Abstract
BACKGROUND AND PURPOSE: Risk of developing ischemia is higher in patients with reduced cerebrovascular reactivity than in those with preserved cerebrovascular reactivity. Therefore, we assessed cerebral hemodynamic modifications in patients with unilateral stenosis of the internal carotid artery by using perfusion-weighted MR imaging to determine if these modifications underlie or anticipate ischemic signs and symptoms.
METHODS: Fifteen patients with unilateral 70–90% carotid artery stenosis were studied with digital subtraction angiography and perfusion-weighted MR imaging. Their findings were compared with those of 15 age- and sex-matched control subjects. Regional cerebral blood volume (rCBV) and mean transit time (MTT) values were calculated in the middle cerebral artery and border zone territories.
RESULTS: No significant difference was noted in rCBV and MTT values between the hemispheres in the symptomatic patients. There was a significant difference in MTT values in the border zones between patients and control subjects. MR images in patients and control subjects did not reveal large territorial infarcts and did reveal similar white matter lesion burdens.
CONCLUSION: There is adequate compensation of unilateral stenosis when the stenosis is less than 90%. The risk of stroke is higher in patients with stenoses exceeding 70%, mostly because of decreased collateral reserve when confronted with emboli.
Internal carotid artery (ICA) stenosis is among the most frequent causes of cerebrovascular ischemia (1, 2). The mechanisms of ischemia are essentially two: hemodynamic, for cerebral hypoperfusion, and embolic, due to distal mobilization of piastrinic or atheromasic material, almost always secondary to plaque ulceration (3).
When perfusion pressure decreases, functional compensation mechanisms activate cerebral autoregulation, avoiding, within certain limits, the manifestation of ischemic lesions. When collateral circulation is insufficient, and thus cerebral vascular reserve capacity is inadequate, the risk for stroke increases, particularly because microemboli have a higher possibility of becoming symptomatic in the hypoperfused areas (4).
In this study of symptomatic patients with 70–90% unilateral stenosis of the ICA, we measured regional cerebral blood volume (rCBV) and mean transit time (MTT) within the middle cerebral artery (MCA) and border zone vascular territories. The purpose was to determine if identifiable hemodynamic disturbances due to the ICA stenosis underlie or anticipate ischemic signs and symptoms.
Methods
We evaluated 15 symptomatic patients (nine men and six women) aged 55–71 years (mean, 63 years), with unilateral stenosis (> 70% by digital subtraction angiography, according to the North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria) of the ICA. All the enrolled patients underwent neurologic and cardiologic examination, electrocardiography, Doppler sonography, and MR angiography of the epiaortic vessels. Seven patients sustained a hemispheric transient ischemic attack, eight an ocular transient ischemic attack; all of them were treated with antiplatelet therapy (usually with low-dose aspirin). In all cases, symptoms were referable to the side of the stenosed ICA. The degree of stenosis ranged from 70% to 90% (Table 1). The contralateral ICA either was normal or showed a stenosis lower than 40%. Fifteen subjects, matched for age and sex, who were proved not to have stenotic lesions by sonographic carotid examination and no signal intensity alterations with MR imaging of the brain, were studied as control subjects. The study protocol was approved by our institutional review board, and informed consent was obtained from patients or guardians in all cases.
