Abstract
BACKGROUND AND PURPOSE: Masses in the parotid tail can be a source of consternation to radiologists and clinicians; inaccurate localization may lead to significant iatrogenic complication. We sought to review the pertinent anatomic localizing features of the parotid tail, relevant facial nerve anatomy, and sources of clinical and radiologic confusion. To conclude, we review imaging features that are helpful in generating a diagnosis in this location.
METHODS: We retrospectively reviewed the imaging and clinical features of 111 parotid tail masses in 103 patients (56 male, 45 female, two of unknown sex; age range, 5 months–81 years). The following imaging findings were noted: size, enhancement, multiplicity of lesions, attenuation on CT scans, signal intensity on MR images, and appearance of the surrounding parotid gland. Diagnosis was confirmed by either surgical resection or biopsy findings or by specific clinical data or characteristic imaging findings.
RESULTS: Seventeen types of parotid tail masses were identified. Benign lesions were: pleomorphic adenoma (n = 15), Warthin tumor (n = 14), infectious process (n = 13), venous malformation (n = 9), and Sjögren disease (n = 9), lymphatic malformations (n = 7), lipoma (n = 6), HIV lymphoepithelial lesion (n = 4), first brachial cleft cyst (n = 3), oncocytoma (n = 2), sarcoid (n = 1), and lymph node (n = 1). Malignant lesions were: Non-Hodgkin lymphoma (n = 14), metastatic disease (n = 7), mucoepidermoid carcinoma (n = 4), acinic cell carcinoma (n = 1), and undifferentiated carcinoma (n = 1). Eight patients had two diagnoses.
CONCLUSION: Understanding normal parotid tail anatomy is important to radiologists, because accurate localization has implications for appropriate management of masses in this location, potentially reducing the occurrence of marginal mandibular nerve injury.
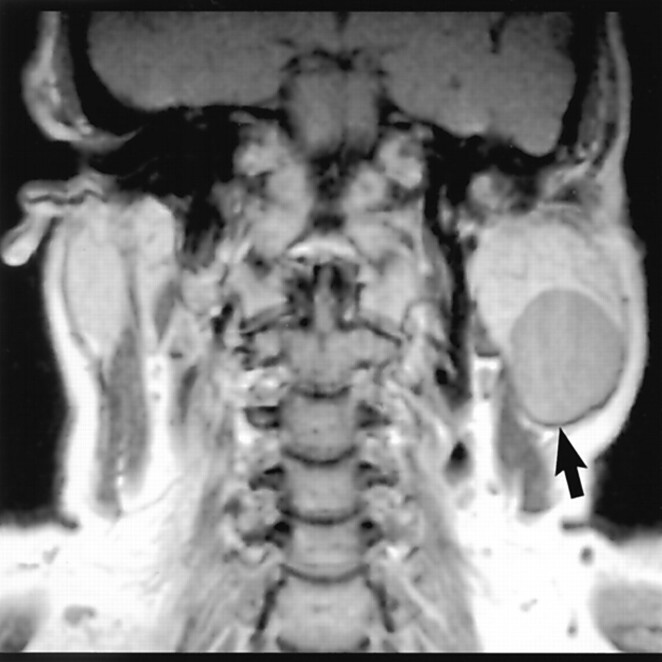
Masses in the parotid tail can be a source of consternation to radiologists and clinicians. A pedunculated parotid tail mass can easily be mistaken for a nonparotid mass on axial images. The location of a parotid tail mass or “earring lesion” is better appreciated on coronal images where localization is less confusing. Also, such lesions can be challenging to localize accurately by palpation.
Inaccurate localization may lead to a significant iatrogenic complication. The risk of damage to the marginal mandibular branch of the facial nerve during excision or biopsy of these lesions is higher if appropriate measures are not taken to achieve facial nerve control.
In an attempt to shed light on this area, we reviewed the images of a large population of patients with known masses in the parotid tail. In the Discussion, we review the pertinent anatomic localizing features of the parotid tail for the clinician and the radiologist. We then discuss the relevant normal facial nerve anatomy. We conclude by reviewing some of the imaging features that are helpful in generating a differential diagnosis in this location.
Methods
We retrospectively reviewed the clinical and imaging characteristics of 111 parotid tail masses in 103 patients by using hard copy images and digitized files kept at our institution over a 17-year period from 1984 to 2001. Clinical data reviewed included demographic information, clinical presentation, and surgical pathologic findings.
Imaging studies were performed by using CT, MR imaging, or both modalities. CT was performed after intravenous administration of contrast material, with use of 3.0-mm-thick axial sections. In four cases, postprocessing was performed to obtain coronal reformations. Standard MR imaging was performed by using axial T1- and T2-weighted and axial and coronal gadolinium-enhanced T1-weighted sequences. Section thickness varied, depending on the reason for the examination. CT was performed in 84 patients and MR imaging in 26 patients.
The images were reviewed and the following characteristics were noted: location, size, enhancement, multiplicity of lesions, attenuation on CT scans, signal intensity on MR images, and appearance of the surrounding parotid gland. The imaging appearance of other salivary glands was noted when relevant. Significant neck lymphadenopathy was noted if present.
For those patients who underwent surgical resection or biopsy (73 of 103), the diagnosis was confirmed by histopathologic review. Diagnosis in the remaining patients (n = 30) was made on the basis of specific clinical data and/or characteristic imaging findings. Only masses with involvement of the inferior 2.0 cm of the parotid gland were considered to be of parotid tail origin and included in the study.
Results
A total of 111 parotid tail masses were identified in 103 patients (56 male patients, 45 female patients, sex unknown in two). Ages ranged from 5 months to 81 years, with age unknown in 10 patients. Eighty-four (76%) of the 111 parotid masses were benign, and 27 (24%) were malignant. Seventeen different types of masses were identified (Table).
