Abstract
BACKGROUND AND PURPOSE: The mechanism underlying the perception of music has been the subject of study for many years. We investigated the role of the anterior portion of the temporal lobes in the perception of music in nonmusicians by use of positron emission tomography (PET).
METHODS: We used the subtraction technique for PET to investigate the role of the anterior portion of the bilateral temporal lobes in music perception. Nonmusicians performed two kinds of musical tasks: harmony listening and soprano part listening.
RESULTS: During the harmony-listening task, the anterior portion of the temporal lobes, cingulate gyri, and cerebellum were bilaterally activated. During the soprano part–listening task, the bilateral superior parietal lobules, and the right precuneus were significantly activated.
CONCLUSION: The anterior portion of the bilateral temporal lobes is vital in the discrimination of melodies and chords. Differences between activated brain regions exist between musicians and nonmusicians when listening to a particular vocal part of a musical phrase.
The mechanism underlying the perception of music has been the subject of study for many years. The literature on brain dysfunction includes cases of patients who lost musical ability because of a condition known as amusia (1). Several reports have suggested that the temporal lobe plays an important role in the perception of music: impairment of the left temporal lobe results in the disturbance of reading musical scores; that is, musical alexia (2). The right temporal lobe has been found to be important in the recognition of timbre (3), and the bilateral temporal lobes have been shown to be involved in the discrimination between familiar and unfamiliar melodies (4). These studies, however, have a number of problems: first, because of artifacts from the pyramidal bones or teeth, many reports failed to characterize the lesions of the temporal lobes in detail. Second, there have been few reports of “pure” amusia. Most amusical patients report accompanying neuropsychological deficits, such as aphasia or apraxia. Thus, in such cases, it is difficult to determine which symptoms are caused solely by amusia. Other studies that have attempted to clarify the mechanism of music perception in the temporal lobes were performed with epileptic patients with unilateral temporal lobectomy. Results of the assessment of musical capacity revealed that right temporal lobectomy affected the discrimination of timbre and of unfamiliar tonal sequences (5–7) and the memory of unfamiliar melodies (8). Patients whose lesions included the posterior part of the right superior temporal gyrus showed more severe disturbances in the perception of pitch and rhythm than did those whose lesions included only the anterior part of that gyrus (9). Judging from the literature on amusic and lobectomized patients, we may infer that the temporal lobe is important for the perception of music. Still unknown, however, is which regions in the temporal lobes play important roles in music perception.
From the results of a previous positron emission tomography (PET) activation study in musicians, we suggest that the anterior portion of the temporal lobes are vital in music perception (10). In this study, we examined music students while they listened to the harmony as a whole (harmony-listening task) and to the alto part of the same piece of music (alto part–listening task) (10). Using the subtraction technique for PET, we compared the brain regions activated during both tasks. The harmony-listening task was associated with bilateral increases of regional cerebral blood flow (rCBF) in the anterior portion of the bilateral temporal lobes, mainly in the bilateral temporal poles. The alto part–listening task was associated with increases of rCBF in the bilateral superior parietal lobules, precunei, premotor areas, and orbital frontal cortices.
In the present study, using the similar musical tasks as those of our previous PET study for musicians, we investigated the mechanism of perception of music in nonmusicians. Activated brain regions were investigated when nonmusicians listened to a harmony as a whole. We also compared the differences in brain regions between musicians and nonmusicians who participated in listening to a certain vocal part of the harmony.
Methods
Subjects
Eleven right-handed male volunteers (age range, 20–30 years; mean age, 21.2 years) participated in the study. All subjects were students at the School of Engineering and Mining, Akita University. None had received formal education or private lessons in music or had any signs or history of neurologic, cardiovascular, or psychiatric disease. All subjects gave written informed consent after the purpose and the procedures of the examination had been fully explained to them. The study was approved by the Ethics Committee of the Research Institute for Brain and Blood Vessels, Akita, Japan. All experiments were conducted in accordance with the Declaration of Helsinki.