TABLE 1:
rCBV and MTT Values, Normalized to Cerebellum, in the Hemispheres Supplied by Normal and Stenotic ICAs in the MCA and Border Zone Territories
| Degree of Stenosis by NASCET (%) | Age (y)/Sex | rCBV MCA |
rCBV Border Zone |
MTT MCA |
MTT Border Zone |
||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Side | Stenotic Side | Normal Side | Stenotic Side | Normal Side | Stenotic Side | Normal Side | Stenotic Side | ||
| 70 | 62/M | 0,77 | 0,81 | 0,71 | 0,68 | 1,29 | 1,28 | 1,26 | 1,28 |
| 75 | 78/M | 1,05 | 1,11 | 0,78 | 0,74 | 1,29 | 1,36 | 1,34 | 1,35 |
| 80 | 56/F | 0,87 | 0,75 | 0,92 | 0,92 | 0,72 | 0,68 | 0,97 | 0,89 |
| 80 | 62/F | 0,66 | 0,76 | 0,75 | 0,75 | 0,93 | 0,91 | 1,45 | 1,41 |
| 80 | 79/F | 1,15 | 1,17 | 0,68 | 0,72 | 1,12 | 1,17 | 1,19 | 1,17 |
| 70 | 74/M | 0,96 | 0,91 | 0,6 | 0,77 | 0,72 | 0,68 | 0,76 | 0,76 |
| 75 | 71/F | 1,15 | 1,17 | 0,96 | 1,02 | 1,22 | 1,12 | 1,35 | 1,43 |
| 75 | 59/F | 0,77 | 0,82 | 0,56 | 0,69 | 1,16 | 1,1 | 1,12 | 1,26 |
| 85 | 65/M | 1,13 | 0,97 | 0,88 | 0,87 | 0,72 | 0,6 | 0,71 | 0,66 |
| 90 | 67/M | 0,92 | 1,01 | 0,74 | 0,74 | 1,04 | 1,47 | 1,04 | 0,96 |
| 80 | 73/M | 0,79 | 0,82 | 0,79 | 0,73 | 0,8 | 0,79 | 0,82 | 0,69 |
| 90 | 64/M | 0,86 | 0,98 | 0,65 | 0,77 | 1,28 | 1,3 | 1,13 | 1,31 |
| 70 | 58/F | 1,15 | 1,08 | 1,01 | 0,89 | 1,5 | 1,26 | 1,5 | 1,53 |
| 80 | 66/M | 1,12 | 1,02 | 0,97 | 0,88 | 1,3 | 1,21 | 1,12 | 1,05 |
| 85 | 73/M | 0,98 | 0,94 | 0,84 | 0,78 | 0,91 | 1,02 | 1 | 0,97 |
MR perfusion study was performed with a 1.5-T unit (Integra; Siemens Medical Systems, Best, the Netherlands) with 23-mT gradient intensity and a dedicated head coil. A preliminary study was carried out to exclude the presence of MCA or border zone infarcts. A T2-weighed sagittal sequence was previously acquired to highlight the bicommisural line necessary to plan the axial images for brain and perfusion studies. T1-weighted axial images (25-cm field of view; matrix, 256 × 128; 200/9 ms [TR/TE]) and axial T2-weighted turbo fluid-attenuated inversion recovery, or FLAIR, images (3800/22 ms; 10-mm section thickness; 230-mm field of view; matrix, 256 × 256) were thus acquired.
A T2*-weighted echo-planar sequence (18/26 ms; section thickness, 3.5 mm; flip angle, 8°; matrix 128 × 64; 40 dynamic series; one signal acquired; imaging time, 1 minute 22 seconds) was used to obtain dynamic susceptibility contrast images along the anteroposterior commisural plane. Nine sections with a thickness of 7 mm were acquired. A single dose of 0.2 mmol/kg gadolinium chelate (Magnevist; Schering, Berlin, Germany) was injected via an antecubital vein, by using a power injector (Spectris; Medrad, Indianola, PA), at the rate of 5 mL/s. Perfusion data were processed and converted into parameter maps for rCBV and MTT.
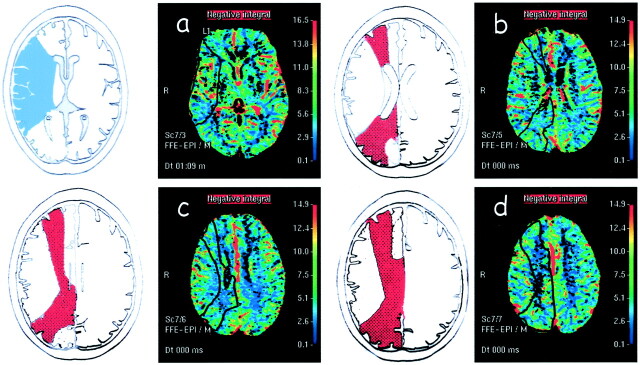
To evaluate MTT and rCBV maps, regions of interest (ROIs) were positioned, bilaterally, in the vascular territory of the distribution of the MCA and border zone (Fig 1) by using the cortical and intracerebral boundaries proposed by van der Zwan et al (5) and modified by Hupperts et al (6). Pixels with signal intensity higher than 2 SDs above the brain mean value, supposed to represent blood vessels, were excluded from all regional analyses.
Fig 1.
A–D, Left, Schematic representations of the MCA (blue in A) and border zone (red in B–D) territories. A–D, Right, Perfusion maps of rCBV show the ROIs (black outlines) positioned in MCA (A) and border zone (B–D) territories.
With use of a method proposed by Kluytmans et al (7), the obtained values were correlated with those obtained in the cerebellum, and thus the hemispheric (MCA and border zone) rCBV-to-cerebellum rCBV ratio and the hemispheric (MCA and border zone) MTT-to-cerebellum MTT ratio were considered for the statistical evaluation. This was done to allow interpatient and control patient comparison.
