Abstract
BACKGROUND AND PURPOSE: Local intraarterial fibrinolysis (LIF) is one of several methods used in treating central retinal artery occlusion (CRAO). We investigated whether LIF is more effective than conservative methods in the treatment of CRAO.
METHODS: In this retrospective study, a total of 178 patients (125 men and 53 women) with CRAO were treated at the Eye Hospital of the University of Freiburg from 1980 to 2000. The average age of the patients was 66.8 years (SD, 12 years). In group I, 116 patients were treated conservatively by anterior chamber paracentesis, massage of the globe, isovolemic hemodilution, acetazolamide, Pentoxifyllin, acetylsalicylic acid, and reduction of arterial hypertension. Some combination but not all of the mentioned conservative methods were used in the conservatively treated patients. In group II, 62 patients receiving LIF received local injection of urokinase or recombinant tissue plasminogen activator into the proximal part of the ophthalmic artery. In case of ipsilateral carotid artery occlusion or high grade stenosis (14 of 62 patients), the thrombolytic agent was administered into the internal maxillary artery.
RESULTS: Among 178 patients, the CRAO was subtotal in 130 (73.0%), incomplete in 39 (21.9%), and total in nine (5.1%). Statistical calculations showed a significantly better visual acuity in group II patients, who were treated with LIF, in comparison with group I patients, who were treated conservatively (P = .0022).
CONCLUSION: For patients with CRAO, LIF is superior to conservative treatment.
Hayreh et al (1) reported that as a general rule, the monkey retina can tolerate no more than 100 minutes of ischemia after ligation of the central retinal artery. In contrast to experimental investigation, fluorescein angiography in humans has shown that in cases of central retinal artery occlusion (CRAO), a partial retinal blood flow exists in most situations. Because of this almost incomplete occlusion, active treatment may be helpful to achieve better blood supply to the retina. In addition to the duration of vessel occlusion, prognosis is dependent on several factors: 1) stage of occlusion, 2) number of occluded retinal branches, 3) occluding material (ie, cholesterol, platelet-fibrin, calcific emboli), 4) duration of occlusion, and 5) patient age.
Several attempts have been made to treat CRAO (Table 1); however, results concerning restoration of visual acuity have remained poor. In this study, we compared medical treatment (including paracentesis) with interventional treatment by local intraarterial fibrinolysis (LIF), which seemed to be superior to all other therapy modalities.
TABLE 1:
Results of conservative treatment of central retinal artery occlusion
| Authors | No. of Patients | Medical Treatment | Final Results |
|---|---|---|---|
| Karjalainen, 1971 (19) (Patients with arteritis were included) | 91 | No comment | 58%blind, 21%good or reduced visual acuity |
| Neubauer et al., 2000 (20) | 65 | Acetazolamide, acetylsalicylic acid, massage of the globe, Pentoxifyllin, beta-blocker, paracentesis | 15%showed distinct improvement (at least three visual acuity gradations) |
| Schmidt et al., 1992 (18) (Control group of conservative treatment in comparison with small number of patients with LIF treatment) | 41 | Ocular massage, Pentoxifyllin, and anterior chamber paracentesis | Virtually no visual improvement; visual acuity improved to 20/50 in only one patient |
| Atebara et al., 1995 (8) | 40 | Paracentesis and Carbogen treatment | Nine (22.5%) patients, visual improvement |
| Augsburger and Magargal, 1980 (21) | 34 | Paracentesis, ocular massage, inhalational therapy (95%oxygen and 5%carbon dioxide), acetazolamide, and aspirin | Helpful in 12 (35%) patients (visual acuity ≥20/100) |
| Duker et al., 1991 (9) | 33 | Carbogen (95%oxygen and 5%carbon dioxide), ocular massage, anterior chamber paracentesis, topical timolol maleate, acetazolamide | 29 patients, low vision (ie, counting fingers or hand movements); one patient, vision deteriorated from 20/40 to hand motions; two patients, final visual acuity of 20/40; one patient, 20/50; one patient, 20/20 |
| Wolf et al., 1989 (10) | 20 | Hypervolemic or isovolemic hemodilution | 10 patients, central vision improved |
| Magargal and Goldberg, 1977 (22) | 20 | Paracentesis | Five patients, significant improvement; five patients, moderate improvement |
| Lorentzen 1969 (23) | 12 | No comment | In no case was there any improvement, rather deterioration |
| (10-year follow-up) | |||
| Rumelt et al., 1999 (14) | 11 | Ocular massage, sublingual isosorbide dinitrate, acetazolamide, mannitol or glycerol, paracentesis, methylprednisolone, streptokinase, and retrobulbar tolazoline | Eight patients, improvement |
| Gombos, 1970 (24) | 7 | Dextran (Rheomacrodex) with papaverine hydrochloride | Two patients, complete recovery; three patients, marked improvement |
| Perkins et al., 1987 (13) | 5 | Acetazolamide, inhalation of 5%carbon dioxide and 95%oxygen, aspirin | Three patients, spontaneous improvement to 20/50 or better; two patients, 20/80 or better after treatment |
| Beiran et al., 1993 (11) | 4 | Hyperbaric oxygenation combined with ocular massage, nifedipine, and glycerol | Three patients, treatment began <100 min, considerable improvement in vision; one patient, treatment began at 6 hr, no improvement |
Methods
From 1980 to 2000, a total of 178 patients with CRAO were treated at the Eye Hospital of the University of Freiburg. Treatment was administered to 125 men (70.2%) and 53 women (29.77%). The average age of all patients was 66.8 years (SD, 12 years). The youngest patient was 18 years old, and the oldest patient was 89 years old.
