Abstract
BACKGROUND AND PURPOSE: Dizziness is a symptom that develops with internal ear disturbances and with dysfunctions of the brain stem and cerebellum, in particular with blood flow disturbances of the brain stem and cerebellum (posterior circulation ischemia [PCI]). Patients with PCI often present with various neurologic signs and symptoms. To examine the usefulness of contrast-enhanced 2D cine phase MR angiography in the diagnosis of PCI, we examined quantitative blood flow of the basilar artery in patients with PCI who had primarily complained of dizziness.
METHODS: We quantitatively measured the blood flow volume rate of the basilar artery by using a contrast-enhanced 2D cine phase MR angiographic technique in 21 patients diagnosed with PCI and in 16 age- and sex-matched control participants.
RESULTS: Maximum and mean average flow velocities of the basilar artery in the PCI group were significantly lower than those of the control group (29.2 ± 9.2 cm/s versus 38.5 ± 8.2 cm/s [P < .005] and 18.0 ± 5.6 cm/s versus 22.6 ± 5.0 cm/s [P < .01], respectively). The flow volume rates of the basilar arteries were also significantly lower in the PCI group (103.3 ± 37.3 mL/min versus 148.8 ± 40.0 mL/min [P < .001]).
CONCLUSION: The flow volume rate of the basilar artery in patients with PCI during intermittent ischemic attacks with dizziness was chronically reduced compared with that in the control participants. This suggests that flow volume rates may influence the development of the clinical signs and symptoms of PCI. We think that contrast-enhanced 2D cine phase MR angiography is a valuable method for the diagnosis of PCI.
Dizziness is a symptom that develops with internal ear disturbances and with dysfunctions of the brain stem and cerebellum, called posterior circulation ischemia (PCI), that present with various neurologic signs and symptoms, including transient hemiplegia, sensory disturbance, dysarthria, and dizziness (1–10). In particular, dizziness often occurs as an isolated sign of PCI (5–8). The term vertebrobasilar insufficiency has been widely used by physicians. The clinical signs and symptoms of vertebrobasilar insufficiency were first described in 1946 by Kubik and Adams (1). Caplan (2) advocated the name posterior circulation ischemia and indicated, in detail, the clinical symptoms. Several other investigators have since sought to define the clinical criteria for PCI (3, 4), and recently, the National Institute of Neurological Disorders and Stroke (NINDS) categorized PCI as a transient ischemic attack of the vertebrobasilar circulation (11). Although it is relatively easy to diagnose PCI in patients who exhibit characteristic neurologic signs and symptoms, it is often difficult to distinguish this diagnosis from internal ear disturbances if only dizziness is displayed. It has been reported that isolated dizziness as the symptom of PCI occurs more frequently than previously thought (5–8). Dizziness with PCI includes an element of peripheral vertigo due to ischemia of the internal ear, so detailed information regarding dizziness, the nature, duration, cause, and neurologic examination findings of positional nystagmus, and difficulty of equilibration is necessary to correctly distinguish PCI from pure peripheral vertigo, such as benign paroxysmal positional vertigo, vestibular neuronitis, Ménière disease, and so on. Recent approaches to monitor degeneration of the brain stem or stenosis of the basilar or vertebral arteries with MR imaging or MR angiography have provided valuable means for the diagnosis of PCI (8–10). However, these means are limited in that their evaluations are qualitative rather than quantitative. Recent advances in MR angiography hardware and software have enabled measurements of blood flow velocity and flow volume rate (12–16). In this study, we used contrast-enhanced 2D cine phase MR angiography to measure quantitative blood flow of the basilar artery in patients with PCI who had complained primarily of dizziness and who had the diagnosis of PCI on the basis of clinical findings.