Distribution of 111 parotid tail masses in 103 patients
| Lesion | Number |
|---|---|
| Benign (n = 84) | |
| Pleomorphic adenoma (BMT) | 15 |
| Warthin tumor | 14 |
| Infectious process | 13 |
| Venous malformation | 9 |
| Sjögren disease | 9 |
| Lymphatic malformation | 7 |
| Lipoma | 6 |
| HIV lymphoepithelial lesion | 4 |
| First branchial cleft cyst | 3 |
| Oncocytoma | 2 |
| Sarcoid | 1 |
| Lymph node | 1 |
| Malignant (n = 27) | |
| Non-Hodgkin lymphoma | 14 |
| Metastatic disease | 7 |
| Mucoepidermoid carcinoma | 4 |
| Acinic cell carcinoma | 1 |
| Undifferentiated carcinoma | 1 |
Note.—Eight patients had two diagnoses (see text). BMT indicates benign mixed tumors; HIV, human immunodeficiency virus.
Pleomorphic adenomas, also called benign mixed tumors (BMT), accounted for 15 lesions and were the most common solitary mass identified. Warthin tumor was the second most common solitary mass lesion (n =14), equal in frequency to the most common malignancy, lymphoma (n = 14). Metastatic lesions were the second most common malignant lesion (n = 7). Most metastatic lesions were due to skin squamous cell carcinomas.
In the pediatric population (aged 5 months to 21 years), venous malformation (n = 9) was the most common mass, followed by lymphatic malformation (n = 7). Infectious processes (n = 13) were seen in the pediatric and adult populations. Among these processes, appearances varied, resulting in inflammatory pseudomasses in six cases, frank abscess in three cases, infected benign tumor in two cases, sialocele rupture in one case, and tuberculous nodal disease in one case. Multinodular enlargement of the parotid gland, with enlarged lymphoid aggregates, was most likely due to lymphoma (n = 14) or Sjögren disease (n = 9).
Eight patients were found to have two different types of masses involving the parotid tail. Three patients with Sjögren disease also had non-Hodgkin lymphoma. Another patient with Sjögren disease had a coexistent parotid tail lipoma. One patient with a mucoepidermoid carcinoma had a BMT in the contralateral parotid tail. Three patients had superimposed infection of underlying lesions: two with tumors (one Warthin tumor, one BMT), and a third with infection of a lymphatic malformation.
Discussion
The parotid tail is an anatomically challenging area to clinicians and radiologists. For clinicians, it can be difficult to correctly localize a mass at the angle of the mandible as parotid in origin versus submandibular gland or extrinsic lymph node in origin. This is particularly true with “earring lesions” in which a mass is pedunculated, arising from the inferior margin of the parotid gland (Fig 1). Subsequently, this may lead to biopsy, which in the case of an intraparotid mass carries the risk of marginal mandibular nerve injury. Similarly, radiologists may misdiagnose a mass in this location and contribute to the anatomic misunderstanding.
Fig 1.
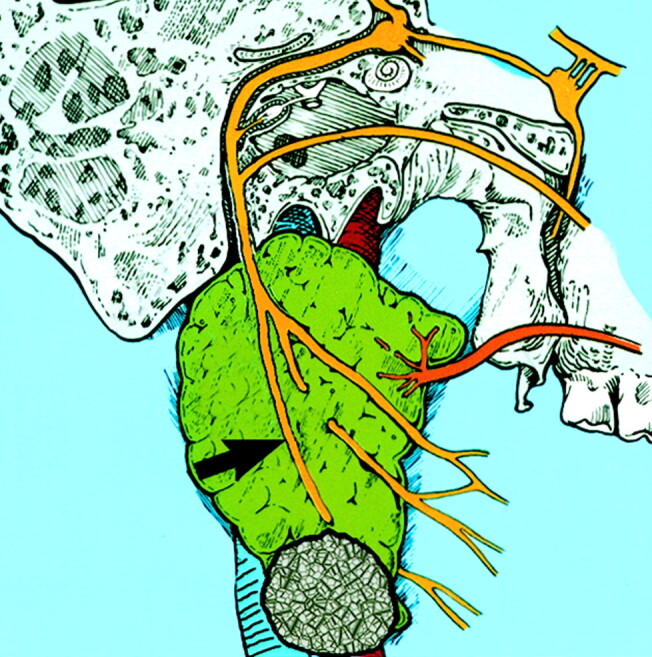
Diagram of parotid gland with a typical mass in the tail. Course of the facial nerve is demonstrated. Note the relationship of the marginal mandibular nerve (arrow) as it traverses the parotid tail mass. (Reprinted with permission from reference 1.)
Parotid Anatomy
The parotid gland is a fascial-covered organ that is suspended from the zygomatic arch. The superficial layer of the deep cervical fascia invests the parotid gland and is contiguous with masseter fascia anteriorly and with sternocleidomastoid muscle (SCM) and digastric muscle fascia posteriorly. Medially, the styloid process separates the deep lobe of the parotid gland from the parapharyngeal space. The gland is bordered laterally by platysma muscle, posteriorly by the SCM, and inferomedially by the posterior digastric muscle. The posterior digastric muscle separates the parotid gland from the more medially located carotid space (2, 3).
The superficial lobe is considered by surgeons and anatomists to represent the palpable, dominant portion of the gland, usually accounting for approximately 80% of the total gland. The parotid tail is a complex anatomic area that is prone to confusion. It is the most inferior aspect of the superficial lobe (3). Note that many surgeons consider the parotid tail to comprise the entire retromandibular part of the parotid gland inferior to the main trunk of the facial nerve, not just the inferior 2.0 cm as we have described herein.
Facial Nerve Anatomy
Although no true fascial division exists, the superficial and deep parotid lobes are separated along the plane of the facial nerve, which has important implications in parotid surgery.
