (31). There was no clinical sign of intracranial hypertension after intraarterial infusion of verapamil in the aforementioned clinical studies (29, 30), suggesting that intraarterially administered verapamil may be safer than other vasodilators.
On the basis of these encouraging results, we started to use verapamil for patients with cerebral vasospasm, either as an adjunct therapy to angioplasty or as the sole treatment. This retrospective study examines the cardiovascular and neurologic side effects, angiographic effects, and immediate clinical effects for patients who received intraarterial administration of verapamil from July 1998 to June 2000.
Methods
We retrospectively reviewed angiography reports, anesthesia records, findings of CT of the head, and results of neurologic examinations of all patients who received intraarterial administration of verapamil as a treatment for vasospasm after subarachnoid hemorrhage from July 1998 to June 2000. Verapamil was intraarterially administered to 33 patients during that period. Four patients were excluded from the study: two did not have acute subarachnoid hemorrhage and two received papaverine as well as verapamil. Thirty-four procedures were performed in the remaining 29 patients (eight men and 21 women; average age, 56 ± 2 years) (Table 1). Eighteen patients had Hunt and Hess grades of I to III, and 11 patients had Hunt and Hess grades of IV and V. The treatment was repeated in five patients >3 days after the first treatment. Four of the five patients experienced vasospasm in different territories during the repeat treatment. Twenty-three procedures were performed with the patients under general anesthesia, and 11 patients received treatment under conscious sedation with midazolam and fentanyl.
TABLE 1:
Summary of patients in this study
| Patient | Age (y) | Sex | H&H | Aneurysm | Treatment | Vasospasm | HHH | Post-treatment Examination(*) |
|---|---|---|---|---|---|---|---|---|
| 1 | 58 | F | III | Left PCOM | Clipped | Mild | No | Better |
| 2 | 56 | F | III | Right SCA, vermian AVM | NBCA glued | Moderate | Yes | Unchanged |
| 3 | 60 | F | I | ACOM | Clipped | Mild | Yes | Better |
| 4†‡ | 63 | F | III | Right MCA | Clipped | Mild | No | Unchanged |
| 4†‡ | 63 | F | III | Right MCA | Clipped | Mild | Yes | Better |
| 5 | 48 | F | IV | Basilar | Coiled | Moderate | No | Better |
| 6 | 51 | F | V | ACOM | Clipped | Severe | Yes | Worse |
| 7 | 39 | F | I | Right PCOM | Clipped | Moderate | Yes | Unchanged |
| 8 | 65 | F | III | Right ACOM | Clipped | Mild | No | Unchanged |
| 9‡ | 39 | F | I | Basilar | Failed clipping and coiling | Severe | No | Worse |
| 10 | 53 | M | III | Right vertebral dissection | Coiled | Severe | Yes | Unchanged |
| 11 | 45 | F | V | ACOM | Clipped | Severe | Yes | Unchanged |
| 11† | 45 | F | V | ACOM | Clipped | Severe | Yes | Unchanged |
| 12‡ | 41 | F | V | ACOM | Coiled | Severe | No | Unchanged |
| 12†‡ | 41 | F | V | ACOM | Coiled | Severe | No | Better |
| 13‡ | 60 | M | I | Left MCA | Clipped | Severe | Yes | Better |
| 14 | 65 | F | III | ACOM | Clipped | Mild | Yes | Better |
| 15‡ | 17 | F | III | Left ACOM | Clipped | Moderate | No | Unchanged |
| 16‡ | 60 | F | III | Basilar | Clipped | Severe | No | Unchanged |
| 17‡ | 43 | M | III | Basilar | Clipped | Moderate | Yes | Unknown |
| 18 | 42 | F | V | Left MCA | Clipped | Severe | Yes | Unchanged |
| 19‡ | 48 | F | V | Left PICA | Coiled | Severe | Yes | Unchanged |