Methods
Participants
Between April 1997 and November 1998, we examined a group of 21 patients (10 men and 11 women; age range, 41–80 years; average age, 61.3 ± 14.2 years) with symptoms of dizziness. The PCI criteria were fulfilled as outlined in previous studies (3–5): 1) presenting paroxysmal or repeated dizziness associated with movement of the head, gait, or action; 2) no abnormality in the labyrinth revealed by otologic examination; 3) no infarctions causing dizziness in the brain stem and cerebellum, except for old infarctions, as revealed by MR imaging; 4) occasional transient signs and symptoms indicating ischemia of the brain stem, such as diplopia or sensory or motor disorder of the face, extremities, and trunk. Eleven men and five women ranging in age from 40 to 85 years (average age, 61.6 ± 11.0 years), with no clinical signs or symptoms of PCI served as control participants.
We calculated body mass index and measured body weight, height, and systolic blood pressure for all patients with PCI and all control participants. In the PCI group, systolic blood pressure was measured twice, at the time of attack and intermittently by using contrast-enhanced 2D cine phase MR angiography. In the control group, systolic blood pressure was measured using only contrast-enhanced 2D cine phase MR angiography. Examinations of positional vertigo by Bárány caloric and Romberg tests and by audiograms were performed for all patients with PCI by a neurotologist to distinguish patients with peripheral vertigo. A Schellong test was performed for all patients with PCI to distinguish patients with orthostatic hypotension. A blood cell count, serum laboratory examination, and electrocardiography were performed for all patients with PCI and control participants. Patients who displayed arrhythmia, such as atrial fibrillation, were excluded from the study, because the method studied was not suited to them. MR imaging was performed at least twice for all patients with PCI to distinguish patients with fresh infarctions and once for each control participant. Contrast-enhanced 2D cine phase MR angiography was performed at the same time as MR imaging during intermittent ischemic attacks in all patients with PCI and for all control participants. Three-dimensional time-of-flight MR angiography was performed in eight of the patients with PCI and in eight control participants, and single photon emission CT was performed in all patients with PCI and four control participants.
Risk factors known to affect cerebral blood flow in both groups were further classified into four subcategories on the basis of the following criteria: 1) hypertension (systolic blood pressure >140 mm Hg before treatment), 2) diabetes mellitus (blood glucose level >200 mg/dL when fasting), 3) hyperlipidemia (blood total cholesterol level >200 mg/dL or neutral fat >160 mg/dL), and 4) smoker (habitual smoking during the study). Informed consent was obtained from all patients and participants before entrance into the study.
Materials Used
Contrast-enhanced 2D cine phase MR angiography was performed on a 1.5-T machine (Advantage 4.8 version; GE Signa, GE Medical Systems, Milwaukee, WI) equipped with a standard head coil. We used contrast-enhanced 2D cine phase MR angiography for quantitative measurement of blood flow. Sequence parameters were as follows: 40/11.8/2 (TR/TE/NEX); flip angle, 30 degrees; matrix, 256 × 192; field of view, 18 cm; section thickness, 5 mm; interpolated phases per RR interval, 32. Velocity encoding was adjusted to a maximum value of 150 cm/s. Flow encoding direction was from caudal to cranial. To determine the transverse imaging plane, MR imaging of the sagittal head was generated from gradient recalled acquisition in the steady state. The imaging plane was determined for an oblique image at the midpontine level to be perpendicular to the basilar artery (Fig 1). Imaging time was approximately 5 to 7 minutes, depending on heart rate. Pixel intensity was measured in the region of interest, which as a minimum oval included the lumen of the basilar artery for all images. Images acquired by using a phase contrast technique provided estimates of flow velocity that were represented by pixel intensity values (cm/s). The flow velocity thus obtained was the average flow velocity in a blood vessel. The flow volume rate through the vessels was calculated by multiplying the averaged flow velocity over all phases of the cardiac cycle and the region of interest area. Because flow velocity was greatly changed by the setup of the region of interest, for most head vascular evaluations, we found that the flow volume rate in mL/min was the most reliable parameter.