The facial nerve trunk exits the skull base through the stylomastoid foramen. It then curves around the lateral base of the styloid process anteriorly to the attachment of the digastric muscle. The main trunk of the facial nerve follows the superior border of the posterior digastric muscle insertion and continues posterolateral to the styloid process. Before piercing the parotid gland, the facial nerve trunk gives off the digastric, posterior auricular, and stylohyoid branches. Approximately 1 cm after exiting the skull base, it enters the parotid gland. Within the gland, the facial nerve trunk divides into a lower cervicofacial branch and an upper temporofacial branch (2–5).
The facial nerve ramifications have a variable branching pattern. Five major branches are recognized: temporal, zygomatic, buccal, mandibular, and cervical. The marginal mandibular branch of the facial nerve runs within the parotid tail (Fig 1).
The facial nerve is not routinely identified on images. The retromandibular vein is a helpful marker in the lower parotid gland, since on axial imaging studies the facial nerve runs adjacent and lateral to the retromandibular vein in the lower gland. It crosses the lateral surface of the retromandibular vein inferiorly. Occasionally, the vein is duplicated, in which case the nerve passes between the two vessels (5).
Definition of Parotid Tail
The parotid tail is the most inferior portion of the superficial lobe. It is composed of a triangular shaped area of tissue deep to the platysma muscle, posterolateral to the posterior belly of the digastric and anterolateral to the SCM (Fig 2). The posterior belly of the digastric separates the parotid tail from the adjacent carotid space. The most inferior aspect of the gland usually terminates at the level of the angle of the mandible, although this is variable. Since the parotid tail is not anatomically distinct from the remainder of the superficial gland, we defined the parotid tail as the inferior 2.0 cm of the gland.
Fig 2.
Axial contrast-enhanced CT scan demonstrates normal parotid tail anatomy. Anatomic boundaries of the parotid tail are the following: lateral and superficial boundary, platysma (white arrow); anteromedial boundary, posterior belly of digastric (black arrow); posteromedial boundary, SCM (curved white arrow). (Reprinted with permission from reference 1.)
The fascia covering the parotid gland is contiguous with the styloid muscles inferiorly and attaches to the styloid process. This deep medial portion of the parotid fascia contributes to the stylomandibular ligament and continues inferiorly to fuse with the fascia of the posterior belly of the digastric and angle of mandible. This portion of the parotid fascia serves to anatomically separate the parotid space from the submandibular space (5).