| 20 | 78 | F | III | Left PICA | Coiled | Mild | Yes | Unknown |
| 21 | 69 | F | IV | ACOM | Clipped | Moderate | Yes | Unchanged |
| 22 | 56 | F | IV | Left MCA | Clipped | Mild | Yes | Unchanged |
| 22† | 56 | F | IV | Left MCA | Clipped | Mild | Yes | Unchanged |
| 23 | 47 | F | I | Basilar,bilateral MCA | Clipped | Severe | No | Worse |
| 23† | 47 | F | I | Basilar,bilateral MCA | Clipped | Severe | Yes | Better |
| 24 | 69 | M | III | Right MCA | Clipped | Moderate | No | Unchanged |
| 25 | 39 | M | III | Right MCA | Clipped | Mild | Yes | Unchanged |
| 26 | 69 | F | III | Left choroidal, bilateral MCA | L choroidal clipped, L MCA wrapped | Moderate | Yes | Unchanged |
| 27‡ | 65 | M | IV | ACOM | Clipped | Mild | Yes | Better |
| 28 | 63 | M | V | ACOM | Clipped | Severe | No | Unknown |
| 29 | 58 | M | IV | Left SCA | Clipped | Moderate | Yes | Better |
Note.—H&H indicates Hunt and Hess grade; HHH, hypertension, hypervolemia, and hemodilution treatment; F, female, M, male; PCOM, posterior communicating artery; SCA, subclavian artery; AVM, arteriovenous malformation; ACOM, anterior communicating artery; MCA, middle cerebral artery; PICA, posterior inferior cerebellar artery; NBCA, 𝓃-butyl-cyanoacrylate.
Post-treatment neurologic examinations were reviewed by an independent stroke neurologist.
Patients who underwent repeat treatment with verapamil.
Patients who underwent repeat control angiography 10 to 15 minutes after verapamil infusion.
Verapamil was administered in three settings: 1) before balloon angioplasty to prevent catheter-induced vasospasm (n = 16); 2) for treatment of mild vasospasm that did not warrant angioplasty (n = 13); and 3) for treatment of moderate to severe vasospasm that could not be safely treated with balloon angioplasty (n = 5). In the first setting, 1 to 2 mg of verapamil was infused as a bolus through a 6F guiding catheter in the internal carotid or vertebral arteries, immediately before the introduction of an angioplasty balloon catheter. In the second scenario, a bolus of 2 mg of verapamil was infused through a 5F diagnostic catheter in the internal carotid or vertebral artery after angiography was performed. For the third indication, verapamil was used as substitute therapy, because the spastic vessels could not be catheterized with the balloon catheter because of vessel tortuosity or distal location. These patients tended to receive higher doses of verapamil. For patients who received multiple doses of verapamil, if successive doses were delivered within 30 minutes, the doses were combined and counted as one dose and the largest effect was chosen as the representative effect. If successive doses were delivered more than 30 minutes apart, the average dose and effect were used.
Because the major reported undesirable effects of IV administered verapamil are hypotension and severe bradycardia (32), blood pressure and heart rate were used as parameters for systemic side effects. The anesthesia records of all patients were analyzed. The average blood pressure and heart rate measured within 5 minutes before verapamil injection were used as baseline measures. Special attention was also paid to the anesthesia record to look for notes of bradycardia and atrioventricular nodal block. The hemodynamic parameters at 10 minutes were used as the measurement of the systemic effect of verapamil, whereas those at 20 minutes were used to assess the recovery of this effect. The data were analyzed statistically with a paired Student t test.