The difference in time to treatment between the two groups was as follows. The median was 9 hours in group I (conservative treatment) with a mean ± SD of 24.2 ± 40.4, because of some extreme outliers. The median was 9 hours in group II (LIF treatment), with a mean ± SD of 10.8 ± 9.5. The difference is not statistically significant (Wilcoxon test, P = .5).
Patients who were treated between 1980 and 1990 were all treated conservatively (group I). We began treatment by LIF in 1990. Since 1990, patients who had no contraindication for LIF were treated by LIF (group II); all others were treated conservatively. The criteria for conservative or intraarterial treatment did not change during the 20 years of the study.
The patients were not referred to the hospital for a special treatment but for any treatment for acute blindness due to CRAO. The decision regarding which treatment had to be administered was always made by one of our physicians (D.S.).
For every patient, a complete ophthalmological examination was performed, including visual acuity (Snellen chart or examination using a Phoropter) and visual field tests (Goldmann perimeter), slit lamp examination, ophthalmoscopy, and, if possible, fluorescein angiography. An improvement in vision was registered if the patient was able to read the next line with smaller optotypes of the Snellen chart compared with the initial visual acuity.
The different stages of CRAO were separated. They are characterized by the following signs and symptoms.
Stages of CRAO
Stage I: Incomplete CRAO.— Stage I
included diminished visual acuity and a residual visual field but no complete visual loss, slight retinal edema together with a slight cherry-red spot of the macula, no increase in retinal signs over several hours, and delayed but not completely interrupted blood flow revealed by fluorescein angiography. However, despite minor retinal findings, spontaneous recovery usually did not occur during follow-up of several hours.
Stage II: Subtotal CRAO.—Stage II
included visual acuity that was highly reduced, a small island for the biggest test marks left in the visual fields, and distinct edema of the central retina with a cherry-red spot of the macula. The retinal arteries were narrow, and a diminished and interrupted blood flow could be observed (sludge phenomenon in arteries and veins, “cattle track” sign of the arteries). Fluorescein angiography showed a distinct delay in arterial blood flow, especially in the perimacular arterioles.
Stage III: Total CRAO.—Stage III
included no light perception, massive edema of the retina occasionally extending from the center (macular area) to the nasal part of the retina, no blood flow in the perimacular arterioles (in some cases with an additional interruption of the choroidal blood flow), and no cherry-red spot. A cattle track sign of the arteries was usually observed. In contrast to the definition in our former publication (2) for stage III of total CRAO, we have included herein only patients with no light perception.
Treatment
In group I, 116 patients were treated conservatively (Table 2 and Table 3). This treatment consisted of anterior chamber paracentesis, massage of the globe, isovolemic hemodilution (replacement of 500 mL of blood with the same quantity of hydroxy-ethyl starch), acetazolamide (500 mg), Pentoxifyllin (400 mg, three times/day or by infusion), acetylsalicylic acid (100 mg/day), and immediate reduction of arterial hypertension if systolic blood pressure was >200 mm Hg and diastolic pressure was >95 mm Hg.
TABLE 2:
Conservative treatment of 29 patients with incomplete central retinal artery occlusion
| Pentoxifyllin | Hemodilution | Aspirin | Acetacolamid (Diamox) | Heparin |
|---|---|---|---|---|
| 2* (7.89%) | 25† (86.2%) | 4 (13.79%) | 3 (10.34%) | 10 (34.48%) |
| Additional treatment or single treatment | ||||
| Pentoxifyllin + additional treatment in one patient* | Hemodilution + additional treatment in 12 patients† | 9 Heparin + 1 Marcumar) | ||
| Pentoxifyllin only in one (3.45%) patient | Hemodilution only in 11 (39.93%) patients | Aspirin only in one (3.45%) patient | Diamox only in one (3.45%) patient | Heparin only in one (3.45%) patient |
Note.—In all patients, an immediate massage of the globe and treatment of high blood pressure were performed.
