Abstract
BACKGROUND AND PURPOSE: Some patients who undergo surgical resection of portions of the supplementary motor area (SMA) have severe postoperative motor and language deficits, whereas others have no deficits. We tested the hypothesis that in some patients with lesions affecting the SMA, the contralateral SMA exhibits some of the activation normally associated with the ipsilateral SMA.
METHODS: Functional MR imaging studies in seven healthy volunteers and 19 patients with frontal lobe tumors or arteriovenous malformations were reviewed retrospectively. The hemisphere in which the SMA activation predominated was tabulated for right and left motor tasks. The relative hemispheric dominance in the SMA for the right and left motor tasks was compared in the healthy and patient groups and with the location of the lesion in the patient group.
RESULTS: None of the control subjects performing a right hand motor task activated predominantly the right SMA. Fifty percent of the patients with lesions overlapping the left SMA performing the right motor task activated predominantly the right SMA. Fifty-seven percent of control subjects performing the left hand motor task activated the left SMA predominantly. One hundred percent of patients with lesions overlapping the right frontal SMA performing the left motor task activated the left SMA predominantly. Differences between patients and controls were statistically significant.
CONCLUSION: A lesion that contacts or overlaps the SMA is associated with an increased functional MR imaging response within the contralateral SMA.
The supplementary motor area (SMA) is thought to play a key role in initiation and control of motor and speech functions (1, 2) Injury to the SMA during surgical excision of medial frontal lobe lesions may also result in severe motor or speech deficits (3–5). These deficits are transient and most patients fully recover from SMA injuries. The closer the lesion to the SMA, the greater is the risk of postoperative deficit from surgical resection (6). Some patients in whom the SMA is surgically resected do not demonstrate postoperative deficits. One possible explanation is that when tumors or vascular malformations affect the SMA, the contralateral SMA assumes some of its normal function.
We reviewed functional MR imaging studies in patients with frontal lobe tumors and arteriovenous malformations (AVMs) to test the hypothesis that in some patients with SMA lesions, the contralateral SMA has some of the function normally associated with the ipsilateral SMA.
Methods
Study Subjects
Radiology logs were reviewed to identify patients at our institution who had a frontal lobe tumor or AVM and underwent preoperative functional MR imaging between August 1999 and November 2001. All patients (n = 19) who performed a complex motor task were included in the study. The 19 patients (10 male, nine female patients; age range, 15–61 years; mean age, 36 years) were grouped according to the location of their lesions: left frontal lobe or right frontal lobe. Medical records were reviewed to determine the type and severity of preoperative neurologic deficits and the histology of the lesion. For a control group, seven healthy volunteers (four men, three women; age range, 20–35 years; mean age, 24.7 years) with no previous history of neurologic or psychiatric illness were recruited by invitation from the investigators. These volunteers gave informed consent for functional MR imaging according to institutional review board guidelines, underwent functional MR imaging with the same techniques as that of the patients, and received a small stipend for participating.
Functional MR Imaging Protocol
Patients and control subjects were imaged with a clinical 1.5-T magnet equipped with high-speed gradients for echo planar imaging (EPI). The participant’s head was positioned within a standard radio-frequency quadrature birdcage coil. Pneumatic earphones were used. Anatomic sequences including fast spin echo, T1- and T2-weighted, and T1–weighted 3D spoiled gradient-recalled acquisition in the steady state (SPGR) (124 axial sections, 1.2 mm thick, 21/7 [TR/TE]) were performed for preoperative assessment. During the performance of tasks, EPI was performed in the coronal plane, with 20 section locations (6-mm-thick sections and 1-mm skip) to acquire nearly whole-brain coverage. EPI parameters were 2000/40 ms, field of view of 24 cm, 64 × 64 matrix, flip angle of 85°, coronal sections.
Self-paced, sequential tapping of the thumb against each finger was used for the motor task. Subjects were signaled to start and stop motor tasks by auditory cues and were observed for correct performance of the motor task. The motor task consisted of four consecutive sequences of rest, right hand movement, and then left hand movement.