Sources of Clinical and Imaging Confusion
Confusion regarding the origin of a mass in the parotid tail can arise on axial imaging studies when such lesions are pedunculated from the inferior aspect of the gland. Axial CT is the most common study performed for the evaluation of a head and neck mass, so familiarity with the normal transaxial anatomy is critical to avoid this pitfall. There are two reasons why confusion tends to occur in this location on axial CT scans. First, a pedunculated mass in the parotid tail may not be surrounded by normal parotid tissue and may not be perceived as arising from the gland. In addition, older patients may have atrophic fat-infiltrated glands, such that a parotid tail mass would have less contrast against adjacent subcutaneous fat. Thus, a mass in the parotid tail might not be perceived as arising from the gland if wide window settings are not used. Second, the parotid tail is in close proximity to the posterior submandibular space, and in some instances, the glands may be in contact with each other. Although separated anatomically by fascia, pedunculated parotid tail masses can protrude into the posterior submandibular space, which may lead to an incorrect imaging impression that such a mass arose from the submandibular space rather than the parotid gland.
Sources of Radiologic Confusion
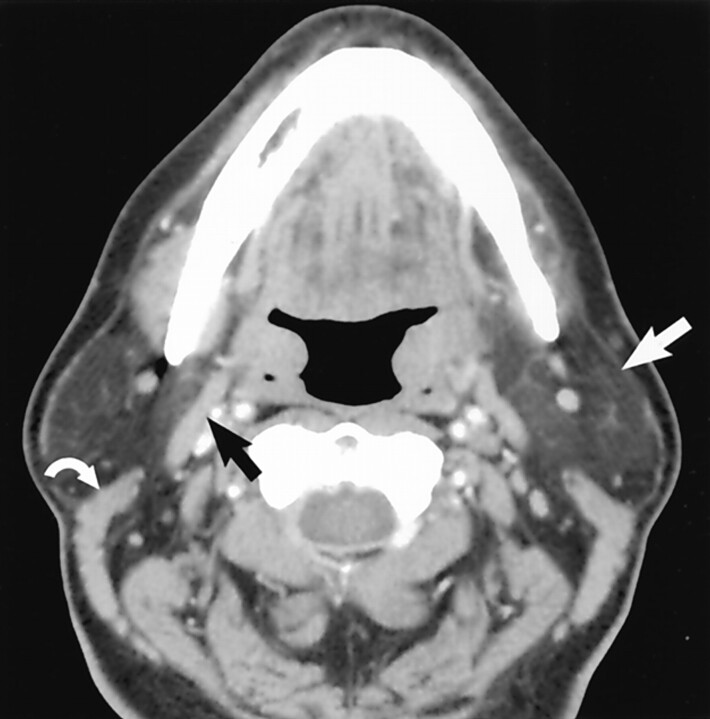
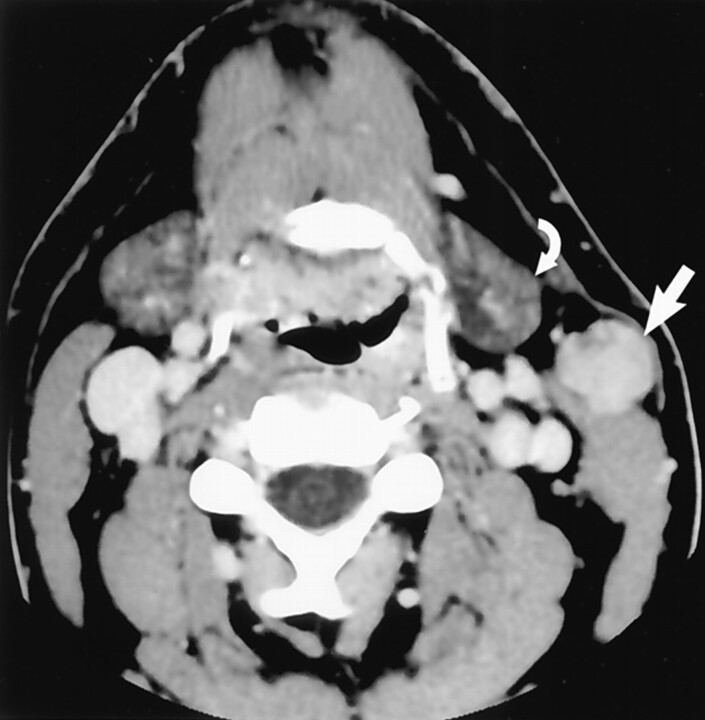
The most frequent source of radiologic confusion occurs when a pedunculated parotid tail mass is mistaken for a lymph node (Fig 3). The relationship of a mass lesion to the SCM is helpful in differentiating parotid tail lesions (anterolateral to the SCM) from jugulodigastric lymph nodes (anteromedial to the SCM). An example of a typical “earring” lesion in the inferior tail illustrates how such a mass might be confused for a necrotic lymph node (Fig 4). The low position of some parotid tail masses (below the angle of mandible) and the frequent absence of normal surrounding parotid tissue are factors responsible for this impression. Axial CT images, which are usually the initial imaging technique used to work up a neck mass, must be carefully assessed to avoid this pitfall. Coronal imaging will usually clarify persistent concerns regarding the origin of a mass in this location (Figs 5 and 6).
Fig 3.
Axial contrast-enhanced CT scan demonstrates normal jugulodigastric lymph nodes bilaterally (curved arrows), which should not be confused with lesions originating in the parotid tail. Note the normal parotid tail tissue located anterolateral to the SCM (straight arrow). The lymph nodes lie anteromedial to the muscle. (Reprinted with permission from reference 1.)
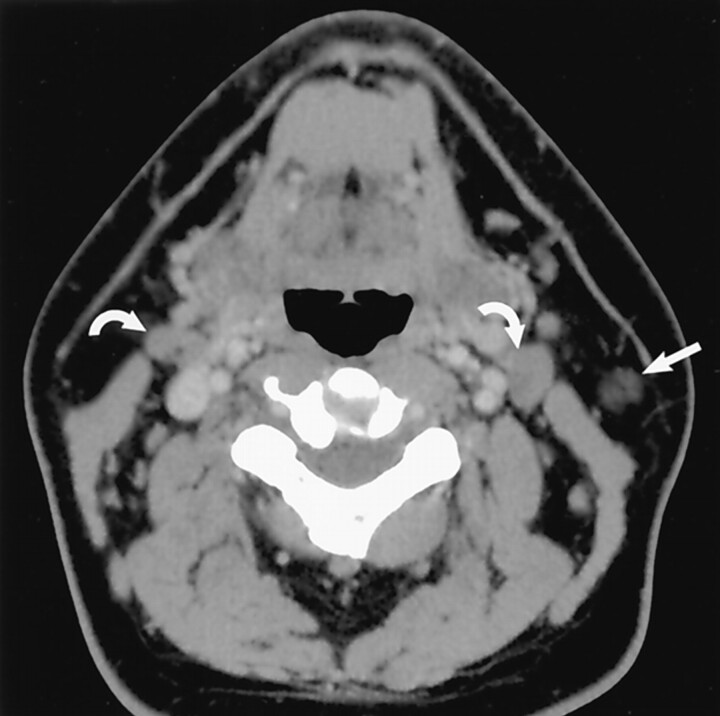
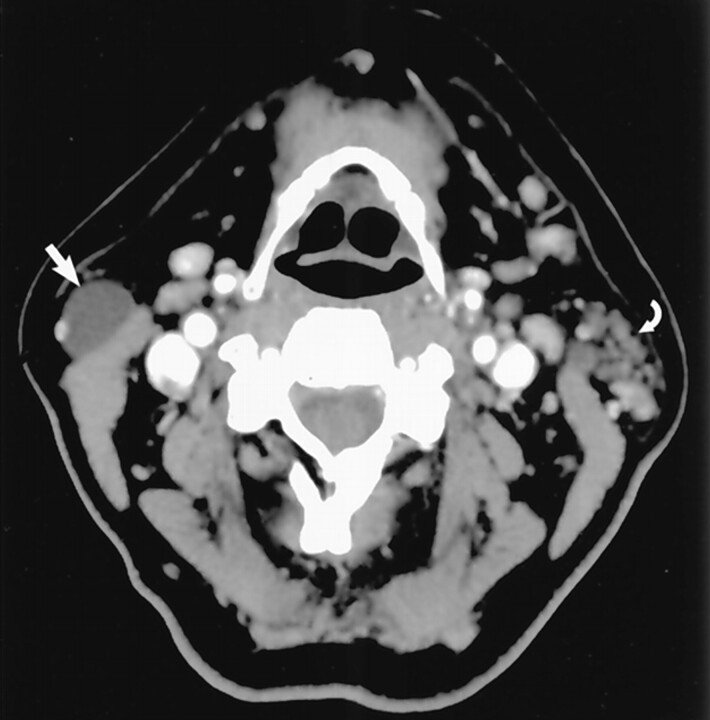
Fig 4.