The patients’ medical records and CT scans of the head obtained before and after treatment were reviewed for signs of neurologic complications, such as increased intracranial pressure, cerebral edema, and hemorrhage. Although Bedford et al (33) reported a 60% rise of intracranial pressure in patients with brain tumor when verapamil was used during anesthesia induction, a previous animal study and several clinical studies did not reveal evidence of clinically significant intracranial hypertension after verapamil infusion. Therefore, we did not directly monitor intracranial pressure in patients with ventriculostomy, because we thought the risk was small. Among the 13 patients who had ventriculostomy before the procedure, 10 had their ventriculostomies opened to a level between 5 and 15 cm H2O, and the other three had their ventriculostomies clamped. We thus used the output of ventriculostomy as indirect indication of intracranial pressure. We also analyzed the anesthesia record for secondary signs of increased intracranial pressure, such as sudden rise of blood pressure and bradycardia. For the 11 patients who were treated under conscious sedation, we examined the procedure reports and patient charts for any record of altered consciousness after verapamil infusion. In addition, CT scans of the head obtained before and after treatment were compared to identify unexpected neurologic complications, such as cerebral edema, ventricular dilatation, herniation, and hemorrhage. All patients underwent CT of the head within 24 hours before the procedure. The average interval until follow-up CT of the head was 3.4 ± 0.5 (n = 34) days. Neuroradiologists unaware of the treatment interpreted the CT scans.
To assess the angiographic effects of intraarterially administered verapamil, repeat angiography was performed 10 to 15 minutes later on 10 occasions. The sex, clinical grade, and degree of vasospasm of these patients are indicated in(Table 1). Six of the patients received intraarterial administration of verapamil as the sole treatment of vasospasm. The 10 angiograms were reviewed. The frontal projections of the arterial phase of these angiograms were digitized by using a Nikon Coolpix 990 digital camera with a fixed magnification. The digital images were imported into MetaMorph 4.6r8 (an image analysis software by Universal Imaging Corp., Downingtown, PA and were magnified 400%, and the diameters of the treated paraclinoid internal carotid artery, M1 segment, and A1 segment were measured by pixels by using the line measurement tool. The projection of a 1-cm marker on the film was used as an internal standard. In four cases, when the markers were not included in the field of view, the ratios of the above vessels to the ophthalmic artery were used to calculate the percent change of vessel diameter after verapamil treatment. The change of the most spastic vessel in each patient was used to calculate the average change among these 10 patients.
To evaluate the immediate clinical effects of intraarterially administered verapamil, the results of the neurologic examinations were compared before and after the procedures among 15 patients who received intraarterial administration of verapamil as the sole treatment during 17 procedures. A stroke neurologist (B.-F.F.) who did not directly participate in the care of these patients reviewed the clinical charts. All patients were admitted to the neurologic intensive care unit after the procedures and underwent daily neurologic examinations by both neurology and neurosurgery teams. The neurologic examinations were determined as better, unchanged, or worse by the stroke neurologist.
Results
The average dose of verapamil per patient was 3 ± 0 mg or 44 ± 5 mcg/kg (Table 2). There was no correlation between the dose and the degree of vasospasm. On average, two vessels were treated in each patient. The largest single dose per vessel was 8 mg (89 mcg/kg). The average dose per vessel was 2 ± 0 mg (29 ± 2 mcg/kg).
TABLE 2:
Change of mean blood pressure and heart rate after verapamil infusion
| Number of Treatments | Dose of Verapamil | Percent Change of MAP at 10 Min*; | Percent Change of MAP at 20 Min* | Percent Change of Heart Rate at 10 Min† | Percent Change of Heart Rate at 20 Mi† | |
|---|---|---|---|---|---|---|
| Not on vasopressor | 11 | 3.4 ± 0.5 mg (51 ± 9 mcg/kg | − 3.1 ± 1.5 | − 0.6 ± 1.7 | 2.4 ± 2.3 | 4.0 ± 2.6 |
| On vasopressor | 23 | 3.0 ± 0.4 (42 ± 6 mcg/kg) | − 4.1 ± 1.3 | − 2.2 ± 1.4 | 1.0 ± 1.2 | 1.0 ± 1.6 |
| Overall | 34 | 3.1 ± 0.3 mg (44 ± 5 mcg/kg) | − 3.8 ± 1.0 | − 1.7 ± 1.1 | 1.5 ± 1.1 | 2.0 ± 1.4 |
The baseline mean arterial pressure of these patients was 112 ± 3 mm Hg †(mean ± standard error of the mean).
The average pretreatment heart rate was 79 ± 3 bpm. (mean ± standard error of the mean).