Postprocessing included spatial and temporal smoothing of the EPI data. A template volume image from each EPI functional MR imaging session was individually coregistered with the same subject’s 3D SPGR anatomic dataset by using linear rigid-body realignment in six axes (7). Identical rigid-body realignment parameters were then applied to the functional MR data from each imaging session, to minimize coregistration errors introduced by either patient motion or EPI distortion. Activation was identified by cross-correlation of the time course in each voxel with a generalized least squares fitting algorithm to a smoothed boxcar reference function modeling the presumed hemodynamic response for the task performance (8). This comparison provided a t statistic. Images of those voxels with a P value less than .005 (uncorrected) were overlaid on the coregistered anatomic brain volume maps.
Imaging results were reviewed retrospectively by two investigators (V.M.H., J.S.) and scored by consensus. Each image was inspected to identify activation in the superior frontal gyrus likely to represent SMA. The following boundaries were used to define presumed SMA location: rostrally, a line 20 mm anterior to the anterior commissure and perpendicular to the anterior commissure-posterior commissure (AC-PC) line; caudally, a line perpendicular to the AC-PC line intersecting the posterior commissure; laterally, the superior frontal sulcus; and inferiorly, the callosomarginal sulcus. Assignment of activation to the left or right hemisphere was made by reference to the interhemispheric fissure.
Activation in the region of the SMA was classified as strongly right dominant (exclusively right SMA activation), weakly right dominant (at least twice as much activation in the right SMA as in the left), mixed hemispheric dominance (the difference between the SMA activation in the hemispheres was less than 100%), weakly left dominant (at least twice as much activation in the left as in the right SMA), or strongly left dominant (exclusively left SMA activation). Activation was classified separately for right and left motor tasks.
Proximity of the tumor or AVM to the SMA was classified as “overlapping” if portions of the tumor or AVM coincided with the region of functional MR imaging activation (Fig 1), as “contiguous” if the activation was adjacent to the border of the lesion (Fig 2), or as “noncontiguous” if normal brain was present between the activation and the lesion (Fig 3).
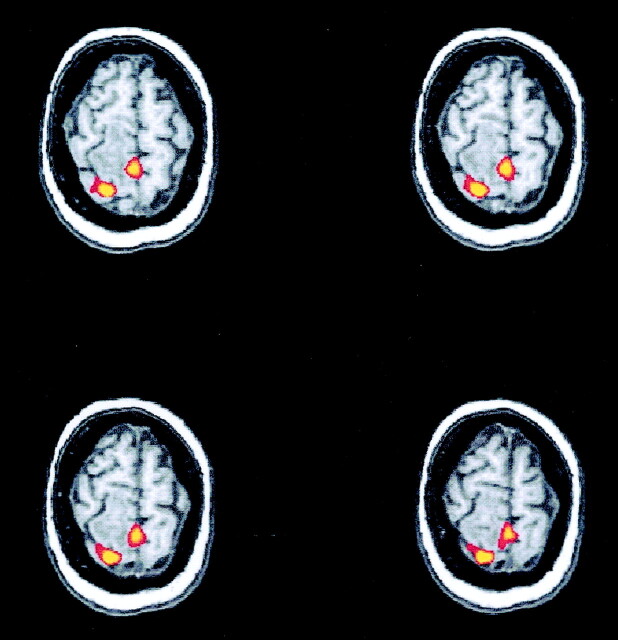
Fig 1.

Patient with left arm weakness and a right frontal lobe AVM overlapping the SMA region. Axial functional MR image illustrates activation of the left SMA (arrow) and right sensorimotor cortex during a left finger-tapping task.
Fig 2.
23-year-old female patient with a right frontal lobe tumor contiguous with the SMA region, who showed preoperative left upper extremity numbness, left pronator drift, and gait disturbance. Axial functional MR images illustrate bilateral SMA activation during a left finger-tapping task.
Fig 3.
35-year-old male patient with a right frontal lobe tumor noncontiguous with the SMA region and no preoperative neurologic deficit. Axial functional MR image illustrates bilateral SMA activation during a left finger-tapping task
Means of the scores for the control group, the group with left frontal lobe lesions, and the group with right frontal lobe lesions were calculated and compared statistically by using the F-test from analysis of variance (ANOVA). Pairwise t tests were performed to compare differences among patients with a right lesion, those with a left lesion, and control subjects. Fisher’s exact test was used to determine the significance of activation patterns occurring exclusively in one of the patient groups.