Earring lesion of the parotid tail. Axial contrast-enhanced CT scan shows typical appearance of a cystic Warthin tumor. Note the nodule of soft tissue (straight white arrow), which is commonly seen. A small amount of parotid tissue (open arrow) is seen anterior to the mass. Note parotid tail anatomic landmarks: SCM posteriorly and platysma (curved white arrow) laterally. (Reprinted with permission from reference 1.)
Fig 5.
Axial nonenhanced T1-weighted MR image demonstrates a large mass (arrow) in the left parotid tail. This mass has little inherent contrast against adjacent normal tissues, making localization more challenging. (Reprinted with permission from reference 1.)
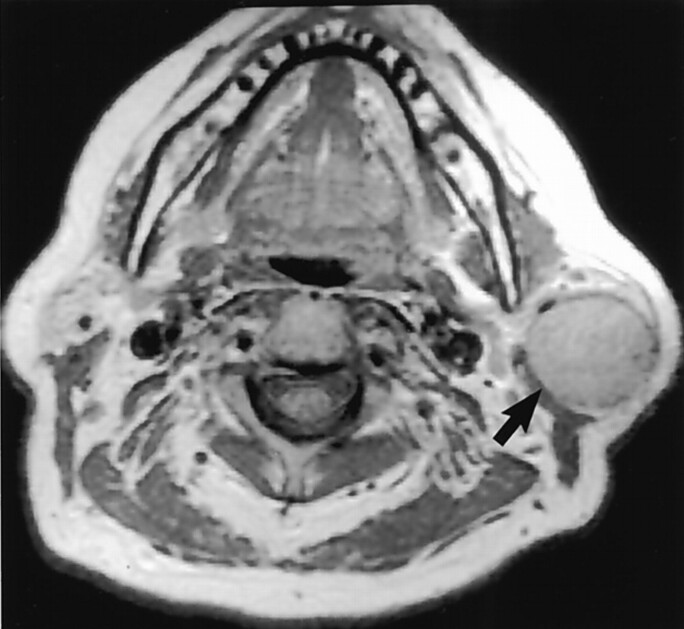
Fig 6.
Coronal nonenhanced T1-weighted MR image clarifies the intraparotid nature of this BMT (arrow). (Reprinted with permission from reference 1.)
Sources of Clinical Confusion
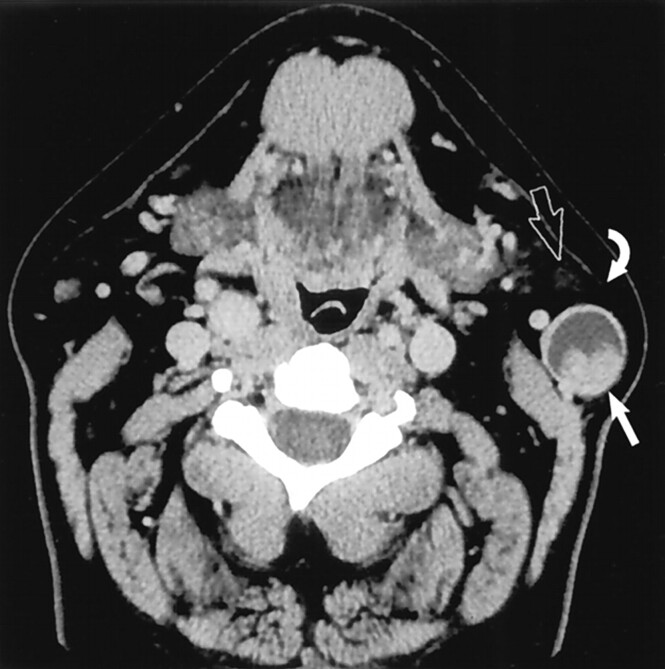
Most parotid tail masses present as “angle of mandible” masses. Although otolaryngologists can usually differentiate an intraparotid mass from an adjacent mass or lymphadenopathy, they do recognize that this distinction can at times be difficult (4, 6). This may be particularly problematic if the patient is not seen by an otolaryngologist. In at least three of our cases, we were aware that the clinician referred the patient to CT for evaluation of a “submandibular mass,” which at imaging proved to be intraparotid in origin (Fig 7). Similarly, masses at the angle of the mandible, such as an enlarged lymph node, may be mistaken for a parotid tail mass (Fig 8).
Fig 7.
Mass was believed to represent a submandibular space or gland tumor by the referring general surgeon, who initially planned to excise the mass. Axial contrast-enhanced CT image reveals the mass (straight arrow) to be deep to platysma and anterior to the SCM, characteristic for a parotid tail “earring lesion.” Note its proximity to the submandibular gland (curved arrow). (Reprinted with permission from reference 1.)
Fig 8.
Otolaryngology referred this patient for imaging after palpating a mass in the “left parotid tail.” Axial contrast-enhanced CT scan through the mass reveals a partly cystic mass in a typical location for a jugulodigastric lymph node (curved arrow). Normal parotid tail tissue is seen (straight white arrow). This was a metastatic node from a clinically occult primary squamous cell carcinoma, which is partly seen on this section in the left faucial tonsil (open arrow). Additional lymph nodes lower in the neck indicated a high-stage tumor. Upon chart review, the referring physician had noted mass effect in the left tonsil; however, a mucosal lesion was not present. (Reprinted with permission from reference 1.)
Surgical Management of Parotid Masses
Surgical treatment of parotid masses has evolved during the last 2 decades. Total parotidectomies are uncommon, except for high-grade malignancies. Imaging features of parotid masses lack the necessary specificity to differentiate benign from malignant masses. Furthermore, fine-needle aspiration, although helpful in surgical planning, lacks the necessary accuracy for differentiating benign from malignant lesions (7). Although most parotid masses are benign, removal is required for histopathologic confirmation because of the clinical and radiologic overlap.