Intraarterial administration of verapamil had very little systemic effect (Table 2). Typically, the mean arterial pressure decreased by a few millimeters of mercury within minutes after the verapamil injection and gradually returned to baseline level. The average changes of mean arterial pressure at 10 and 20 minutes were −5 ± 1 mm Hg and −2 ± 1 mm Hg, respectively, or −3.8 ± 1.0% and −1.7 ± 1.1% of baseline (112 ± 3 mmHg), respectively. The changes were statistically significant (P < .05 by paired Student t test) but were clinically irrelevant. After the administration of verapamil, the heart rate did not change, allowing for normal physiological fluctuation. The average changes in heart rate at 10 and 20 minutes were 1 ± 1 and 1 ± 1 bpm, respectively, or 1.5 ± 1.2% and 2.0 ± 1.4% of pretreatment heart rate (79 ± 3 bpm) (P < .55 by paired t test). There was no severe bradycardia that warranted treatment. Patients who were receiving triple-H therapy had a similar response to verapamil as that of patients who were not receiving triple-H therapy (Table 2). There was no precipitous increase of systemic effect at the higher doses.
A review of the anesthesia records of all patients did not reveal any secondary sign of increased intracranial pressure, such as a sudden rise of blood pressure and bradycardia. For patients who had a ventriculostomy open during the procedure, there was no change of CSF output from the ventriculostomy. Among the 11 patients who received the treatment under conscious sedation, we did not observe sudden deterioration of consciousness or other neurologic changes to suggest elevation of intracranial pressure after verapamil administration. A comparison of CT scans of the head before and after treatment with verapamil revealed no evidence of worsening cerebral edema, increased hydrocephalus, or new hemorrhage.
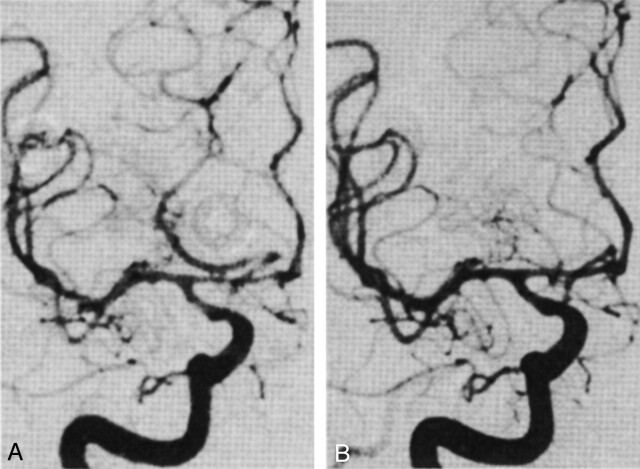
On the 10 occasions when repeat angiograms were obtained 10 to 15 minutes after the administration of verapamil, the average change of diameter of the spastic vessel segments was 44 ± 9%. The most dramatic effects were observed in patients with severe vasospasm. In comparison, the effect on nonspastic vessel segments was minimal (6 ± 3%). One example is shown in Figure 1. This patient had moderately severe vasospasm in the right supraclinoid internal carotid, right middle cerebral, and right anterior cerebral arteries. In addition, there seemed to be high resistance in the anterior circulation, such that the middle cerebral artery and anterior cerebral artery branches were poorly opacified compared with the posterior communicating and posterior cerebral arteries. Because the patient had left-sided weakness, endovascular treatment was indicated. However, there was a loop in her right cervical carotid artery, which would have made placement of a balloon catheter very difficult. Therefore, 2 mg of verapamil was infused into her right internal carotid artery. Fifteen minutes later, the right anterior cerebral artery was visibly dilated (68% increase in diameter in the midsegment). Although there was only a slight increase in the diameter of the right distal internal carotid artery and middle cerebral artery, normal opacification of the anterior circulation was restored and contrast was seen to opacify the distal middle cerebral artery branches before the posterior cerebral artery branches. This latter finding suggests that verapamil may dilate angiographically invisible small resistance arterioles.