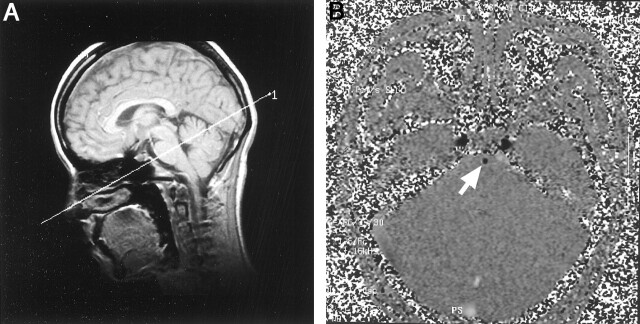
Fig 1.
Gradient recalled acquisition in the steady state and contrast-enhanced 2D cine phase MR images.
A, For the determination of the transverse image plane, a sagittal gradient recalled acquisition in the steady state MR image (20/5; flip angle, 60 degrees) was created. The imaging plane was determined at the midpontine level to be perpendicular to the basilar artery.
B, Contrast-enhanced 2D cine phase MR image (40/11.8; flip angle, 30 degrees), acquired at 32 phase. Note the basilar artery as an area of hypointensity (arrow).
Statistical Methods
Age, systolic blood pressure, and measurements such as flow velocities and flow volume rates of the basilar artery in patients with PCI and control participants were compared by using the Student t test. Comparisons among the rates of risk factors and frequencies of brain stem and cerebellar infarction between two groups were made by the Fisher exact probability test.
Results
Clinical Backgrounds, Signs, Symptoms, and Risk Factors
In the PCI group, five patients displayed paroxysmal vertigo, with three showing nystagmus during the attack. The other 16 displayed dizziness, with only one showing nystagmus (Table 1). Vertigo or dizziness occurred with movement of the head, gait, or action and continued for a duration of a few seconds to a few minutes. For most patients with PCI, positional nystagmus was not shown by examination by the neurotologist. Nystagmus in these patients during the attack was horizontal look nystagmus. In addition, transient neurologic deficits were seen in five patients, hemiplegia in one, sensory disturbances in two, dysarthria in one, and truncal ataxia in one. The results of Bárány caloric and Romberg tests were negative for all patients. Audiograms revealed presbyacusia in two patients with PCI and in two control participants. The results of Schellong test were negative for all patients with PCI. Anemia was not found in any patient with PCI or control participant. Two patients showed ST-T changes on electrocardiograms, but sonographic cardiography revealed no heart dysfunction in these patients.
TABLE 1:
Clinical backgrounds, symptoms, and risk factors in posterior circulation ischemia and control groups
| PCI Group | Control Group | P | |
|---|---|---|---|
| Clinical Backgrounds | |||
| N (male vs female) | 21 (10:11) | 16 (11:5) | NS† |
| Age range, y (mean) | 41–80 (61.3) | 40–85 (61.6) | NS* |
| Body mass index,kg/m2 | 24.8 ± 4.0 | 22.7 ± 3.9 | NS* |
| SBP, mm Hg | |||
| Intermittent | 135.0 ± 18.1 | NS* | |
| Attack | 148.6 ± 19.6 | 132.3 ± 12.9 | <.001 |
| Clinical Symptoms | |||
| Paroxysmal vertigo (with nystagmus) | 5 (3) | 0 | |
| Paroxysmal dizziness (with nystagmus) | 16 (1) | 0 | |
| Hearing loss | 2 | 2 | |
| Headache | 0 | 1 | |
| Chronic neurologic deficit | 0 | 3 | |
| Transient neurologic deficit | 4 | 0 | |
| Risk Factors for Cerebral Blood Flow | |||
| Hypertension | 11 | 3 | NS† |
| Diabetes Mellitus | 1 | 0 | NS† |
| Hyperlipidemia | 7 | 7 | NS† |
| Smoking | 3 | 4 | NS† |
Note.—PCB, posterior circulation ischemia; SBP, systolic blood pressure; hypertension, SBP > 140 mm Hg; diabetes mellitus, fasting blood glucose level > 200 mg/dL; hyperlipidemia, total blood cholesterol level > 200 mg/dL or neutral fat > 160 mg/dL; smoker, habitual smoking during study.