Pentoxifyllin + hemodilution + aspirin, one (3.45%) patient.
Hemodilution + heparin, nine (31.03%) patients; hemodilution + aspirin, two (6.89%) patients; hemodilution + Diamox, two (6.89%) patients.
TABLE 3:
Conservative treatment of 83 patients with subtotal central retinal artery occlusion
| Pentoxifyllin Infusions | Hemodilution | Aspirin | Acetacolamide (Diamox) | Rheomacrodex Infusions | Heparin | Eye Drops for Lowering Eye Pressure | Paracentesis |
|---|---|---|---|---|---|---|---|
| 29 (34.9%) | 46 (54.21%) | 28 + 1 Plavix + 1 Tiklyd: 30 (36.14%) | 9 (10.84%) | 8 (9.64%) | 16 (19.28%) | 11 (13.25%) | 13 (15.66%) |
| Additional treatment or single treatment | |||||||
| Pentoxifyllin + additional treatment*: 21 patients (25.3%) | Pentoxifyllin + additional treatment†: 36 (43.37%) | Aspirin + additional treatment‡: 25 (30.12%) | Acetacolamide + additional treatment§: nine (10.84%) | Rheomacrodex + additional treatment§§: eight (9.64%) | Heparin + additional treatment: 14 (16.87%) | Eye drops + additional treatment: 11 (13.25%) | Paracentesis + additional treatment: 11 (13.25%) |
| Pentoxifyllin only: eight patients (9.64%) | Hemodilution only: 10 (12.05%) | Aspirin only: five (6.02%) | Acetacolamide only | Rheomacrodex only | Heparin only: two (2.41%) | Eye drops only | Paracentesis only: two (2.41%) |
Note.—In all patients, immediate massage of the globe and treatment of high blood pressure were performed.
Pentoxifyllin + aspirin, five (6.02%) patients; Pentoxifyllin + Diamox, one (1.2%) patient; Pentoxifyllin + aspirin + Diamox, one patient; Pentoxifyllin + Rheomacrodex, two (2.41%) patients; Pentoxifyllin + hemodilution, one patient; Pentoxifyllin + Paracentesis, three (3.61%) patients; Pentoxifyllin + hemodilution + aspirin + paracentesis, one patient; Pentoxifyllin + eye drops + paracentesis, two (2.41%) patients; Pentoxifyllin + hemodilution + eye drops + paracentesis, one patient; Pentoxifyllin + hemodilution + aspirin + heparin: one patient; Pentoxifyllin + Diamox + Rheomacrodex + paracentesis, one patient; Pentoxifyllin + hemodilution + eye drops, one patient; Pentoxifyllin + Diamox + paracentesis, one patient.
Hemodilution + aspirin, 12 (14.45%) patients; hemodilution + eye drops, three (3.61%) patients; hemodilution + heparin, 10 (12.05%) patients; hemodilution + aspirin + heparin, one (1.2%) patient; hemodilution + aspirin + eye drops, one patient; hemodilution + Diamox + Rheomacrodex + paracentesis, one patient; hemodilution + aspirin + Diamox + heparin, one patient; hemodilution + eye drops + Diamox, one (1.2%) patient.
Aspirin + Heparin, one patient; aspirin + Rheomacrodex, one patient.
Diamox + Rheomacrodex + eye drops, two patients.
Rheomacrodex + paracentesis, one patient.
In group II, 62 patients received local intraarterial injection of urokinase (total amount between 200,000 and 1.3 million IU) or recombinant tissue plasminogen activator (between 40 and 80 mg), respectively, into the proximal part of the ophthalmic artery. In case of occlusion of the ipsilateral internal carotid artery, the thrombolytic agent was administered into the internal maxillary artery. High grade stenosis (>70%) or occlusion of the ipsilateral internal carotid artery was present in 14 (22.6%) of 62 patients. In two additional patients, the origin of the ophthalmic artery was in the middle meningeal artery and not in the internal carotid artery. These two patients also were treated via the middle meningeal artery.
Patients with a cilioretinal artery were excluded from LIF treatment. None of the 178 patients had any inflammatory vascular conditions, such as giant cell arteritis or Wegener granulomatosis. Globe massage and treatment to reduce high blood pressure were administered before LIF for every patient. Before treatment began, immediate reduction of arterial hypertension had been achieved in group II patients treated with LIF.