Pleomorphic adenomas, the most common parotid tumor overall, have a 5% or more frequency of malignant degeneration, which is an additional rationale for removing all parotid masses (4, 8). Incomplete resection of pleomorphic adenomas also carries the risk of local recurrence, which is frequently multifocal (9).
Most otolaryngologists perform superficial parotidectomy for benign lesions and low-grade malignancies. There are three main reasons for this approach. One, most parotid tumors are benign well-encapsulated lesions, and as such, are usually completely removed with a subtotal resection. Second, most parotid masses are located in the superficial gland, without a deep lobe component. Third, superficial parotidectomy is clearly associated with a lower rate of facial nerve injury. Superficial parotidectomy is usually both diagnostic and curative. Even for low-grade malignant tumors, a subtotal resection, which includes a cuff of normal tissue, has also proved to be curative, while decreasing the risk of permanent facial nerve injury. Obviously involvement of the deep lobe or facial nerve itself represents specific problems that cannot be addressed with a subtotal approach. High-grade malignancies or masses involving the deep lobe still require total parotidectomy (8).
Surgical Complications in Parotid Surgery
The most significant common surgical complication in parotid surgery is facial nerve injury. This may involve a solitary branch, several branches, or the whole nerve. The branch most frequently injured in any parotid surgery is the marginal mandibular nerve, which is responsible for lower lip depression. Permanent facial nerve paralysis is uncommon with standard technique, reported at less than 3% for superficial parotidectomy (8).
Biopsy of a mass in the parotid tail without recognition of its intraparotid nature can lead to injury of the marginal mandibular nerve. The referring surgeon must be aware that a mass in this location may be of parotid origin, since the surgical approach and treatment of these lesions are different.
Review of Imaging Features
Most parotid masses to undergo imaging are evaluated by using contrast-enhanced CT. Recently published data in the otolaryngology literature support the routine use of CT scanning in parotid mass work-up for a variety of reasons, including the discovery that masses in this location are occasionally extraparotid. CT also reveals the presence of additional masses, either in the same lobe or in the contralateral gland, that were clinically silent. Finally, CT changes the perceived tumor extent (superficial versus deep lobe involvement), which has altered surgical approach in a number of cases (6).
Differential Clinical Features
Previous series indicate most tumors in the parotid gland are benign (70–80%), in contrast to tumors in other salivary glands (10). Our data, in which 76% of cases were benign, are similar to those of prior published reports. Helpful objective clinical features include age and sex. The frequency of parotid malignancy in the pediatric population is similar to or lower than that in adults. Older series report a frequency of 25%, whereas more recent series suggest a lower rate of 16%. Of malignancies in the pediatric group, mucoepidermoid carcinoma is most common, accounting for 42–55% (11, 12).
Benign tumors or infectious or inflammatory lesions account for most pediatric lesions. Pleomorphic adenomas, followed by hemangiomas, are the most commonly reported benign tumors (11, 12).
Interestingly, the solitary case of tuberculous nodal disease in the current series is unusual, since more than 90% of cervical lymphadenitis is due to nontuberculous mycobacteria (usually Mycobacterium avium-intracellulare) in the pediatric population. This is in contradistinction to adults, in whom tuberculous cervical lymphadenitis is more common (13).
Patient sex was significant in two disease categories: Sjögren disease, in which all known cases in this series were female patients (nine of nine patients), and Warthin tumor, which was nearly exclusively seen in male patients (13 of 14 patients).
Differential Imaging Features
Imaging features that were evaluated include mass location, multiplicity of lesions, lesion margins, attenuation or signal intensity, enhancement characteristics, and ancillary imaging findings such as the presence of neck lymphadenopathy and the appearance of the salivary gland tissue in general.
The location of a parotid mass is of some significance. Most parotid glandular masses are BMTs. In this series that included only masses with parotid tail involvement, Warthin tumors (14 of 103 patients) were nearly equal in frequency to the most common solitary mass in the parotid tail, BMT (15 of 103 patients).
The number of masses also helps to narrow differential considerations. Warthin tumor is known to frequently be multifocal and/or bilateral (14). In this series, Warthin tumors were multicentric in five of 14 cases and bilateral in three. In contradistinction, all primary BMTs in this series were unifocal. Three of 15 BMT postsurgical recurrences were multifocal and believed secondary to spillage at the time of surgery or inadequate resection, a well-described phenomenon (8, 9).
Parotid lymphoma was the lesion most likely to present with multifocal and/or bilateral masses (seven of 14 cases) and was the most common malignancy in the parotid tail. This is inconsistent with results of prior series, which suggest that both primary and secondary parotid lymphoma are relatively uncommon. Secondary lymphoma is slightly more common and accounts for 1–8% of parotid lymphoma (3). The reason for the high frequency of parotid lymphoma in this series is unknown, but likely reflects selection bias. Interestingly, seven (50%) of the 14 parotid lymphomas in this series were unilateral masses, and only 50% of the lymphomas had associated significant neck lymphadenopathy.
Lesion margins have some prognostic value, although not highly specific. Generally speaking, masses with well-circumscribed margins tend to be benign (multiple lymph node masses in lymphoma being an exception). Common masses such as BMT and Warthin tumor were highly likely to be well-circumscribed lesions. Notable exceptions to this were two pathologically proved infected tumors, one a BMT and the other a Warthin tumor; both demonstrated irregular or ill-defined margins, mimicking malignancy.
Infiltrating margins, particularly when associated with soft-tissue or muscle invasion, raise concern for malignancy. Irregular margins were most commonly observed in parotid metastases (seven of seven cases). Adjacent soft-tissue invasion was additionally present in two of seven cases.
On the contrary, lesion margins were not helpful in the less common mucoepidermoid carcinomas and oncocytomas. Mucoepidermoid carcinomas were noted to have irregular, infiltrative margins in two of four cases and were well circumscribed in two cases. Similarly in two cases of oncocytoma, one was well circumscribed and one was infiltrative. An infiltrating or poorly circumscribed appearance is also characteristic of lymphatic malformations. These also tend to be transpatial, as are venous malformations.