Student t test.
Fisher exact probability test.
In the control group, one participant complained of headaches. Chronic neurologic deficits were present in three, hemiplegia in two, and aphasia due to an old infarction in the region of the middle cerebral artery in one, but the others showed no neurologic signs or symptoms. Only one control participant exhibited an ST-T change on electrocardiograms, but sonographic cardiography revealed no heart dysfunction.
Hypertension was observed in 11 patients with PCI and three control participants; all were treated with antihypertensive agents. Intermittent testing revealed that the average systolic blood pressure in the PCI group was 135.0 ± 18.1 mm Hg and was 148.6 ± 19.6 mm Hg during attack, significantly higher than in the control group (P < .01). Systolic blood pressure did not show any extreme decreases during attacks of dizziness for any patient with PCI. Blood serum laboratory examination revealed diabetes mellitus in only one patient with PCI, but hyperlipidemia was observed in seven patients with PCI and seven control participants. The frequencies of the risk factors were statistically analyzed, but, as seen in Table 1, prevalence rates were not significantly different between the groups.
Neuroimaging Findings
After an attack of dizziness, no new infarctions were found by repeated MR imaging in the PCI group. Three patients the PCI group possessed old infarctions in the region of the middle cerebral artery. Six patients displayed old pontine infarctions but not in the cerebellum or the vestibular nuclei; these were unlikely to have caused the dizziness. Flow voids of the unilateral vertebral artery were absent in two patients. Three-dimensional time-of-flight MR angiography showed narrowings of the unilateral vertebral artery in six patients, absences of the vertebral artery in two, narrowings of the basilar artery in two, and stenoses of the middle cerebral artery in eight. Hypoperfusion of the brain stem or cerebellum was evident in two patients by single photon emission CT. Hypoperfusion in the area supplied by the middle cerebral artery was observed in eight patients.
In the control group, old cerebral infarctions in the region of the middle cerebral artery were observed on the MR images of seven participants. No signs of brain stem or cerebellar infarction were evident in any of the control participants. Three-dimensional time-of-flight MR angiography showed stenosis of the middle cerebral artery in four participants but no abnormality of the vertebral or basilar artery. By single photon emission CT, an area of hypoperfusion was observed in the region of the middle cerebral artery in four participants. However, there was no evidence of abnormal perfusion in either the brain stem or cerebellum. Overall, significantly higher frequencies of brain stem or cerebellar infarction were observed more in the PCI group than in the control group (P < .01).
Flow Velocity and Flow Volume Rate in the Basilar Artery
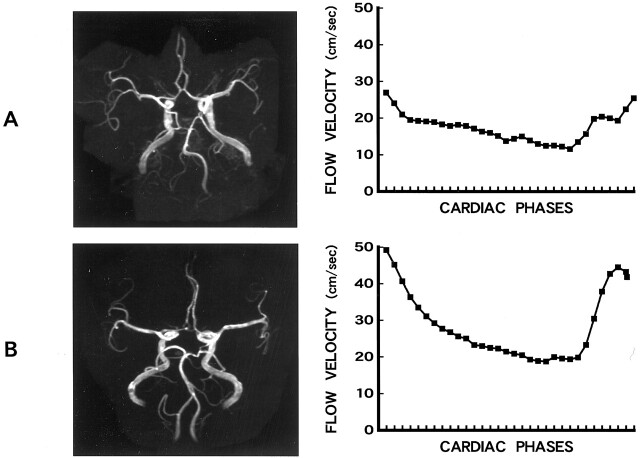
As illustrated in Figure 2, the 3D time-of-flight MR angiograms showed that the right vertebral artery and the basilar artery were narrow in patients with PCI (Fig 2A), the maximums of the average flow velocity of the basilar artery in patients with PCI were considerably lower than those of normal control participants (Fig 2B), and changes of blood flow velocities between systolic and diastolic phases were smaller in patients with PCI than in normal control participants.