The additional anticoagulation with heparin (partial thromboplastin time between 50 and 60 s) was started during the administration of LIF and was continued for at least 2 days. After discharge from the hospital, patients received additional treatment with acetylsalicylic acid (aspirin) or, rarely, Phenprocoumon (Marcumar), to avoid recurrence of arterial obstruction.
We performed additional isovolemic hemodilution in five patients. These patients did not achieve improvement of visual acuity after LIF or after initial isovolemic hemodilution.
The LIF procedure has been described in detail in our previous publications (3, 4). A 5F guiding catheter was introduced through the femoral artery with the patient under local anesthesia and was placed in the proximal, extracranial part of the internal carotid artery. Angiography of the common carotid artery was performed for every patient before the catheter was introduced into the internal carotid artery. Angiographic follow-up during and at the end of LIF (Fig 1) was not mandatory for evaluating the effectiveness of treatment. Therefore, it was not continuously performed. A volume of 1 mL/min urokinase was manually injected through a microcatheter (Tracker 18) that was introduced coaxially through the guiding catheter (total amount between 200,000 and 1.3 million IU). The mean dose of urokinase was 938,666 IU (median dose, 1 million IU). The mean dose of recombinant tissue plasminogen activator was 55 mg (median dose, 50 mg). Urokinase was administered for durations of 1 hour 10 minutes to 2 hours 30 minutes. Most patients received a total amount of 800,000 to 1 million IU of urokinase. During treatment, the eyes were examined (visual acuity and ophthalmoscopy) every 10 minutes by the same ophthalmologist to assess for improvement in vision or better blood flow. The dosage of treatment with recombinant tissue plasminogen activator or urokinase depended on visual improvement. If the fundus showed better arterial flow of the retinal arteries and visual improvement of one or two lines (Snellen chart), the injection of the fibrinolytic agent could be finished.
Fig 1.
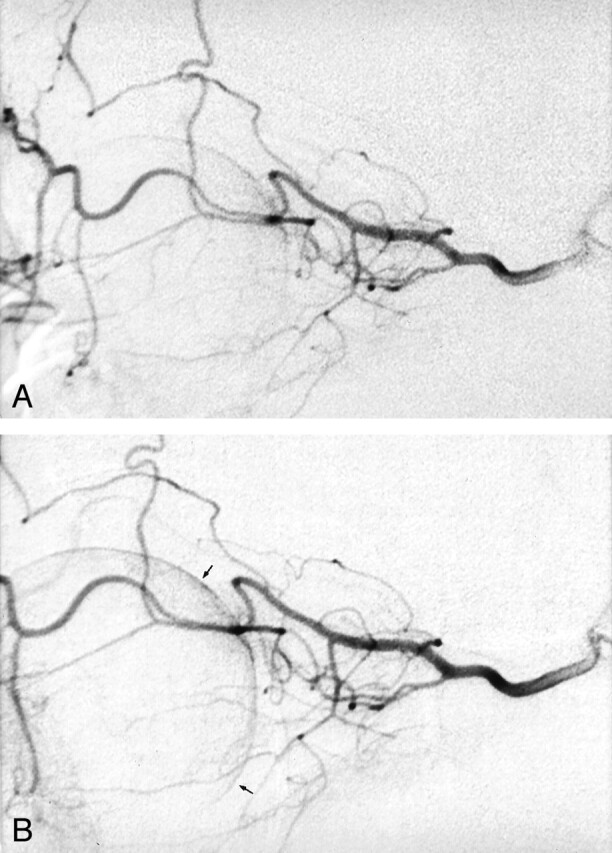
Superselective angiography of the ophthalmic artery in a patient with acute blindness due to CRAO.
A, Before the administration of LIF.
B, After the administration of LIF, no significant changes were noted, except for slightly better choroidal-retinal perfusion (arrows).
Statistical Methods
To judge the treatment effect as independently as possible from other influences, we used the procedure of ordinal logistic regression. The target variable, on the one hand, was the level of visual acuity after treatment, and, on the other hand, the before and after difference in visual acuity was chosen. The independent variables were initial visual findings (degree of visual acuity), stage of occlusion (incomplete, subtotal, total CRAO), age, sex, duration of occlusion, and type of therapy (conservative or LIF treatment). With backward elimination, the number of influence variables was reduced to essential variables. The judgment of significance was calculated by using Wald’s χ2 test. For statistical purposes, we subdivided the measurements of visual acuity of patients into five groups (Table 4).