Results
Seven patients with frontal lobe AVMs and 12 patients with frontal lobe tumors underwent preoperative functional MR imaging within the study period. In two patients, SMA activation was not identified at the thresholds chosen. The AVMs were graded as Spetzler-Martin grade 4 or less, and all were smaller than 3.3 cm. All AVMs lacked evidence of steal phenomenon during cerebral angiography. The pathologic diagnoses in the 12 patients with tumor were meningioma (one patient), mixed glial neoplasm grade IV (one patient), astrocytomas grade II (one patient), astrocytoma grade III (one patient) astrocytoma grade IV (two patients), oligodendroglioma grade II (five patients), and oligodendroglioma grade III (one patient). The preoperative clinical deficits included motor deficits in four patients and speech deficit in one. All patients and healthy control subjects were right handed.
For each of the seven control subjects performing the right hand motor task, functional MR imaging activation predominated in the left hemisphere (strong or weak left hemisphere dominance) in 100% (Fig 4). For the control subjects performing the left hand motor task, the SMA activation predominated in the right hemisphere in 43% (strongly or weakly) and in the left hemisphere in 57% (weakly).
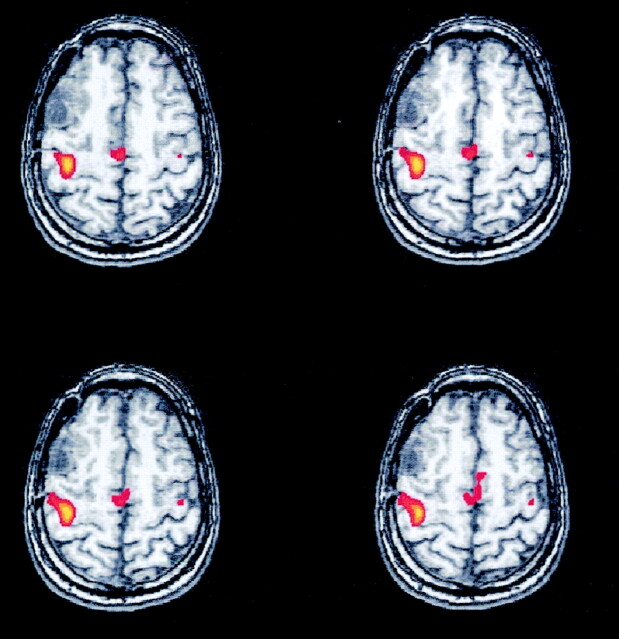
Fig 4.
A and B, Axial functional MR images show activation in sensorimotor cortex and SMA for left finger (A) and right finger (B) motor tasks in a healthy volunteer. All control subjects demonstrated left SMA dominance for the right finger task; 43% of the control subjects showed right SMA dominance for the left finger task.
Of the 10 patients with left frontal lobe tumors and AVMs, four had lesions overlapping the SMA, one had a lesion that was contiguous, and five had lesions that were noncontiguous with the SMA. Of the nine patients with right frontal lobe tumors and AVMs, three had lesions overlapping the SMA activation, one patient had a lesion that was contiguous, and five patients had lesions that were noncontiguous with the SMA.
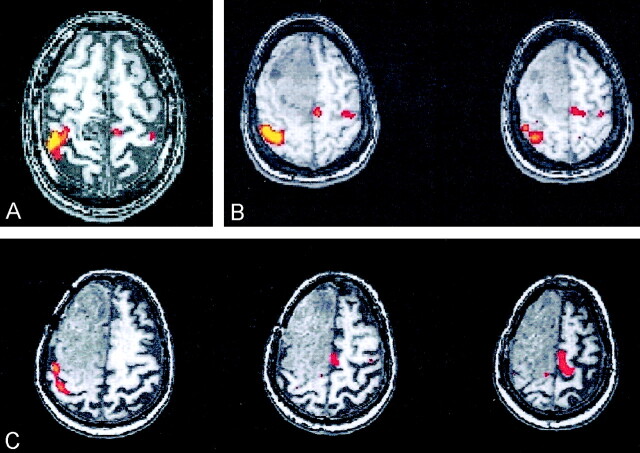
In two (50%) of the four patients with lesions overlapping the left frontal SMA, functional MR imaging activation for the right hand motor task predominated in the right SMA (Fig 5). In all three patients (100%) with right frontal lesions overlapping the SMA, activation for the left hand motor task predominated in the left SMA (Fig 6).