Fig 2.
3D time-of-flight MR angiograms and blood flow velocity data plots.
A, 3D time-of-flight MR angiogram (33/4; flip angle, 20 degrees) of a 49-year-old male patient with PCI and dizziness shows narrowing of the right vertebral and basilar arteries. Change of blood flow velocity between systolic and diastolic phases was greater in the patient than in the normal control volunteer (see B).
B, 3D time-of-flight MR angiogram (33/4; flip angle, 20 degrees) of a 54-year-old female control participant with no clinical signs or symptoms shows mild meandering of the basilar artery.
As shown in Table 2, the maximum and mean average flow velocities of the basilar arteries in the PCI group (29.2 ± 9.2 cm/s and 18.0 ± 5.6 cm/s, respectively) were significantly lower than those of the control group (38.5 ± 8.2 cm/s and 22.6 ± 5.0 cm/s, respectively) (P < .005 and P < .01, respectively). Similarly, the flow volume rates of the basilar artery in the PCI group (103.3 ± 37.3 mL/min) were significantly lower than those of the control group (148.8 ± 40.0 mL/min) (P < .001). However, the minimum average flow velocity of the basilar artery in the PCI group (10.0 ± 4.0 cm/s) was not significantly different from that of the control group (11.3 ± 5.0 cm/s). It has been suggested that the reduction of blood flow in the basilar arteries of patients with PCI is due to stenosis or occlusion of the vertebral or subclavian arteries or of the brachiocephalic trunk. However, this has not yet been proved.
TABLE 2:
Average flow velocity and flow volume rate of the basilar artery in posterior circulation ischemia and control groups
| PCI Group | Control Group | P* | |
|---|---|---|---|
| Range of interest, cm2 | 0.10 ± 0.03 | 0.11 ± 0.03 | NS |
| Maximum average velocity, cm/s | 29.2 ± 9.2 | 38.5 ± 8.2 | <.005 |
| Minimum average velocity, cm/s | 10.0 ± 4.0 | 11.3 ± 5.0 | NS |
| Mean average velocity, cm/s | 18.0 ± 5.6 | 22.6 ± 5.0 | <.01 |
| Flow volume rate, mL/min | 103.3 ± 37.3 | 148.8 ± 40.0 | <.001 |
Note.—PCI indicates posterior cerebral ischemia.
Student t test.
Discussion
The cine phase MR imaging technique has been previously described by Dumoulin and Hart (17) and, as shown in both patients and phantoms, has the accuracy to measure flow velocity of targeted vessels based on signal intensity (18–22). In addition, this technique enables measurement of blood flow velocity in real time by synchronizing heart rate (12–15, 21–23). A number of alternative methods have been used to assess cerebral blood flow, including xenon-enhanced CT, positron emission tomography, and single photon emission CT, but these methods are often limited and incapable of determining blood flow velocity of targeted vessels (24–26). In contrast, the Doppler sonography system can measure blood flow velocity in the cervical or cerebral artery in real time (27–29) but possesses limitations and inaccuracies for the measurement of blood flow volume rates, because errors regarding the diameters of the targeted vessels are produced (30).