TABLE 4:
Five groups of visual impairment
| 0 | Blindness, no light perception |
| I (high degree of visual loss) | Perception of light, finger counting, recognition of hand motions |
| II (pronounced degree of visual loss) | 1/50–1/15 |
| III (distinct degree of visual loss) | 0.1–0.3 |
| IV (minor degree of visual loss) | 0.4–1.0 |
Results
Of 178 patients with CRAO, 116 (65.2%) were treated conservatively and 62 (34.8%) were treated with LIF. Of 178 patients, 130 (73.0%) had subtotal occlusion, 39 (21.9%) had incomplete occlusion, and nine (5.1%) had total occlusion (Table 5). The average duration of blindness up to the beginning of treatment was 19.49 hours (minimal, 1 hour; maximal, 312 hours; SD, 33.58 hours).
TABLE 5:
178 Patients with central retinal artery occlusion
| Stage of Occlusion | No. of Patients |
|---|---|
| Subtotal | 130 (73.0%) |
| Incomplete | 39 (21.9%) |
| Total | 9 (5.1%) |
The difference in time to treatment between the two groups was as follows. The median was 9 hours in group I (conservative treatment), with a mean ± SD of 24.2 ± 40.4, because of some extreme outliers. The median was 9 hours in group II (LIF treatment), with a mean ± SD of 10.8 ± 9.5. The difference was not statistically significant (Wilcoxon test, P = .5).
The statistical calculations showed that a significant superiority existed in group II patients who were treated with LIF compared with group I patients who were treated conservatively (P = .0022). The much better results in group II were shown independently from the statistical models used.
As expected, patients with incomplete CRAO showed a significantly better prognosis (P = .0001) than those with more pronounced occlusion of the central retinal artery, but the very small patient group with total occlusion could not be separated from the other groups (P values according to different models, .23-.97). Patient sex had no influence on the values; however, visual prognosis decreased with advancing age (P = .01). The mean age of the conservatively treated patients (group I) was higher than that of the patients treated with LIF (group II). The statistical evaluation of the age groups of patients treated with LIF compared with the conservatively treated patients showed that if the high and low age data of patients were eliminated, the significant superiority of the patients treated with LIF did not change.
The latency period (delay from visual loss to beginning of treatment) did not show a significant difference between the two groups. The negative influence of the duration of blindness was not significant (P = .07-.08). An improvement in vision was registered if the patient was able to read the next line with smaller optotypes of the Snellen chart, compared with the initial visual acuity. Initial visual acuity was of great importance when analyzing the final results after treatment (P = .0001), but it had no influence on the visual changes (P = .5) (Table 6 and Table 7).
TABLE 6:
Influence of treatment on 178 patients with central retinal artery occlusion: results of ordinal logistic regression for visual acuity after treatment
| Effect | No. of Patients | Wald’s χ2 | P | |
|---|---|---|---|---|
| Treatment | Conservative | 116 (65.17%) | ||
| LIF | 62 (34.3%) | 9.38 | b | |
| 0.0022 | ||||
| Gender | Male | 125 (70.2%) | b | |
| Female | 54 (30.3%) | 0.17 | 0.6834 | |
| e | ||||
| Occlusion | Subtotal | 130 (73.0%) | b | |
| Incomplete | 39 (21.9%) | 26.49 | 0.0001 | |
| Complete | 9 (5.1%) | 4.53 | 0.0332 | |
| Age | 18–89 years | 8.71 | 0.0032 | |
| Latency | 1–312 hr | 3.97 | 0.0459 | |
| Initial visual acuity | 0–5 | 43.31 | 0.0001 |
Note.—LIF indicates local intra-arterial fibrinolysis; b, baseline (reference) class; e, removed by backward elimination; (given P values are probability to remove values).
TABLE 7:
Results for ordinal logistic regression for change of visual acuity under treatment: subpopulation of subtotal occlusions
| Effect | Classes/Range | Wald’s χ2 | P | ||
|---|---|---|---|---|---|
| Treatment | Conservative, | 5.70 | b | ||
| 83 (63.8%) | b | 0.0169 | |||
| LIF, 47 (36.15%) | |||||
| (36.15%) | |||||
| Gender | Male 93 | 0.01 | b | ||
| (71.5%) | 0.9061 | e | |||
| Female, 37 (28.5%) | |||||
| (28.5%) | |||||
| Age | 18–89 years | 8.85 | 0.0029 | ||
| Latency | 1–312 hr | 3.60 | e | 0.0579 | e |
| Initial visual acuity | 1–3 | 4.28 | 0.0385 |
Note.—LIF indicates local intra-arterial fibrinolysis; b, baseline (reference) class; e, removed by backward elimination.
The biggest group of patients (n = 130) was that with subtotal CRAO. In that group, 83 patients were treated conservatively and 47 with LIF. Comparing the two treatment modalities, we noted significantly better final visual acuity in the patients treated with LIF, in contrast to those treated conservatively. Because of the smaller number of patients in the LIF group (group II), the effect of treatment was significant (P = .0169) on a lower level.