Fig 1.
Frontal projection angiograms of the right internal carotid artery.
A, Before treatment with verapamil. Before verapamil infusion, moderately severe vasospasm can be seen in the right anterior cerebral, middle cerebral, and distal internal carotid arteries. Better and more rapid opacification of the right posterior cerebral artery through the right posterior communicating artery can be seen, suggesting high resistance in the anterior circulation.
B, After treatment with verapamil. The diameters of the right distal internal carotid artery, right M1 section, and right A1 section increase by 6%, 12%, and 68%, respectively. Normal opacification of the right middle and anterior cerebral arteries is restored, even though there is no significant change of the diameters of the distal internal carotid and right middle cerebral arteries.
There was no deterioration revealed by the neurologic examinations of the 17 patients treated with verapamil alone (Table 3). Five of them had improved results of their neurologic examinations immediately after the procedure and did not require additional intervention.
TABLE 3:
Change in Results of Neurologic Examination After Verapamil Infusion
| Clinical Grade (Hunt & Hess) | Vasospasm | Number of Treatments | Neurologic Examination Results |
|||
|---|---|---|---|---|---|---|
| Better | Unchanged | Worse | ||||
| I, II, III | Mild | 8 | 3 | 5 | 0 | |
| Moderate | 3 | 0 | 3 | 0 | ||
| Severe | 1 | 1 | 0 | 0 | ||
| IV, V | Mild | 3 | 1 | 2 | 0 | |
| Moderate | 1 | 0 | 1 | 0 | ||
| Severe | 1 | 0 | 1 | 0 | ||
| Total | Mild | 12 | 4 | 8 | 0 | |
| Moderate | 4 | 0 | 4 | 0 | ||
| Severe | 2 | 1 | 1 | 0 | ||
| Total | 17 | 5 | 12 | 0 | ||
Discussion
This study showed that low dose verapamil intraarterially administered to patients with vasospasm has no significant effects on blood pressure or heart rate that would compromise the treatment of vasospasm. In our patients, there was no change in hemodynamic parameters, findings of CT of the head, or results of neurologic examinations that would suggest an increase in intracranial pressure. A small number of patients experienced immediate angiographic and clinical improvement. These data support the safety of intraarterially administered verapamil to patients with vasospasm and prompt further study of its efficacy.
Although the exact effect of intraarterially administered verapamil on intracranial pressure is not known without direct monitoring of intracranial pressure, it is likely to be small and transient, not significant enough to cause any secondary sign or clinical consequence. The animal study of rats showed only a 9% increase of intracranial pressure by intravenous administration of verapamil compared with 77% by nitroprusside (34). When verapamil was administered intraarterially to patients undergoing balloon test occlusion or embolization for cerebral arteriovenous malformations (29, 30), there was no clinical manifestation of intracranial hypertension. The observation by Bedford et al (33) may not be generalized to patients with vasospasm because their patients had compromised cerebral compliance from the mass effect and edema of large brain tumors and altered autoregulation from chronic hypertension. In our study, most patients with vasospasm had normal cerebral compliance. A small increase of cerebral blood flow and cerebral blood volume after verapamil treatment could have been well tolerated without significant elevation of intracranial pressure. For patients who had ventriculostomy, the lack of significant displacement of CSF suggested that there was no dramatic or prolonged elevation of intracranial pressure.
In this study, we used intraarterially administered verapamil as a treatment for vasospasm caused by subarachnoid hemorrhage and as a preventive measure for potential catheter-induced vasospasm. Transient vasospasm can occur at the tip of the guiding catheter or in the intracranial vessels in response to manipulation of guidewires and catheters. In addition to the vasospasm from subarachnoid hemorrhage, catheter-induced vasospasm can occasionally cause flow arrest. We first noticed the potential benefit of verapamil in the treatment of vasospasm during the treatment of catheter-induced vasospasm in the external carotid artery and its branches, which are more prone to vasospasm. Our anecdotal experiences showed that catheter-induced vasospasm occurred approximately one quarter of the time when the external carotid branches were catheterized for treatment of epistaxis or dural AVF. This frequency could be lower with new hydrophilically coated catheters and wires. The catheter-induced vasospasm resolved rapidly (usually within 5–10 minutes) after the infusion of 1 to 2 mg of verapamil, and the spasm was virtually prevented in vessels pretreated with verapamil. We currently routinely administer 1 to 2 mg of verapamil after the placement of the guiding catheter before navigation of microcatheters for angioplasty, and we rarely see catheter-induced vasospasm.