In this study, we measured blood flow in the basilar artery of the midpons, because it was easy to determine the imaging plane perpendicular to the basilar artery. In the control group, we obtained 22.6 ± 5.0 cm/s and 148.8 ± 40.0 mL/min as the mean flow velocity and flow volume rates, respectively, of the basilar artery. In normal control participants, flow velocity and flow volume rates of the basilar artery have been reported. Using a similar approach, Davis (16) reported a peak flow velocity of 54.8 ± 14.0 cm/s and a flow volume rate of 167.1 ± 25.2 mL/min. In addition, studies by Tominaga et al (15) reported a mean average flow velocity and flow volume rate of 30.4 ± 9.0 cm/s and 129.3 ± 56.2 mL/min, respectively. Furthermore, by using transcranial Doppler sonography, Dewitt and Wechsler (27) observed a mean flow velocity of 42 ± 10 cm/s. Direct comparisons of these results with ours are difficult, because measuring methods differed for flow velocity. As for blood flow in a blood vessel, it becomes parabolic and is fastest at the center (31). Davis (16) and Dewitt and Wechsler (27) measured the fastest velocity in the basilar artery, whereas our study and that presented by Tominaga et al (15) measured the average velocity and so obtained a lesser value. The signal intensity decreased as the region of interest at the basilar artery was determined more widely to include the peripheral signal intensity void area; the mean flow velocity was thus correspondingly reduced. These aberrations to real flow velocity may vary considerably among investigations. In contrast to the flow velocity, the flow volume rate could be obtained accurately independent of the methodologies because aberrations of flow velocities and regions of interest were canceled out in calculations of flow volume rates (14). Considering the above, the flow volume rate should be a more useful measure for comparison between the PCI and control groups.
In the PCI group, the flow volume rate we obtained from the basilar artery was 103.3 ± 37.3 mL/min, which was markedly lower than that of the control group. In support of these data, studies by Tominaga et al (15) found that the flow volume rate of the basilar artery reduced significantly to 91.9 ± 46.0 mL/min in patients with PCI. However, because they did not use cine mode, the quantitative measurement of the blood flow volume rate would have been inadequate and the changes of blood flow velocities between systolic and diastolic phases were not obtained. It would have been helpful for diagnoses of PCI to know that these changes of velocities were smaller in the patients than in the normal control participants.
In this study, we showed that the blood flow volume rate of the basilar artery in patients with PCI reduced markedly during the intermittent stages of an ischemic attack such as dizziness. In this context, we propose that the clinical signs and symptoms of PCI are caused by ischemia of the vestibular nuclei in the brain stem, where the blood flow volume in vertebrobasilar circulation has been chronically reduced and then further reduced by inducements including standing, rotation of the head, fluctuation of blood pressure, microemboli, etc. (29). The precise mechanism underlying isolated dizziness in association with PCI has yet to be clarified; however, it is thought to be due to particular vulnerabilities to ischemia (28, 29) in areas supplied by branches of the anterior-inferior cerebellar or the posterior-inferior cerebellar arteries that belong to vertebrobasilar circulation (28). These vessels are so anomalous that determination of the supplying artery in this area was difficult and quantitative measurement of blood flow was unfeasible. Considering this, measurement of the flow volume rate in the basilar artery would allow one to quantitatively assess PCI. It has been suggested herein that the reduction of blood flow in the basilar artery is due to stenosis or occlusion of the vertebral artery or stenosis of the basilar artery itself. On one hand, it has been thought that the reduction of blood flow in the basilar artery is due to an inborn, abundant flow from the posterior communicating artery to the posterior cerebral artery. Hence, PCI cannot be diagnosed from only a reduction of it. However, perhaps in some patients with PCI, dizziness would be developed by ischemia to the vestibular nucleus after a reduction of the basilar artery. It is often difficult to distinguish a diagnosis of PCI from internal ear disturbances such as benign paroxysmal positional vertigo if only dizziness is displayed, particularly in elderly patients with a risk factor of cerebrovascular disease, because dizziness with PCI includes elements of peripheral vertigo due to ischemia of the internal ear. The contrast-enhanced 2D cine phase MR angiographic technique along with MR imaging can quantitatively evaluate the basilar artery and may assist in the diagnosis of PCI. It should be noted, however, that the clinical value of blood flow in the basilar artery as it relates to diagnosis of PCI might vary among individual patients. Thus, for a more accurate diagnosis, it might be necessary to measure blood flow in patients with peripheral vertigo with many variations of the posterior circulation system at the time of a PCI attack and at some locations of the basilar and vertebral arteries.