Visual improvement after LIF, according to three different stages, is shown in Table 8. In comparison, visual improvement after conservative treatment according to three different stages is shown in Table 9. Obviously, the results after treatment with LIF are better than with conservative treatment. The better results were achieved in patients who were treated during the first 6 hours after acute loss of vision (Table 8). Patients who were treated conservatively (Table 9) did not show distinct improvement of visual acuity when treated early.
TABLE 8:
Visual improvement after intra-arterial fibrinolysis: three different stages in 62 patients
| Stage of CRAO | Group with Distinct Visual Improvement n = 10 patients (16.13%) | Group with Partial Visual Improvement n = 26 patients (41.93%) | Group without Visual Improvement n = 26 patients (41.93%) |
|---|---|---|---|
| Stage I (incomplete) | 5 (50%) | 3 (30%) | 2 (20%) |
| Stage II (subtotal) | 5 (10.64%) | 19 (40.43%) | 23 (48.94%) |
| Stage III (total) (no light perception) | 0 | 4 (80%) | 1 (20%) |
| Treatment of 62 patients with intra-arterial fibrinolysis: time interval of treatment after blindness | |||
|---|---|---|---|
| Treatment <6 hr after Visual Loss (n = 10) | Treatment ≥6–14 hr after Visual Loss (n = 26) | Treatment >14 hr after Visual Loss (n = 26) | |
| Distinct improvement | 4 (30.77%) | 5 (13.16%) | 1 (9.09%) |
| Partial improvement | 6 (46.15%) | 15 (39.47%) | 5 (45.45%) |
| No improvement | 3 (23.08%) | 18 (47.37%) | 5 (45.45%) |
Note.—CRAO indicates central retinal artery occlusion.
TABLE 9:
Visual improvement after conservative treatment: three different stages in 116 patients
| Stage of CRAO | Group with Distinct Visual Improvement n = 7 patients (6.5%) | Group with Partial Visual Improvement n = 27 patients (23.28%) | Group without Visual Improvement or with Deterioration n = 82 patients (70.69%) |
|---|---|---|---|
| Stage I (incomplete) | 5 (17.24%) | 14 (48.28%) | 10 (34.48%) |
| Stage II (subtotal) | 2 (2.41%) | 13 (15.66%) | 68 (81.93%) |
| Stage III (total) (no light perception) | 0 | 0 | 4 (100%) |
| Conservative treatment of 116 patients: time interval of treatment after blindness | |||
|---|---|---|---|
| Treatment <6 hr after Visual Loss n = 31 (26.72%) | Treatment: ≥6 to 14 hr after Visual Loss n = 44 (37.93%) | Treatment: ≥14 hr after Visual Loss n = 41 (35.34%) | |
| Distinct improvement | 1 (3.23%) | 3 (6.82%) | 3 (7.32%) |
| Partial improvement | 7 (22.58%) | 11 (25.00%) | 9 (21.95%) |
| No improvement | 23 (74.19%) | 30 (68.18%) | 29 (70.73%) |
Note.—CRAO indicates central retinal artery occlusion.
Complications of LIF Treatment
Two complications of LIF treatment occurred (transient aphasia in one patient and hemiparesis in another). These were thromboembolic complications due to catheter manipulation. In both patients, fibrinolytic treatment had been administered immediately through the same microcatheter and a complete resolution of the paresis and aphasia occurred. Both patients were >80 years old. These episodes occurred at the beginning of our study. After these events, we no longer treated patients who were >75 years of age. The only exception for treatment of patients who are >75 years of age is CRAO of the only eye.
Discussion
In the treatment of patients with CRAO, new aspects arose. Conventional conservative methods, such as anterior chamber paracentesis, massage of the globe, isovolemic hemodilution, acetazolamide, Pentoxifyllin, acetylsalicylic acid, and inhalational therapy did not show any significant amelioration for most patients (Table 1).
Our results with the LIF method, accumulated in Freiburg since 1990, show that LIF is more effective than conservative treatment modalities (Table 6). Similar beneficial results in the treatment of CRAO with LIF in a smaller number of patients have been published by other authors (5, 6).
Weber et al (5) compared two groups of patients with CRAO in a non-randomized, retrospective study. In one group, 17 patients were treated with LIF, and in a control group, 15 patients were treated conservatively. The authors showed that the patients treated with LIF had significantly better visual outcomes compared with those in the control group.
Richard et al (6) treated 46 patients with CRAO and seven patients with branch retinal arterial occlusion by using LIF. However, they did not compare their results with a control group. Twenty-three patients experienced increase in visual acuity of two or more visual acuity gradations (50%).