The improved clinical examination and angiographic findings in a small number of patients are encouraging but far from proving the beneficial effects of intraarterially administered verapamil. As represented in Figure 1, in addition to the improvement of proximal vessel diameter, there is better and more rapid opacification of the distal branches. These findings are consistent with a microvascular component of cerebral vasospasm, as suggested by several animal studies (6–8, 35). Measurement of cerebral hemodynamics with position emission tomography also indicates failure of autoregulatory vasodilation of the parenchymal vessels in patients with vasospasm (9). The smaller resistance arterioles, which cannot be treated by angioplasty, may be more responsive to calcium channel blockers. Furthermore, like nimodipine, we do not know whether the beneficial effect of verapamil in this small number of patients is due to improved vasospasm or cerebral metabolic effects. These issues may be resolved by cerebral blood flow studies of patients receiving intraarterial administration of verapamil.
As reported in a dose-escalating study of patients undergoing carotid balloon test occlusion (30), verapamil is more effective in the 6 to 8 mg per vessel range, which is the highest dose used in that study. To assure a large safety margin, we used a low dose of verapamil in the current study, which might not be optimal for patients with cerebral vasospasm. Four patients with severe vasospasm that could not be treated by angioplasty received 4–8 mg of verapamil in one internal carotid artery delivered within 30 minutes. They did not experience a precipitous change of hemodynamics or signs of intracranial hypertension, suggesting that higher doses of verapamil could be safe. Since this study, we have encountered anecdotal experiences of dramatic relief of severe vasospasm and sustained clinical improvement when an 8-mg bolus of verapamil was infused into one internal carotid artery. A dose-escalating study with direct monitoring of intracranial pressure is underway to determine the safety and efficacy of higher doses of verapamil for patients with vasospasm.
The duration of the effect of intraarterially administered verapamil is not known at present. Its systemic effect is short, as evidenced by the rapid recovery of the small blood pressure drop. However, its effect on cerebral blood vessels could be longer (7). When intraarterially administered, the local concentration of verapamil is much larger than when intravenously administered; it may take longer for local drug concentrations to fall below the therapeutic range. Verapamil is an amphiphilic drug, which may have a large distribution in brain tissue (36). Verapamil may thus be slowly released from brain and exert a prolonged effect. The pharmacokinetics of intraarterially administered verapamil need to be addressed in future studies.
Conclusion
Low dose intraarterially administered verapamil is safe and well tolerated by patients with cerebral vasospasm after subarachnoid hemorrhage. Beneficial effects are achieved in some patients. A prospective study using cerebral blood flow as a surrogate end point is under way to further evaluate the safety and efficacy of intraarterially administered verapamil for patients with cerebral vasospasm.
Acknowledgments
We thank Drs. Sundeep Mangla, Shailendra Joshi, and Eui Jong Kim for critical review of the manuscript.