Conclusion
Although it is relatively easy to diagnose PCI in patients who exhibit characteristic neurologic signs and symptoms, it is often difficult to distinguish PCI from internal ear disturbances such as benign paroxysmal positional vertigo if patients display only dizziness. It has been reported that isolated dizziness as a symptom of PCI occurs more frequently than previously thought (5–8). In this study, by using a cine phase MR angiographic technique, we showed that the blood flow volume rates of the basilar artery in patients with PCI, clinically diagnosed by MR imaging and neurotologic examinations, reduce markedly during the intermittent stages of an ischemic attack with dizziness. On the basis of our results, we conclude that reduction of blood flow in the basilar artery influences development of dizziness in association with PCI. This technique is a relatively easy, noninvasive, time-sparing approach to distinguish PCI from pure peripheral vertigo, such as benign paroxysmal positional vertigo.
Acknowledgments
The authors thank Dr. Michiko Sakai and radiologic technologists Tatsuji Sato, Yasunobu Miyake, Koji Nishiwaki, and Junji Okuda for practical assistance.
Footnotes
Supported by the Department of Radiology and Otology, Kasugai Municipal Hospital, Kasugai, Japan.
References
- 1.Kubik CS, Adams RD. Occlusion of the basilar artery: a clinical and pathological study. Brain 1946;69:73–121 [DOI] [PubMed] [Google Scholar]
- 2.Caplan L. Posterior circulation ischemia: then, now, and tomorrow: The Tomas Willis Lecture—2000. Stroke 2000;31:2011–2023 [DOI] [PubMed] [Google Scholar]
- 3.Williams D, Wilson TG. The diagnosis of the major and minor syndromes of basilar insufficiency. Brain 1962;85:741–774 [DOI] [PubMed] [Google Scholar]
- 4.Corvera J, Benitez LD, Lopez-Rios G, Rabiela MT. Vestibular and oculomotor abnormalities in vertebrobasilar insufficiency. Ann Otol Rhinol Laryngol 1980;89:370–376 [DOI] [PubMed] [Google Scholar]
- 5.Troost BT. Dizziness and vertigo in vertebrobasilar disease: part II. central causes and vertebrobasilar disease. Stroke 1980;11:413–415 [DOI] [PubMed] [Google Scholar]
- 6.Ausman JI, Shrontz CE, Pearce JE, Diaz FG, Crecelius JL. Vertebrobasilar insufficiency: a review. Arch Neurol 1985;42:803–808 [DOI] [PubMed] [Google Scholar]
- 7.Gomez CR, Cruz-Flores S, Malkoff MD, Sauer CM, Burch CM. Isolated vertigo as a manifestation of vertebrobasilar ischemia. Neurology 1996;47:94–97 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe M, Takahashi A, Arahata Y, Motegi Y, Inafuku S. MRI findings in patients with vertigo and dizziness possibly arising from vertebrobasilar insufficiency. Clin Neurol 1994;34:32–37 [PubMed] [Google Scholar]
- 9.Day JJ, Freer CE, Dixon AK, et al. Magnetic resonance imaging of the brain and brain-stem in elderly patients with dizziness. Age Aging 1990;19:144–150 [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi S, Kaga K, Yamasoba T, Higo R, O’Uchi T, Tokumaru A. Slow blood flow of the vertebrobasilar system in patients with dizziness and vertigo. Acta Otolaryngol 1993;113:257–260 [DOI] [PubMed] [Google Scholar]
- 11.The National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular disease III. Stroke 1990;21:637–676 [DOI] [PubMed] [Google Scholar]
- 12.Bendel P, Buonocore E, Bockisch A, Besozzi MC. Blood flow in the carotid arteries: quantification by using phase-sensitive MR imaging. AJR Am J Roentgenol 1989;152:1307–1310 [DOI] [PubMed] [Google Scholar]
