Abstract
Summary: We have developed a model of reversible cerebral ischemia in a high-level nonhuman primate. By using endovascular techniques, the posterior cerebral artery is permanently occluded with coils, and the ipsilateral middle cerebral artery is temporarily occluded with a balloon. The balloon can be deflated and/or removed to reestablish flow at precise time intervals. Functional imaging of the brain can be performed during occlusion and reperfusion, since the balloon can be deflated or removed in a scanner.
The treatment of acute ischemic stroke in humans has been the subject of much interest in the past several years (1–4). Modern imaging now includes the ability to obtain physiologic measurements in addition to anatomic detail, and it holds great promise as a prognostic and triaging tool to determine whether brain tissue is irreversibly damaged in patients with acute stroke (5–8). The prognostic value of some of the physiologic parameters, however, remains unclear and has been difficult to study in human subjects. For example, the presence or absence of abnormal signal intensity on diffusion-weighted MR images has not been a consistent marker for the final extent of infarction. Therefore, we have developed a model of reversible cerebral ischemia in a high-level nonhuman primate. The model appears to be useful because a well-defined ischemic cerebral insult can be observed over time and reversed with precision.
Description of the Technique
These studies were conducted under a protocol approved by the institutional animal care and use committee.
Pigtail monkeys (Macaca nemestrina) (3 Springs Scientific, Perkasie, PA) were anesthetized with ketamine (10 mg/kg IM) and intubated, at which point anesthesia was maintained by using intravenous fentanyl (25 μg/kg/h) and diazepam (2.5 mg/h titrated to the blood pressure with a systolic maximum of 140 mm Hg). A peripheral venous catheter was inserted for fluid replacement with a normal sodium chloride solution at a rate of 5 mL/kg/h. In addition, a central venous catheter was inserted via the femoral vein for drug infusion. The monkeys were paralyzed with 0.06 mg/kg/h pancuronium bromide and then transported to the angiography suite.
In the angiography suite, the femoral artery was surgically exposed. By using a standard 18-gauge angiographic puncture needle, an 0.038-inch guidewire was introduced into the femoral artery. A 10-cm 5F or 6F vascular sheath (Meditech; Watertown, MA) was then passed over the guidewire and secured. The sidearm port of the vascular sheath was used for perfusion of the sheath lumen and also to monitor arterial pressure. A standard 5F cerebral catheter (Merit Medical Systems, South Jordan, UT) was then introduced through the sheath. Catheter manipulations in this model were usually observed by using live subtraction and road mapping. The 5F catheter was positioned into the ascending aorta for initial angiography. A posteroanterior (PA) view of the skull with the superior orbital roof superimposed on the petrous ridge was selected for the position of the first angiogram. This view approximated the standard angiographic projection used in human clinical settings. A 10–12-mL injection of radiographic contrast material (Optiray 320; Mallinckrodt Medical Inc., St. Louis, MO) was used. Images were digitally acquired at a rate of three to six frames per second.
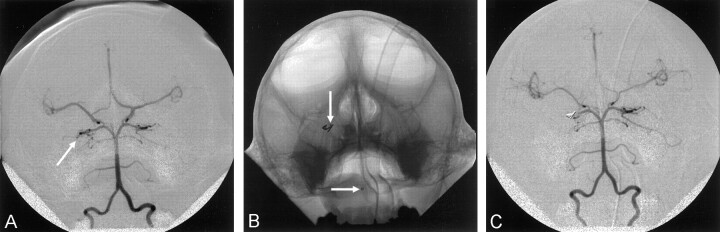
After initial angiography was performed, the catheter was positioned into the more accessible of the two vertebral arteries. Selective angiography of the vertebral artery was performed in the PA and lateral projections (Fig 1A). By using standard microcatheter techniques and live subtraction fluoroscopy, a microcatheter (Tracker 18 Turbo; Target Therapeutics, Fremont, CA) was coaxially inserted through the base catheter, and via the vertebrobasilar system, the posterior cerebral artery was catheterized to the level of the P2 segment distal to the posterior communicating artery (Fig 1B). Endovascular coils (Cook, Bloomington, IN) were deposited to occlude the posterior cerebral artery at this level. The microcatheter was removed, and an angiogram was obtained through the base catheter in the vertebral artery to confirm occlusion of the posterior cerebral artery (Fig 1C).
Fig 1.
PA angiograms.
A, View of a left vertebral artery. Both posterior cerebral arteries are opacified, and via the posterior communicating arteries, so are the bilateral anterior and middle cerebral arteries. Arrow indicates the right posterior cerebral artery.
B, View of the microcatheter (arrows) in the right posterior cerebral artery obtained during coil deposition.
C, View of the left vertebral artery after coil embolization of the right posterior cerebral artery.