References
- 1.Fisher CM, Roberson GH, Ojemann RG. Cerebral vasospasm with ruptured saccular aneurysm: the clinical manifestations. Neurosurgery 1977;1:245–248 [DOI] [PubMed] [Google Scholar]
- 2.Eskridge JM, McAuliffe W, Song JK, et al. Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery 1998;42:510–517 [DOI] [PubMed] [Google Scholar]
- 3.Higashida RT, Halbach VV, Dowd CF, Dormandy B, Bell J, Hieshima GB. Intravascular balloon dilatation therapy for intracranial arterial vasospasm: patient selection, technique, and clinical results. Neurosurg Rev 1992;15:89–95 [DOI] [PubMed] [Google Scholar]
- 4.Zubkov YN, Nikiforov BM, Shustin VA. Balloon catheter technique for dilatation of constricted cerebral arteries after aneurysmal SAH. Acta Neurochir (Wien) 1984;70:65–79 [DOI] [PubMed] [Google Scholar]
- 5.Firlik AD, Kaufmann AM, Jungreis CA, Yonas H. Effect of transluminal angioplasty on cerebral blood flow in the management of symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 1997;86:830–839 [DOI] [PubMed] [Google Scholar]
- 6.Park KW, Metais C, Dai HB, Comunale ME, Sellke FW. Microvascular endothelial dysfunction and its mechanism in a rat model of subarachnoid hemorrhage. Anesth Analg 2001;92:990–996 [DOI] [PubMed] [Google Scholar]
- 7.Takayasu M, Bassett JE, Dacey RG. Effects of calcium antagonists on intracerebral penetrating arterioles in rats. J Neurosurg 1988;69:104–109 [DOI] [PubMed] [Google Scholar]
- 8.Meyer FB. Calcium antagonists and vasospasm. Neurosurg Clin N Am 1990;1:367–376 [PubMed] [Google Scholar]
- 9.Yundt KD, Grubb RL Jr, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab 1998;18:419–424 [DOI] [PubMed] [Google Scholar]
- 10.Katoh H, Shima K, Shimizu A, et al. Clinical evaluation of the effect of percutaneous transluminal angioplasty and intra-arterial papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. Neurol Res 1999;21:195–203 [DOI] [PubMed] [Google Scholar]
- 11.Firlik KS, Kaufmann AM, Firlik AD, Jungreis CA, Yonas H. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol 1999;51:66–74 [DOI] [PubMed] [Google Scholar]
- 12.Polin RS, Hansen CA, German P, Chadduck JB, Kassell NF. Intra-arterially administered papaverine for the treatment of symptomatic cerebral vasospasm. Neurosurgery 1998;42:1256–1267 [DOI] [PubMed] [Google Scholar]
- 13.Elliott JP, Newell DW, Lam DJ, et al. Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 1998;88:277–284 [DOI] [PubMed] [Google Scholar]
- 14.Livingston K, Guterman LR, Hopkins LN. Intraarterial papaverine as an adjunct to transluminal angioplasty for vasospasm induced by subarachnoid hemorrhage. AJNR Am J Neuroradiol 1993;14:346–347 [PMC free article] [PubMed] [Google Scholar]
- 15.Numaguchi Y, Zoarski GH. Intra-arterial papaverine treatment for cerebral vasospasm: our experience and review of the literature. Neurol Med Chir (Tokyo) 1998;38:189–195 [DOI] [PubMed] [Google Scholar]
- 16.Tsurushima H, Kamezaki T, Nagatomo Y, Hyodo A, Nose T. Complications associated with intraarterial administration of papaverine for vasospasm following subarachnoid hemorrhage: two case reports. Neurol Med Chir (Tokyo) 2000;40:112–115 [DOI] [PubMed] [Google Scholar]
- 17.Barr JD, Mathis JM, Horton JA. Transient severe brain stem depression during intraarterial papaverine infusion for cerebral vasospasm. AJNR Am J Neuroradiol 1994;15:719–723 [PMC free article] [PubMed] [Google Scholar]
- 18.McAuliffe W, Townsend M, Eskridge JM, Newell DW, Grady MS, Winn HR. Intracranial pressure changes induced during papaverine infusion for treatment of vasospasm. J Neurosurg 1995;83:430–434 [DOI] [PubMed] [Google Scholar]