Attenuation on CT scans and/or signal intensity on MR images are also helpful imaging characteristics. Complex lesions, with a mixed cystic-nodular appearance or centrally necrotic areas (in otherwise normal-appearing parotid glands) were most likely to represent Warthin tumor (nine of 14 cases), unlike in BMT where cystic changes were seen only in three of 15 cases (Fig 4). Metastatic disease was complex and/or cystic in all cases (seven of seven), but usually had associated features, such as infiltrating or invasive margins, that distinguished malignancy from benign lesions such as Warthin tumor.
When encountering a cystic mass in the parotid gland, there is a wide differential list. Clearly, different considerations should be thought of if the process is multifocal or bilateral than if a solitary cyst is present.
Multiple cysts without associated nodularity or complexity usually reflect a benign process. When multiple, these include lymphoepithelial lesions. These cysts tend to be well circumscribed, without invasion or infiltration, which differentiates them from malignant processes. Imaging and histologic findings are nonspecific, however, and this can be seen in human immunodeficiency virus (HIV), Sjögren disease, and chronic recurrent parotitis of childhood (15–17). Clinical history is usually revealing in such cases.
Multiple cysts and/or small soft-tissue nodules characterize lymphoepithelial lesions. The cysts are usually simple and multiple, although lymphoepithelial lesions can manifest with a solitary or dominant cyst or mass. Few HIV lymphoepithelial lesions were seen in this series (four total), which likely reflects the relative paucity of HIV-positive patients in our geographic location. Another contributing factor may be the decline in HIV lymphoepithelial lesions since the introduction of protease inhibitors. In three of four cases, the diagnosis of acquired immunodeficiency syndrome was known before imaging. In one case, CT was the first diagnostic procedure to suggest the disease.
Sjögren disease also commonly manifests with multiple cysts (or a solitary or dominant cyst), seen in five of nine cases. Sjögren disease is usually distinguished from other multicystic pathologic conditions by the abnormal appearance of the salivary glandular tissues (Fig 9). Glandular calcifications may be present and are more typical of Sjögren disease (17). All nine cases of Sjögren disease in this series demonstrated abnormal increased attenuation of the parotid gland, with multiple small nodules, usually thought to represent lymphoid aggregates, an appearance consistent with lymphoepithelial lesions. The radiographic appearance may be indistinguishable from lymphoepithelial lesion due to HIV or recurrent parotitis.
Fig 9.
Axial contrast-enhanced CT scan through the parotid tail demonstrates a dominant cyst (straight arrow). Nodular lymphoid aggregates (curved arrow) in the contralateral gland should suggest a systemic process, in this case, Sjögren disease. The appearance is typical of Sjögren disease or HIV lymphoepithelial lesion, which cannot be distinguished on a histopathologic basis. (Reprinted with permission from reference 1.)
Potential causes for solitary cystic masses include abscess, infected lymph node, solitary lymphoepithelial lesion (of any cause), Warthin tumor, granuloma, branchial cleft cyst, cystic schwannoma, or a nonseptated lymphatic malformation.
Well-circumscribed simple cysts without nodularity or invasive features will be of a benign or inflammatory etiology in most cases. Peripheral cyst enhancement suggests primary or secondary infectious or inflammatory process. If associated nodularity is present, however, this should raise concern for Warthin tumor. Adjacent soft-tissue stranding can be a clue with infectious causes. Branchial cleft cyst may be difficult or impossible to differentiate from an infectious process such as nontuberculous nodal infection if a sinus tract or other developmental clue is not present. Either a first or second branchial cleft cyst may manifest in the parotid gland. A first branchial cleft cyst may manifest as an intraparotid cyst, sinus tract, or fistula (18). Three first branchial cysts were found in the current series, although one case was not surgically confirmed.
Cystic masses with septations or ill-defined margins are more likely to represent lymphatic malformations, particularly if transpatial. Imaging features suggestive of lymphatic malformations include little or no enhancement and fluid levels reflecting prior hemorrhage. Conversely, venous malformations are characterized by delayed fairly homogeneous enhancement and phleboliths, and do not typically hemorrhage (19–21). In this series, pheboliths were seen in four of nine cases of venous malformations.
Three of the nine patients with Sjögren disease in this series developed non-Hodgkin lymphoma. Patients with primary Sjögren disease are at a significantly increased risk of developing non-Hodgkin lymphoma, estimated at 44 times greater than the risk in the general population (22). This is the most serious complication in Sjögren disease, believed to account for 20% of deaths in primary Sjögren disease (23). Neck lymphadenopathy is frequently present, but not necessary to consider this diagnosis. Dominant or enlarging masses in patients with Sjögren disease are concerning and usually require biopsy.
Finally, CT attenuation is diagnostic in the homogeneous fat attenuation seen in parotid lipoma, similar to lipomas elsewhere in the body. Of six parotid lipomas in this series, one underwent surgical removal for unknown reasons, and histopathologic examination confirmed this characteristic imaging appearance. One patient with Sjögren disease in this series had a coexistent parotid tail lipoma, for which she underwent imaging, although there is no known association between these two processes. This was thought to be incidental.
Conclusion
The parotid tail is a challenging area to radiologists and surgeons. Understanding normal parotid tail anatomy is important to radiologists because accurate localization has implications for appropriate management of masses in this location, potentially reducing the occurrence of marginal mandibular nerve injury. Permanent facial nerve paralysis is an uncommon complication of superficial parotidectomy (<3%) when appropriate measures are taken to avoid injuring the nerve. Correct understanding of transaxial anatomy is important so radiologists can convey accurate information to the referring surgeon and thus contribute to appropriate surgical planning.