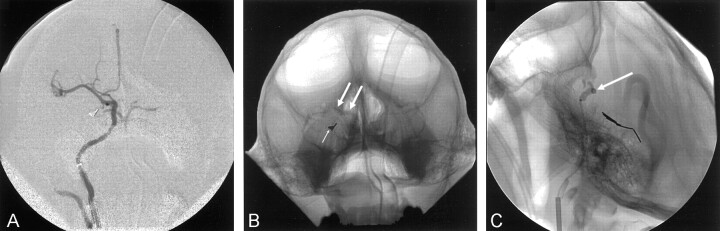
The base catheter was then positioned into the ipsilateral internal carotid artery, and angiography was performed. The animal was systemically anticoagulated with heparin (500 U intravenous bolus followed by an intravenous infusion of 100 U/h). Activated clotting time was used to monitor anticoagulation. The standard catheter was then exchanged for a guiding catheter (5F or 6F Envoy; Cordis Corporation, Miami, FL). A nondetachable endovascular balloon (Endeavor NDSB 8501; Target Therapeutics) was then coaxially inserted through the guiding catheter. The balloon was positioned at the intracranial junction of the internal carotid artery with the anterior and middle cerebral arteries (Fig 2). The balloon was inflated to achieve occlusion, which was confirmed by means of angiography through the guiding catheter. The volume required to achieve occlusion was recorded. The balloon was either deflated and maintained in position or remained inflated in position, depending on the particular experiment.
Fig 2.
Angiograms.
A, PA view of the right internal carotid artery. Coils in the right posterior cerebral artery are from prior embolization.
B, PA view. Coils (small arrow) are in the right posterior cerebral artery, and an endovascular balloon (large arrows) is occluding the distal right internal carotid and proximal right middle cerebral arteries.
C, Lateral view. Coils are in the right posterior cerebral artery and an endovascular balloon (arrow) is occluding the distal right internal carotid and proximal right middle cerebral arteries.
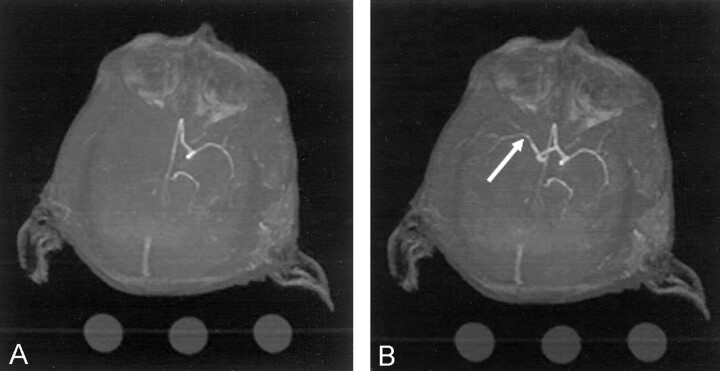
The catheter system was taped in position and secured, and the animal was transported to the MR unit. Continued anesthesia, monitoring, and ventilatory support were provided during transportation and in the MR suite. At the appropriate experimental time in the MR suite, the balloon was either inflated to the predetermined volume or deflated and removed. The heparin infusion was terminated when the balloon was removed. Conventional angiograms and MR angiograms (Fig 3A) were correlated during the occlusive phase. After balloon deflation and removal, cerebral reperfusion was confirmed by means of repeat MR angiography (Fig 3B).
Fig 3.
MR angiograms.
A, Image obtained during balloon occlusion of the right middle cerebral artery. The right posterior cerebral artery was occluded with coils. No flow is apparent in the right middle or posterior cerebral arteries.
B, Image obtained after deflation and removal of the balloon. Flow (arrow) has returned in the right middle cerebral artery.
After the procedure, the catheters were removed. The femoral arteriotomy was closed with several small sutures until a water-tight seal was obtained. The soft tissues and skin were then stapled closed.
Discussion
The treatment of acute stroke, whether by intravenous, intra-arterial, or combination routes, has become an accepted intervention. Clinical outcomes are, however, largely determined by the physiologic status of the affected brain at the time of treatment. While MR imaging is useful in determining tissue parameters, such as the intracellular sodium concentration, the perfusion of the tissue, and the apparent diffusion coefficient of the tissue, these values have not been consistently prognostic, particularly in the setting of treatments designed to reperfuse acutely ischemic cerebral parenchyma. Thus, confusion remains regarding the implications of such values. Furthermore, they have been difficult to study in the human. Therefore, determining whether to treat a patient with acute stroke has continued to rely primarily on an arbitrary time window. To this end, we have tried to develop a model that can be used to investigate physiologic parameters. Our next investigative effort will be directed at characterizing such parameters so that they can be applied in the human clinical context.