For each variable, rCBV and MTT, one-way analysis-of-variance tests were performed to assess differences between the stenotic and contralateral sides and between patients and control subjects. Excel 97 (Microsoft, Bellview Redmond, WA) was used for data analysis. By means of a semiautomatic segmentation intensity-based analysis, we also measured the volume of the hyperintense lesions found in the supratentorial white matter of each hemisphere. Lesions were outlined as ROIs, and lesion volume was calculated by multiplying the total ROI by the section thickness.
Results
Findings are reported in Tables 1 and 2 and Fig 2 From data analysis, no statistically significant differences were noted between the cerebral hemispheres in the MCA and border zone territories in the patients. Comparing the mean values of the two hemispheres with those of control subjects showed no statistically significant differences concerning rCBV in both the MCA and border zone territories. Considering MTT data, a statistically significant difference was noted between patients and control subjects for the values in the border zone territory (P < .05) but not for those in the MCA vascular territory. No significant differences were evident between patients and control subjects concerning the white matter lesions.
TABLE 2:
Mean rCBV and MTT Values, Normalized to Cerebellum, of Both Hemispheres in MCA and Border Zone Territories in Control Subjects and Patients
| Subjects | MCA |
Border Zone |
||
|---|---|---|---|---|
| rCBV | MTT | CBV | MTT | |
| Controls | 1,02 | 0,96 | 0,85 | 0,95* |
| Patients | ||||
| Normal side | 0,94 | 1,06 | 0,77 | 1,12* |
| Stenotic side | 0,95 | 1,05 | 0,79 | 1,13* |
A statistically significant difference was found between MTT in the border zone of patients and control subjects (P = .05).
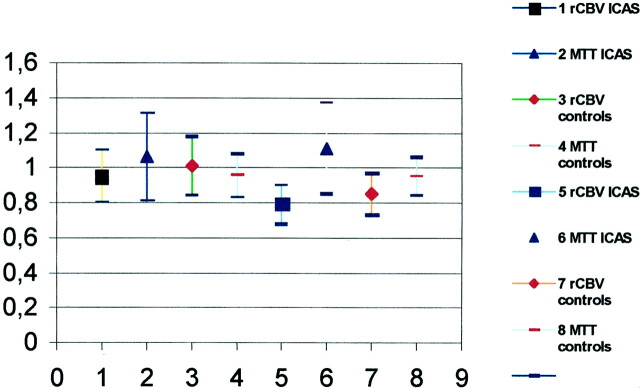
Fig 2.
Comparison of perfusion data in the MCA (symbols 1–4) and border zone (symbols 5–8) territories between the control group (3, 4, 7, 8) and patients with ICA stenosis (1, 2, 5, 6).
Discussion
Although so-called hemodynamically significant stenoses (meaning, capable of causing local hemodynamic change at the point of stenosis and considered over 70% based on NASCET criteria) are frequently encountered in clinical practice, hemodynamic stroke conversely is rather rare. Nevertheless, the risk of developing an ischemic event is higher in patients with reduced cerebrovascular reactivity than in those in whom cerebrovascular reactivity is preserved (4). For this reason, different tools have been proposed to test cerebral hemodynamics. Transcranial Doppler sonography represents a noninvasive and reliable technique that has been used in the last few years to measure, in the intracranial arteries, the flow variations in response to dilatatory stimuli (acetazolamide, CO2 inhalation, apnea) (8). Other imaging techniques to study cerebral hemodynamics are PET, SPECT, and, recently, perfusion-weighted MR imaging. Perfusion-weighted MR imaging allows the evaluation of some fundamental parameters to study cerebral hemodynamics, such as rCBV, regional cerebral blood flow, and MTT. Perfusion-weighted MR imaging can be easily performed as completion of a standard brain MR examination, with a small increase in the time and costs of the imaging technique.