- 13.Marks MP, Pelc NJ, Ross MR, Enzmann DR. Determination of cerebral blood flow with a phase-contrast cine MR imaging technique: evaluation of normal subjects and patients with arteriovenous malformations. Radiology 1992;182:467–476 [DOI] [PubMed] [Google Scholar]
- 14.Kashimada A, Machida K, Honda N, et al. Measurement of cerebral blood flow in normal subjects by phase contrast MR imaging [in Japanese]. Nippon Igaku Hoshasen Gakkai Zasshi 1994;54:1116–1125 [PubMed] [Google Scholar]
- 15.Tominaga S, Seo T, Ishikura R, Tabuchi Y, Nakao N. Quantative flow measurement of the vertebro-basilar circulation for positional vertigo by using 2D phase contrast technique [in Japanese]. Nippon Igaku Hoshasen Gakkai Zasshi 1996;56:257–263 [PubMed] [Google Scholar]
- 16.Davis WL. Flow analysis applications in carotid and vertebral disease. Signa Applications Guide 1994;6:35–42 [Google Scholar]
- 17.Dumoulin CL, Hart HL. Magnetic resonance angiography. Radiology 1986;161:717–720 [DOI] [PubMed] [Google Scholar]
- 18.Firmin DN, Nayler GL, Kilner PJ, Longmore DB. The application of phase shifts in NMR for flow measurement. Magn Reson Med 1990;14:230–241 [DOI] [PubMed] [Google Scholar]
- 19.Caputo GR, Kondo C, Masui T, et al. Right and left lung perfusion: in vitro and in vivo validation with oblique-angle, velocity-encoded cine MR imaging. Radiology 1991;180:693–698 [DOI] [PubMed] [Google Scholar]
- 20.Pelc LR, Pelc NJ, Rayhill SC, et al. Arterial and venous blood flow: noninvasive quantitation with MR imaging. Radiology 1992;185:809–812 [DOI] [PubMed] [Google Scholar]
- 21.Nayler GL, Firmin DN, Longmore DB. Blood flow imaging by cine magnetic resonance. J Comput Assist Tomogr 1986;10:715–722 [DOI] [PubMed] [Google Scholar]
- 22.Van Rossum AC, Sprenger M, Visser FC, Peels KH, Valk J, Roos JP. An in vivo validation of quantitative blood flow imaging in arteries and veins using magnetic resonance phase-shift techniques. Eur Heart J 1991;12:117–126 [DOI] [PubMed] [Google Scholar]
- 23.Mohiaddin RH, Wann SL, Underwood R, Firmin DN, Rees S, Longmore DB. Vena caval flow: assessment with cine MR velocity mapping. Radiology 1990;177:537–541 [DOI] [PubMed] [Google Scholar]
- 24.Shirahata N, Henriksen L, Vorstrup S, et al. Regional cerebral blood flow assessed by 133Xe inhalation and emission tomography: normal values. J Comput Assist Tomogr 1985;9:861–866 [DOI] [PubMed] [Google Scholar]
- 25.Meyer JS, Hayman LA, Amano T, et al. Mapping local blood flow of human brain by CT scanning during stable xenon inhalation. Stroke 1981;12:426–437 [DOI] [PubMed] [Google Scholar]
- 26.Burt RW, Witt RM, Cikrit DF, Reddy RV. Carotid artery disease: evaluation with acetazolamide-enhanced Tc-99m HMPAO SPECT. Radiology 1992;182:461–466 [DOI] [PubMed] [Google Scholar]
- 27.Dewitt LD, Wechsler LR. Transcranial Doppler. Stroke 1988;19:915–921 [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga T. Correlation between vertigo and abnormal hemodynamics of the vertebral artery. Jibirinsho 1992;85:1531–1541 [Google Scholar]
- 29.Grad A, Baloh RW. Vertigo of vascular origin: clinical and electronystagmographic features in 84 cases. Arch Neurol 1989;46:281–284 [DOI] [PubMed] [Google Scholar]
- 30.Taylor KJ, Holland S. Doppler US: part I. basic principles, instrumentation and pitfalls. Radiology 1990;174:297–307 [DOI] [PubMed] [Google Scholar]
- 31.Goldsmith HL, Turitto VT. Rheological aspects of thrombosis and haemostasis: basic principles and applications. Thromb Haemost 1986;55:415–435 [PubMed] [Google Scholar]