We were able to find some conditions for noneffective LIF in CRAO. The prognosis remained poor when total occlusion of the central retinal artery existed or if, in addition to the CRAO, a distinct hypoperfusion of the choroidal vessels was present, as shown by fluorescein angiography (2, 7). In addition to arterial hypertension, CRAO may be due to subintimal hemorrhage, intimo-intimal intussusception, or dissection of the central retinal artery. In cases of CRAO resulting from these causes, LIF cannot succeed.
In the literature, the conservative treatment of patients with CRAO varies extremely from author to author. In most publications, only a small number of patients are involved and comparisons with control groups are not shown. Even with prospective studies (8–10), statistical evaluation of treatment was impossible because of small patient numbers. Different drugs and procedures (single and in combination) were used (Table 1). Therefore, a single most effective drug for improving vision cannot be selectively identified from these reports.
Case presentations in all studies do not differentiate the degree of CRAO severity (stage of CRAO); therefore, it is impossible to compare the data. In the reports presenting successful treatment, the patients had not been divided into groups according to their CRAO stage (ie, incomplete, subtotal, or total). One cannot ignore the possibility that those patients enjoying good treatment results, as reported in the literature, had incomplete CRAO before treatment.
In many patients, the time delay of treatment after visual loss was not mentioned. Therefore, very different results after treatment were found. Early treatment of patients within a few hours after visual loss was emphasized by some authors because results in this group were better (11–14).
General diseases, such as arterial hypertension or embolic event or both, and their treatment have not been taken into consideration in many of the publications as a possible cause for CRAO. Emboli may be caused by stenosis or occlusion of the internal carotid artery or by heart problems such as arrhythmia, valve disease, or patent foramen ovale. Other rare embolic sources also exist, such as talc, atrial myxoma, or thrombi from the heart or aneurysm. Embolism severity in the retinal arteries depends on the type, quantity, and size of the emboli.
Systemic treatment with urokinase or recombinant tissue plasminogen activator is possible (15, 16) but can induce cerebral hemorrhages (17), especially if a high dosage is administered as a bolus within a short time. Local administration of the thrombolytic agent into the ophthalmic artery within 60 to 90 minutes reduces the risk of bleeding, as verified with our patients in whom no retinal or cerebral hemorrhage occurred. A further important main advantage of this procedure is the agent’s high concentration close to the occlusive material in the artery. Furthermore, local flushing supports the recanalization of occluded vessels.
There are some contraindications for local fibrinolytic therapy, such as myocardial infarction, cardiac insufficiency, infective endocarditis (endocarditis lenta), aneurysm of the heart wall, auricular fibrillation, and absolute arrhythmia. Hemorrhagic diathesis, cirrhosis of the liver, gastric or duodenal ulcer, untreatable hypertension, and the administration of oral anticoagulants are also contraindications. The procedure should be avoided if the patient has undergone major surgery within the previous 4 weeks or has suffered a stroke within the last 2 months. In cases of thromboembolic complications due to catheter manipulation (this occurred in two of our patients), fibrinolytic treatment can be administered immediately, through the same microcatheter (2, 18).
Conclusion
If no contraindication for thrombolysis exists, LIF can be recommended as the treatment of choice for CRAO, supported by anticoagulation therapy. Massage of the globe and medical treatment as described should also be used. During and after LIF, additional treatment with heparin and later with acetylsalicylic acid (aspirin) and/or Clopidogrel and, in selected cases, with Phenprocoumon, is essential to avoid the recurrence of vascular occlusions.
TABLE 3a:
Conservative treatment of four patients with a total central retinal artery occlusion
| Pentoxifyllin Infusions | Hemodilution | Aspirin | Acetacolamide (Diamox) | Rheomacrodex Infusions | Heparin | Eye Drops for Lowering Eye Pressure | Paracentesis |
|---|---|---|---|---|---|---|---|
| One patient* | Two patients | One patient | One patient |
Note.—In all patients, immediate massage of the globe and treatment of high blood pressure were performed.
One patient was treated with Pentoxifyllin infusions + Diamox + paracentesis.