We evaluated the cerebral hemodynamic modifications in patients with symptomatic unilateral carotid stenosis by using perfusion-weighted MR imaging. Our data show that MTT and rCBV have no variability between the two hemispheres in patients with 70–90% carotid stenosis. Only mild and insignificant increased rCBV was observed in the hemisphere supplied by the stenosed ICA. These results were already suggested by Kluytmans et al (7) who described that patients with a severe stenosis of the ICA are not hemodynamically impaired on that side. This finding is confirmed in our study, considering both the MCA and border zone territories. Our data partially disagree with those recently reported by Maeda et al (9). These authors indeed describe differences in MTT between the normal and the symptomatic hemisphere in distal vascular territories of cerebral arteries in patients with ICA stenosis. This evaluation was performed by positioning ROIs in areas that already visibly presented signal intensity differences in the MTT maps between the two hemispheres. Also, when analyzing the patients in their series, only two patients had an ICA stenosis lower than 90%: one had no CBV asymmetry, and the other had a CBV decrease in an area of watershed infarction. All the other patients from that series had ICA occlusion or stenosis over 90% (for this reason, this degree of stenosis was chosen as the upper limit for our patient selection). Hemodynamic asymmetries between the hemispheres in patients with symptomatic carotid occlusion were also reported by Kim et al (10), who also correlated the prolongation of MTT with the vascular reserve capacity data obtained with SPECT before and after intravenous administration of acetazolamide. Based on our results and the mentioned data from other studies, we suggest that hemodynamic changes probably occur in the cerebral hemispheres only in cases with a high degree of stenosis or occlusion of the ICA. Also, the presence of contralateral significant stenoses or occlusions may lead to hemodynamic impairment. It is likely that in the range between 70% and 90% the vascular reserve capacity of the brain enables significant hemodynamic changes.
A potential bias to this observation is that only patients without evidence of stroke were selected, which may reflect subjects with an intact circle of Willis or other means of collateral circulation. It is likely that in patients with poor collateral circulation, hemodynamic changes may occur even with a 70–90% ICA stenosis.
Because no differences were found in the hemodynamic between the hemispheres of patients, we assessed whether these parameters differ from those of the control group. For this reason, the mean values of the MTT of both hemispheres (which is the most sensitive in cases of carotid stenosis) were compared with those calculated in subjects not affected by carotid stenosis. Our data analysis (Fig 2) demonstrated a statistically significant increase (P < .05) in MTT in the border zone territories in patients with respect to that in the control subjects. Prolonged MTT in the hemispheres of patients with carotid stenosis could be related to microvascular impairment affecting small vessels on both sides. Assuming that microvascular disease may lead to signal intensity alterations in the white matter, we evaluated white matter lesions in the two groups, but no differences were found in patients and control subjects. This observation may lead to the conclusion that prolonged MTT in patients with stenosis of ICA is not related to microvascular impairments, more likely reflecting a hemodynamic compensation between the two hemispheres, especially in the border zone areas.
Conclusion
The present study documents that there are no significant differences in cerebral perfusion between the hemispheres supplied by the stenotic and the normal carotid arteries in a group of symptomatic patients with ICA stenosis greater than 70% but lower than 90%. It is likely, as demonstrated in the literature in cases with ICA occlusion, that these alterations emerge for higher values of stenosis at least in patients without stroke and thus, presumably, with an intact circle of Willis or other means of collateral circulation. A hemodynamic compensation mechanism between the two hemispheres, particularly for the distal border zone territories that tend to have a slower MTT with respect to the control subjects, seems to be demonstrated as well.
References
- 1.Wolf PA, Kannel WB, Mc Gee PC. Epidemiology of strokes in North America. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM. Stroke: pathophysiology, diagnosis and management. New York: Churchill Livingstone,1968;1:19–29 [Google Scholar]
- 2.Kurtzke JF. Epidemiology of cerebrovascular disease. In: Prevention des accidentes vasculaires cerebraux. Paris: Academie Nationale de Medicine,1983. :11–24
- 3.Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke 1999;30:593–598 [DOI] [PubMed] [Google Scholar]
- 4.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–1482 [DOI] [PubMed] [Google Scholar]
- 5.Van der Zwan A, Hillen B, Tulleken CAF, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg 1992;77:927–940 [DOI] [PubMed] [Google Scholar]
- 6.Hupperts RMM, Lodder J, Heuts-van Raak, Wilmink JT, Kessels AGH. Borderzone brain infarcts on CT taking into account the variability in vascular supply areas. Cerebrovasc Dis 1996;6:294–300 [Google Scholar]
- 7.Kluytmans M, van der Grond, Eikelboom BC, Viergever. Long term hemodynamic of carotid endarterectomy. Stroke 1998;29:1567–1572 [DOI] [PubMed] [Google Scholar]
- 8.Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of C02-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke 1988;19:963–969 [DOI] [PubMed] [Google Scholar]
- 9.Maeda M, Yuh WTC, Ueda T, et al. Severe occlusive carotid artery disease: hemodynamic assessment by MR perfusion imaging in symptomatic patients. AJNR Am J Neuroradiol 1999;20:43–51 [PubMed] [Google Scholar]
- 10.Kim JH, Lee SJ, Shin T, et al. Correlative assessment of hemodynamic parameters obtained with T2*-weighted perfusion MR imaging and SPECT in symptomatic carotid artery occlusion AJNR Am J Neuroradiol 2000;21:1450–1456 [PMC free article] [PubMed] [Google Scholar]