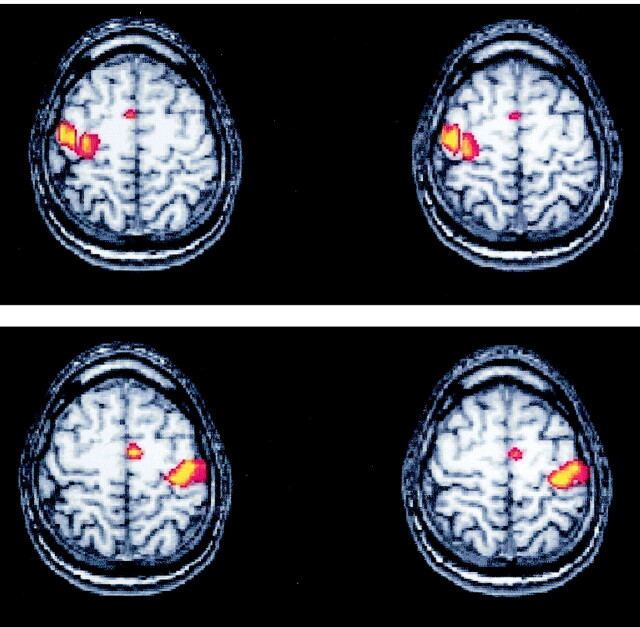
Fig 5.
Axial functional MR images show activation from right motor tasks in the two patients with left frontal masses overlapping the SMA in whom reorganization of SMA was detected.
A, Activation in the SMA (red arrows) is contralateral to the sensorimotor cortex activation (black arrows) in a patient with a large meningioma.
B, In a patient with a grade II oligodendroglioma, activation secondary to the right finger task is noted in the right SMA (red arrow) and in the left sensorimotor cortex (black arrow)
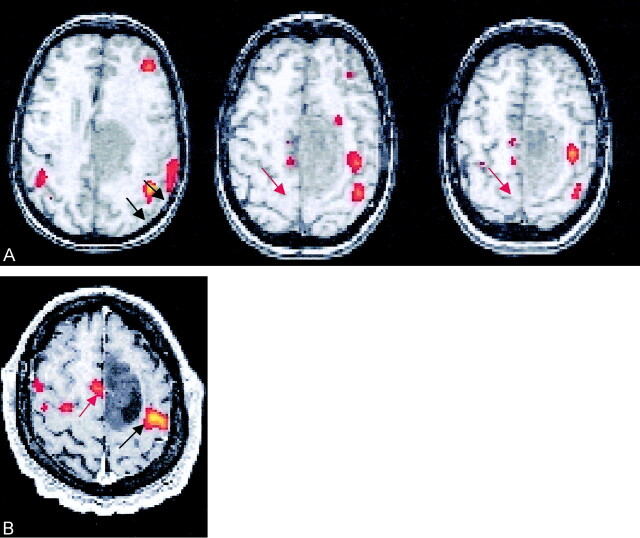
Fig 6.
A–C, Axial functional MR images show activation from left motor tasks in three patients with right frontal AVM (A) or tumor (B and C) contiguous with the SMA. Activation in the SMA was identified in the left hemisphere in each case.
Differences between patient and control groups for the motor tasks were in general significant. For the right hand motor task, two of 10 patients with left frontal lobe tumors, zero of seven patients with right frontal lesions, and zero of seven control subjects had right SMA dominance. For the right hand motor task, the F-test from ANOVA applied to the three groups had a P value of .036, indicating significant differences among mean scores of the three groups. Comparison of the 22 individuals (patients and controls) out a lesion overlapping the left SMA to those with a lesion overlapping the left SMA was significant. None of the 22 had right SMA predominance, but two of the four patients with overlapping tumors did. The result had a P value of .0185 (Fisher exact test).