References
- 1.Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology 1980;87:75–78 [DOI] [PubMed] [Google Scholar]
- 2.Schmidt D, Schumacher M. Stage-dependent efficacy of intra-arterial fibrinolysis in central retinal artery occlusion (CRAO). Neuroophthalmology 1998;20:125–141 [Google Scholar]
- 3.Schumacher M, Schmidt D, Wakhloo AK. Intra-arterial fibrinolysis in central artery occlusion [in German]. Radiologe 1991;31:240–243 [PubMed] [Google Scholar]
- 4.Schumacher M, Schmidt D. Local fibrinolysis in central retinal artery occlusion: follow-up in 36 cases. In: Takahashi M, Korogi Y, Moseley I, eds. Proceedings of the XVth Symposium of Neuroradiology Kumamoto. Berlin: Sringer-Verlag;1995. :458–460
- 5.Weber J, Remonda L, Mattle HP, et al. Selective intra-arterial fibrinolysis of acute central retinal artery occlusion. Stroke 1998;29:2076–2079 [DOI] [PubMed] [Google Scholar]
- 6.Richard G, Lerche RC, Knospe V, Zeumer H. Treatment of retinal arterial occlusion with local fibrinolysis using recombinant tissue plasminogen activator. Ophthalmology 1999;106:768–773 [DOI] [PubMed] [Google Scholar]
- 7.Schmidt D, Schumacher M. Intra-arterial fibrinolysis in 51 patients with central retinal artery occlusion [in German]. Z Prakt Augenheilk 1999;20:205–212 [Google Scholar]
- 8.Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology 1995;102:2029–2035 [DOI] [PubMed] [Google Scholar]
- 9.Duker JS, Sivalingam A, Brown GC, Reber R. A prospective study of acute central retinal artery obstruction: the incidence of secondary ocular neovascularization. Arch Ophthalmol 1991;109:339–342 [DOI] [PubMed] [Google Scholar]
- 10.Wolf S, Hoberg A, Bertram B, Jung F, Kiesewetter H, Reim M. Video fluorescein angiography follow-up of patients with retinal artery occlusion [in German]. Klin Monatsbl Augenheilkd 1989;195:154–160 [DOI] [PubMed] [Google Scholar]
- 11.Beiran I, Reissman P, Scharf J, Nahum Z, Miller B. Hyperbaric oxygenation combined with nifedipine treatment for recent-onset retinal artery occlusion. Eur J Ophthalmol 1993;3:89–94 [DOI] [PubMed] [Google Scholar]
- 12.Chen JC, Cheema D. Repeated anterior chamber paracentesis for the treatment of central retinal artery occlusion. Can J Ophthalmol 1994;29:207–209 [PubMed] [Google Scholar]
- 13.Perkins SA, Magargal LE, Augsburger JJ, Sanborn GE. The idling retina: reversible visual loss in central retinal artery obstruction. Ann Ophthalmol 1987;19:3–6 [PubMed] [Google Scholar]
- 14.Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol 1999;128:733–738 [DOI] [PubMed] [Google Scholar]
- 15.Wiegand W, Siegert J, Scholz R, Kroll P. Lysetherapie bei Zentralarterienverschlüssen: Sitzungsbericht 153 Vers [in German]. Verein RW Augenärzte 1991;383–389
- 16.Schinzel H, Kern M, Kelbel C, Weilemann LS, Benning H, Grehn F. Fibrinolytische Therapie bei akuten Zentralarterienverschlüssen des Auges [in German]. Intensivmed 1992;29:170–172 [Google Scholar]
- 17.Kase CS, O’Neal AM, Fisher M, Girgis GN, Ordia JI. Intracranial hemorrhage after use of tissue plasminogen activator for coronary thrombolysis. Ann Intern Med 1990;112:17–21 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt D, Schumacher M, Wakhloo AK. Microcatheter urokinase infusion in central retinal artery occlusion. Am J Ophthalmol 1992;113:429–434 [DOI] [PubMed] [Google Scholar]
- 19.Karjalainen K. Occlusion of the central retinal artery and retinal branch arterioles: a clinical, tonographic and fluorescein angiographic study of 175 patients. Acta Ophthalmologica Suppl 1971;109:1–95 [PubMed] [Google Scholar]
- 20.Neubauer AS, Mueller AJ, Schriever S, Grüterich M, Ulbig M, Kampik A. Minimal invasive Therapie bei klinisch komplettem Zentralarterien-verschluβ- eigene Ergebnisse und Literaturvergleich [in German]. Klin Monatsbl Augenheilkd 2000;217:30–36 [DOI] [PubMed] [Google Scholar]
- 21.Augsburger JJ, Magargal LE. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol 1980;64:913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magargal LE, Goldberg RE. Anterior chamber paracentesis in the management of acute nonarteritic central retinal artery occlusion. Surg Forum 1977;28:518–521 [PubMed] [Google Scholar]
- 23.Lorentzen SE. Occlusion of the central retinal artery: a follow-up. Acta Ophthal (Copenh) 1969;47:690–703 [DOI] [PubMed] [Google Scholar]
- 24.Gombos GM. A new treatment of central retinal artery occlusion. Ann Ophthalmol 1970;2:893–896 [Google Scholar]