- 19.Fogarty-Mack P, Pile-Spellman J, Hacein-Bey L, et al. Superselective intraarterial papaverine administration: effect on regional cerebral blood flow in patients with arteriovenous malformations. J Neurosurg 1996;85:395–402 [DOI] [PubMed] [Google Scholar]
- 20.Joshi S, Hashimoto T, Ostapkovich N, et al. Effect of intracarotid papaverine on human cerebral blood flow and vascular resistance during acute hemispheric arterial hypotension. J Neurosurg Anesthesiol 2001;13:146–151 [DOI] [PubMed] [Google Scholar]
- 21.Vajkoczy P, Horn P, Bauhuf C, et al. Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke 2001;32:498–505 [DOI] [PubMed] [Google Scholar]
- 22.Ghani GA, Sung YF, Weinstein MS, Tindall GT, Fleischer AS. Effects of intravenous nitroglycerin on the intracranial pressure and volume pressure response. J Neurosurg 1983;58:562–565 [DOI] [PubMed] [Google Scholar]
- 23.Pluta RM, Afshar JK, Thompson BG, Boock RJ, Harvey-White J, Oldfield EH. Increased cerebral blood flow but no reversal or prevention of vasospasm in response to 𝓁-arginine infusion after subarachnoid hemorrhage. J Neurosurg 2000;92:121–126 [DOI] [PubMed] [Google Scholar]
- 24.Allen GS, Ahn HS, Preziosi TJ, et al. Cerebral arterial spasm: a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med 1983;308:619–624 [DOI] [PubMed] [Google Scholar]
- 25.Petruk KC, West M, Mohr G, et al. Nimodipine treatment in poor-grade aneurysm patients: results of a multicenter double-blind placebo-controlled trial. J Neurosurg 1988;68:505–517 [DOI] [PubMed] [Google Scholar]
- 26.Taniyama Y, Ito H, Iwakura K, et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J Am Coll Cardiol 1997;30:1193–1199 [DOI] [PubMed] [Google Scholar]
- 27.Babbitt DG, Perry JM, Forman MB. Intracoronary verapamil for reversal of refractory coronary vasospasm during percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1988;12:1377–1381 [DOI] [PubMed] [Google Scholar]
- 28.Pomerantz RM, Kuntz RE, Diver DJ, Safian RD, Baim DS. Intracoronary verapamil for the treatment of distal microvascular coronary artery spasm following PTCA. Cathet Cardiovasc Diagn 1991;24:283–285 [DOI] [PubMed] [Google Scholar]
- 29.Joshi S, Young WL, Pile-Spellman J, et al. Manipulation of cerebrovascular resistance during internal carotid artery occlusion by intraarterial verapamil. Anesth Analg 1997;85:753–759 [DOI] [PubMed] [Google Scholar]
- 30.Joshi S, Young WL, Pile-Spellman J, et al. Intra-arterial nitrovasodilators do not increase cerebral blood flow in angiographically normal territories of arteriovenous malformation patients. Stroke 1997;28:1115–1122 [DOI] [PubMed] [Google Scholar]
- 31.Bauer JH, Reams GP. The role of calcium entry blockers in hypertensive emergencies. Circulation 1987. :75(6 Pt 2):V174–V180 [PubMed] [Google Scholar]
- 32.Krebs R. Adverse reactions with calcium antagonists. Hypertension 1983;5(4 Pt 2):II125–II129 [DOI] [PubMed] [Google Scholar]
- 33.Bedford RF, Dacey R, Winn HR, Lynch C III. Adverse impact of a calcium entry-blocker (verapamil) on intracranial pressure in patients with brain tumors. J Neurosurg 1983;59:800–802 [DOI] [PubMed] [Google Scholar]
- 34.Thiagarajah S, Azar I, Lear E, Albert D. Intracranial pressure changes during infusions of verapamil as compared with sodium nitroprusside. Bull N Y Acad Med 1985;61:650–656 [PMC free article] [PubMed] [Google Scholar]
- 35.Dietrich HH, Dacey RG Jr. Molecular keys to the problems of cerebral vasospasm. Neurosurgery 2000;46:517–530 [DOI] [PubMed] [Google Scholar]
- 36.Cheymol G. Clinical pharmacokinetics of drugs in obesity: an update. Clin Pharmacokinet 1993;25:103–114 [DOI] [PubMed] [Google Scholar]