Footnotes
Presented at the 40th annual meeting of the American Society of Neuroradiology, Vancouver, British Columbia, May 11–17, 2002.
References
- 1.Harnsberger HR. Head and Neck Digital Teaching File. Salt Lake City, UT: Amirsys, Inc.;2002
- 2.Bannister LH. Alimentary system. In: Williams PL, Bannister L, Berry M, et al, eds. Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery. 38th ed. Edinburgh, Scotland: Churchill Livingstone1995. :1691–1692
- 3.Som PM, Brandwein M. Salivary glands. In: Som PM, Curtin HD, eds. Head and Neck Imaging. 3rd ed, vol 2. St. Louis, MO: Mosby-Year Book, Inc.;1996. :824–830, 898–907
- 4.Hoffman H, Funk G, Endres D. Evaluation and surgical treatment of tumors of the salivary glands. In: Thawley SE, Panje W, Batsakis L, Robert J, eds. Comprehensive Management of Head and Neck Tumors. 2nd ed, vol 2. Philadelphia, PA: W. B. Saunders Company;1999. :1147–1177
- 5.Gates G, Johns M. Embryology and anatomy of the salivary glands. In: Paparella MM, Shumrick D, eds. Otolaryngology: Basic Sciences and Related Disciplines. 2nd ed, vol 1. Philadelphia, PA: W. B. Saunders Company;1980. :150–151
- 6.Urquart A, Hutchins LG, Berg RL. Pre-operative computed tomography scans for parotid tumor evaluation. Laryngoscope 2001;111(Pt 1):1984–1988 [DOI] [PubMed] [Google Scholar]
- 7.Zbaren P, Schar C, Hotz MA, Loosli H. Value of fine needle aspiration cytology of parotid gland masses. Laryngoscope 2001;111(Pt 1):1989–1992 [DOI] [PubMed] [Google Scholar]
- 8.Eisele DW, Johns ME. Complications of surgery of the salivary glands. In: David E, ed. Complications in Head and Neck Surgery. St. Louis, MO: Mosby-Year Book, Inc.;1993. :183–200
- 9.Buchman C, Stringer SP, Mendenhall WM, Parsons JT, Jordan JR, Cassisi NJ. Pleomorphic adenoma: effect of tumor spill and inadequate resection on tumor recurrence. Laryngoscope 1994;104:1231–1234 [PubMed] [Google Scholar]
- 10.Sungur N, Akan IM, Ulusoy MG, Ozdemir R, Kilinc H, Ortak HT. Clinicopathological evaluation of parotid gland tumors: a retrospective study. J Craniofacial Surg 2002;13:26–30 [DOI] [PubMed] [Google Scholar]
- 11.Orvidas LJ, Kasperbauer JL, Lewis JE, Olsen KD, Lesnick TG. Pediatric parotid masses. Arch Otolaryngol Head Neck Surg 2000;126:177–184 [DOI] [PubMed] [Google Scholar]
- 12.Jacques DA, Krolls SO, Chambers RG. Parotid tumors in children. Am J Surg 1976;132:469–471 [DOI] [PubMed] [Google Scholar]
- 13.Kwan KL, Stottmeier KD, Sherman IH, McCabe WR. Mycobacterial cervical lymphadenopathy: relation of etiologic agents to age. JAMA 1984;251:1286–1288 [DOI] [PubMed] [Google Scholar]
- 14.Maiorano E, Muzio L, Lo F, Piattelli GA. Warthin’s tumour: a study of 78 Cases with emphasis on bilaterality, multifocality, and association with other malignancies. Oral Oncol 2002;38:35–40 [DOI] [PubMed] [Google Scholar]
- 15.Huisman TA, Holzmann D, Nadal D. MRI of chronic recurrent parotitis in childhood. J Comput Assist Tomogr 2001;25:269–273 [DOI] [PubMed] [Google Scholar]
- 16.Kirshenbaum KJ, Nadimpalli SR, Friedman M, Kirshenbaum GL, Cavallino RP. Benign lymphoepithelial parotid tumors in AIDS patients: CT and MR findings in nine cases. AJNR Am J Neuroradiol 1991;12:271–274 [PMC free article] [PubMed] [Google Scholar]
- 17.Takashima S, Nagareda T, Noguchi Y, et al. CT and MR appearances of parotid pseudotumors in Sjögren syndrome. J Comput Assist Tomogr 1992;16:376–383 [DOI] [PubMed] [Google Scholar]
- 18.Triglia JM, Nicollas R, Ducroz V, Koltai PJ, Garabedian EN. First branchial cleft anomalies: a study of 39 cases and a review of the literature. Arch Otolaryngol Head Neck Surg 1998;124:291–295 [DOI] [PubMed] [Google Scholar]
- 19.Baker LL, Dillon WP, Hieshima GB, Dowd CF, Frieden IJ. Hemangiomas and vascular malformations of the head and neck: MR characterization. AJNR Am J Neuroradiol 1993;14:307–314 [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson RL, Robson MB, Barnes PB, Burrows PE. Head and neck vascular anomalies of childhood. Neuroimaging Clin N Am 1999;9:115–132 [PubMed] [Google Scholar]
- 21.Burrows PE, Mulliken JB, Fellows KE, et al. Childhood hemangiomas and vascular malformations: angiographic differentiation. AJR Am J Roentgenol 1983;141:483–488 [DOI] [PubMed] [Google Scholar]
- 22.Zulman J, Jaffe R, Tahal N. Evidence that the malignant lymphoma of Sjögren’s syndrome is a monoclonal B-cell neoplasm. N Engl J Med 1978;299:1215–1220 [DOI] [PubMed] [Google Scholar]
- 23.Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum 2002;46:741–747 [DOI] [PubMed] [Google Scholar]