Other animal models of ischemic stroke have been described. Stroke has been induced in rats by means of middle cerebral artery occlusion with surgical clipping and suture occlusion. These models have been used to evaluate the efficacy of a variety of pharmacologic agents, but they have proved disappointing when the agents were then applied in clinical trials (9). Stroke models involving craniotomy in larger animals such as dogs, cats, and nonhuman primates have involved direct occlusion of the middle cerebral artery (10–12). Importantly, simple middle cerebral artery occlusion has not been successful in creating a consistent, reproducible stroke in primates; this limitation had made the evaluation of any subsequent intervention problematic. In fact, this inconsistency has led to the development of a monkey model in which multiple intracranial vessels are occluded. Initially, investigators showed that multiple artery occlusion via the transorbital route in a nonhuman primate consistently and reproducibly created a stroke in the middle cerebral artery territory. More recently, a similar model of multiple artery occlusion has been developed by using an endovascular approach; this has also been successful at creating a consistent stroke in the middle cerebral artery territory (13).
All of these methods have typically been models in which a permanent ischemic insult was inflicted. In contrast, the model we are reporting is reversible. One should be able to simulate in the monkey model treatment for an acute stroke in the human with reperfusion of an occluded cerebral artery. A well-defined ischemic cerebral insult of specific duration with reperfusion at a precise moment can be observed over time by using a variety of instruments.
Conclusion
The rationale for our model stems from previous experience in creating permanent strokes in high-level nonhuman primates, coupled with the basic anatomy. Prior investigators have been unable to consistently create a stroke with endovascular occlusion of the internal carotid artery at the origin of the middle cerebral artery. In contrast, we have been able to consistently create a permanent ischemic stroke in the monkey when we occlude, with endovascular coils, the P2 segment of the posterior cerebral artery and the ipsilateral internal carotid artery at the level of the internal carotid artery bifurcation above the level of the origins of the posterior communicating artery and ophthalmic artery. Ischemia was achieved despite the presence of an anterior communicating artery collateral vessel from the contralateral hemisphere to the ipsilateral anterior cerebral artery. We hypothesized that coil occlusion of the P2 segment of the posterior cerebral artery plus removable occlusion in the ipsilateral internal carotid artery at the level of the internal carotid artery bifurcation are sufficient to induce ischemia that can be reversed with great precision. Our results have borne this out.
References
- 1.Hacke W, Kaste M, Fieschi C, et al for the ECASS study group. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025 [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 3.Furlan A, Higashida R, Wechsler LR, et al for the PROACT Investigators. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA 1999;282:2003–2011 [DOI] [PubMed] [Google Scholar]
- 4.Lewandowski CA, Frankel M, Tomsick TA, et al, and the EMS bridging trial investigators. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: emergency management of stroke (EMS) bridging trial. Stroke 1999;30:2598–2605 [DOI] [PubMed] [Google Scholar]
- 5.Lo EH, Matsumoto K, Pierce AR, Garrido L, Luttinger D. Pharmacologic reversal of acute changes in diffusion-weighted magnetic resonance imaging in focal cerebral ischemia. J Cereb Blood Flow Metab 1994;14:597–603 [DOI] [PubMed] [Google Scholar]
- 6.Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME. Evaluation of early reperfusion and IV tPA therapy using diffusion- and perfusion-weighted MRI. Neurology 1999;52:1792–1798 [DOI] [PubMed] [Google Scholar]
- 7.Albers GW. Expanding the window for thrombolytic therapy in acute stroke: the potential role of acute MRI for patient selection. Stroke 1999;30:2230–2237 [DOI] [PubMed] [Google Scholar]
- 8.Firlik AD, Kaufmann AM, Wechsler LR, Firlik KS, Fukui MB, Yonas H. Quantitative cerebral blood flow determinations in acute ischemic stroke: relationship to computed tomography and angiography. Stroke 1997;28:2208–2213 [DOI] [PubMed] [Google Scholar]
- 9.Grotta J. Neuroprotection is unlikely to be effective in humans using current trial designs. Stroke 2002;33:306–307 [PubMed] [Google Scholar]
- 10.Symon L, Pasztor E, Branston NM. The distribution and density of reduced cerebral blood flow following acute middle cerebral artery occlusion: an experimental study of the technique of hydrogen clearance in baboons. Stroke 1974;5:355–363 [DOI] [PubMed] [Google Scholar]
- 11.Yonas H, Wolfson SK, Dujovny M, Boehnke M, Cook E. Selective lenticulostriate occlusion in the primate: a highly focal cerebral ischemia model. Stroke 1981;12:567–572 [DOI] [PubMed] [Google Scholar]
- 12.Del Zoppo GJ, Copeland BR, et al. Experimental acute thrombotic stroke in baboons. Stroke 1986;17:1254–1265 [DOI] [PubMed] [Google Scholar]
- 13.Horowitz M, Kassam A, Nemoto E, Arimoto J, Jungreis C. An endovascular primate model for the production of a middle cerebral artery ischemic infarction. Intervent Neuroradiol 2001;7:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]