For the left hand motor task, three of nine patients with right frontal lobe lesions compared with zero of seven with left frontal lobe lesions and three of seven control subjects had left hemispheric dominance (Fig 4B). The F-test from ANOVA gave a P value of .08, suggesting a possible difference among the three group means. Of the 23 individuals (patients and controls) without a lesion overlapping the right SMA, four had left SMA predominance; and of the three patients with a lesion overlapping the right SMA, three had left SMA predominance. The result was significant at the .0135 level (Fisher’s exact test).
We also compared SMA hemispheric dominance in patients with high-grade tumors versus patients with either low-grade tumors or AVMs without shunt surgery. For patients with left frontal lobe lesions performing right motor tasks, one of the two with high-grade tumors had right SMA dominance, and two of the eight with low-grade tumors or AVMs had right SMA dominance. This was not statistically significant by using Fisher’s exact test (P = .99). For patients with a right frontal lobe lesion, two of three with high-grade tumors had a laterality switch (left SMA dominance during performance of the left hand motor task) versus one of six for the low-grade tumors. Again, the result was not significant by using Fisher’s test (P = .23)
Discussion
In healthy subjects, the SMA activation predominated in the left (dominant) hemisphere more commonly than in the right. It predominated in the left hemisphere in all subjects performing the right hand motor task and in 57% of subjects during the left hand motor task. This study showed that when a lesion overlapped the SMA, laterality tended to shift to the contralateral hemisphere. Frontal lobe lesions that did not overlap with the SMA did not appear to affect the relative hemispheric predominance of functional MR imaging activation. These results imply that in patients with lesions affecting the SMA in one hemisphere, the SMA in the opposite hemisphere is recruited to perform motor tasks.
The most important limitation in the study is the possible bias because of a local effect of cerebral tumors and AVMs on the hemodynamic linkage between blood flow and neuronal activity. A vascular lesion may artifactually reduce the blood oxygen level–dependent (BOLD) effect in adjacent eloquent cortex, obscuring activation where neuronal function may be present (9–11). This effect has been noted in AVMs with shunt surgery into carotid artery branches but not in AVMs lacking the shunting (10). None of our patients had such shunting. Reduced BOLD effect in the vicinity of glial tumors (12) and absence of BOLD effect in the presence of glioblastoma multiforme have been reported (13). We found no significant difference in the activation patterns in the high-grade tumors versus AVMs and low-grade tumors. Although glial tumors in the Schreiber et al study (12) may have reduced the activation in adjacent SMA by as much as 36% on average, that effect we believe does not explain the change observed in hemispheric dominance in our study.
The current retrospective analysis was undertaken as a pilot study. Samples were small and therefore the statistical power was limited. In our statistical comparison, we compared those patients in whom a shift of SMA predominance was hypothesized with all patients and control subjects in whom a shift was not hypothesized. For example, the patients with a tumor overlapping the left SMA were compared with the patients with nonoverlapping tumors in the left frontal lobe, those with right frontal lobe tumors, and control subjects. Significance was not achieved when only the control subjects were used in the comparison.
Additional weaknesses were present in this retrospective study. Patients with a diversity of lesions were included. Motor tasks, which were self-paced and effort-dependent, did not ensure equal levels of performance with each hand, although large differences in performance were not likely. Mirror movements in the opposite limb or failure to relax the opposite limb on command may alter the relative amount of activation in the two hemispheres. The patients were observed to ensure an overt performance of the motor task for each hand, but slight mirror movements might have gone unnoticed. The confounding effects of such possible mirror movements on the relative hemispheric dominance of SMA functional MR imaging activation are not known.
Since the study was retrospective, threshold is an uncontrolled variable. Thresholds were chosen to provide the clinically required motor functional MR imaging activation maps with no reference to SMA activation, therefore no systematic bias was likely introduced as a result of the threshold variability. At the chosen threshold, SMA functional MR imaging activation was not seen in all cases. We lacked exact criteria for anatomic localization of the presupplementary motor area and did not attempt to distinguish activation of presupplementary motor area from that of SMA, as others have (14–16). We used a parcellation technique to identify the SMA region rather than using Talairach coordinates, since the effects of tumors may render that method of localization less reliable.
To our knowledge, the laterality of SMA functional MR imaging activation for motor tasks in patients with frontal lobe lesions has not been studied in depth. However, SMA laterality for language function has been studied (4–6). A shift in SMA laterality may be a factor in the risk of neurologic deficit from surgery in the region of the SMA.
Prospective studies and more quantitative measures of laterality are needed to improve knowledge of patterns of SMA functional MR imaging activation and effects of cerebral lesions on laterality. In addition, future studies might address potential reorganization of SMA function during language tasks. As patterns of SMA reorganization in the presence of chronic brain lesions are better understood, the ability to predict postoperative motor and language deficits may be improved.
Conclusions
Relative hemispheric dominance for the SMA may change in patients who have tumors or AVMs that overlap the SMA. The change may affect the risk of postoperative motor deficits in patients who undergo surgery for ablation of the lesion. Functional MR imaging is a useful tool for assessing preoperative patterns of SMA activity and thus also may be useful in predicting postoperative outcomes. The particular effects of the tumor or AVM that affect the relative hemispheric dominance deserve further study.
References
- 1.Freund HJ, Hummelsheim H. Lesions of premotor cortex in man. Brain 1985;108:697–733 [DOI] [PubMed] [Google Scholar]
- 2.Orgogozo JM, Larsen B. Activation of the supplementary motor area during voluntary movement in man suggests it works as a supramotor area. Science 1979;206:847–850 [DOI] [PubMed] [Google Scholar]
- 3.Krainik A, Lehericy S, Duffau H, et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 2001;57:871–878 [DOI] [PubMed] [Google Scholar]
- 4.Rostomily RC, Berger MS, Ojemann GA, et al. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg 1991;75:62–68 [DOI] [PubMed] [Google Scholar]
- 5.Zentner J, Hufnagel A, Pechstein U, et al. Functional results after resective procedures involving the supplementary motor area. J Neurosurg 1996;85:542–549 [DOI] [PubMed] [Google Scholar]
- 6.Nelson L, Lapsiwala S, Haughton VM, et al. Preoperative imaging of the supplementary motor area in patients with medial frontal lobe brain tumors. J Neurosurg In press [DOI] [PubMed]
- 7.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173 [DOI] [PubMed] [Google Scholar]
- 8.Lowe MJ, Russell DP. Treatment of baseline drifts in fMRI time series analysis. J Comput Assist Tomogr 1999;23:463–473 [DOI] [PubMed] [Google Scholar]
- 9.Ozdoba C, Nirkko AC, Remonda L, Lövblad KO, Schroth G. Whole-brain functional magnetic resonance imaging of cerebral arteriovenous malformations involving the motor pathways. Neuroradiology 2002;44:1–10 [DOI] [PubMed] [Google Scholar]
- 10.Lehericy S, Biondi A, Sourour N, et al. Arteriovenous brain malformations: is functional MR imaging reliable for studying language reorganization in patients? Initial observations. Radiology 2002;223:672–682 [DOI] [PubMed] [Google Scholar]
- 11.Rueckert L, Appollonio I, Grafman J, et al. Magnetic resonance imaging functional activation of left frontal cortex during covert word production. J Neuroimaging 1994;4:67–70 [DOI] [PubMed] [Google Scholar]
- 12.Schreiber A, Hubbe U, Ziyeh S, Hennig J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol 2000;21:1055–1063 [PMC free article] [PubMed] [Google Scholar]
- 13.Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000;21:1415–1422 [PMC free article] [PubMed] [Google Scholar]
- 14.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 1996;6:342–353 [DOI] [PubMed] [Google Scholar]
- 15.Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Pütz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci 1999;19:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunieda T, Ikeda A, Ohara S, et al. Different activation of presupplementary motor area, supplementary motor area proper, and primary sensorimotor area, depending on the movement repetition rate in humans. Exp Brain Res 2000;135:163–172 [DOI] [PubMed] [Google Scholar]