Task Procedures
In music theory, chords are classified roughly into two groups: consonance and dissonance. Consonance is defined as the harmonious sounding of two or more notes together (11). Dissonance refers to two or more notes sounding together and forming an unstable sound (11). The melody can be subdivided into phrases, or subdivisions, of a melodic line (11). The stimuli in this experiment were three new musical pieces of harmonious style with three vocal parts. The musical pieces had the style of homophony in classical music, in which there was a clear distinction between melody and accompanying harmony (11). All pieces were composed by one of the authors and were 24 bars in length. Each musical piece consisted of six phrases and six dissonances that sounded unstable and unpleasant compared with the preceding and succeeding chords. These musical pieces were played by a professional pianist and recorded on a minidisk. All subjects performed the following two tasks during the PET measurements: 1) harmony-listening task—subjects were required to listen to the harmony as a whole. If subjects heard dissonance, they were instructed to make a sign with the index finger of their right hand. (2) Soprano part–listening task—subjects were required to listen to the tone of the soprano part of the harmony. If the subjects regarded some length of the tonal sequence as one phrase, they had to make the same sign described above. Task 1 was first performed for all musical pieces, followed by task 2 in the same order. All stimuli were presented binaurally by means of inset stereo earphones. The instructions for each task were as follows: 1) Close your eyes. Now, you will listen to the first (second, third) musical piece of harmonious style with three vocal parts. Please listen to the harmony as a whole. If you hear a dissonance, please make a sign with the index finger of your right hand. 2) Close your eyes. Now, you will listen to the first (second, third) musical piece of harmonious style with three vocal parts, which is the same music as task 1. Now, please listen to the tone of the soprano part, namely, the highest part of the harmony. If you find the end of subdivision of a melodic line—that is, a phrase—please make a sign the same as task 1.
PET Measurements
The protocol used in this study has been described in detail elsewhere (10). PET data were acquired in a three-dimensional acquisition mode by using Headtome V (Shimazu, Kyoto, Japan). Scans were performed with subjects lying supine in a darkened room with their eyes closed. Six CBF measurements were determined for each subject—three during the harmony-listening task and three during the soprano part–listening task. Employing the 15O-labeled water (H215O) intravenous bolus technique (12), we collected emission data for 90 seconds in each measurement following the intravenous bolus injection of 15 mL (40 mCi) of H215O. Each piece of music was started 15 seconds before data acquisition, repeated twice, and continued for about 120 seconds. Emission data were corrected for attenuation by acquiring 10 minutes of transmission data using a 68Ge orbiting rod source before the activation scans. A washout period of approximately 10 minutes was allowed between successive scans. For anatomic reference, all subjects underwent axial T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI), by using a 1.5-T MR imaging system (Vision; Siemens, Erlangen, Germany). T1WI (TR/TE = 665/14 ms) and T2WI (TR/TE = 3600/96 ms) were obtained by using a section thickness of 5 mm with intersection gap of 1 mm.
Data Analysis
PET data analysis was performed on a SGI Indy running IRIX 6.5 (Silicon Graphics, Mountain View, CA), by using an automated PET activation analysis package (13) composed of six main processing stages described elsewhere (10). The six main stages consist of intrasubject coregistration, intrasubject normalization, automatic detection of the anterior commissure–posterior commissure line, detection of multiple stretching points and surface landmarks on intrasubject averaged image sets, intersubject summation and statistical analyses, and superimposition of the statistical results onto the stereotactic MR image. Deformation of the individual brains to correspond to the standard atlas brain was achieved by spatially matching the individual landmarks to the corresponding predefined standard surface landmarks and minimizing correlation coefficients of regional profile curves between the stretching centers. Activation foci were considered to be significantly activated if the corresponding P value was less than a predetermined threshold (P < .01, uncorrected for multiple comparisons). Anatomic identification of the activation foci was achieved by referring the stereotactic coordinates of the peak-activated pixels to the standard Talairach brain atlas (14). For further details, see the report by Satoh et al (10).
Results
Subjects performed the experimental tasks reasonably well. In the harmony-listening task, the mean correct response was almost 80%; in the soprano part–listening task, it was almost 85%. The regions activated during the harmony-listening task, but not during the soprano part–listening task, are listed in Table 1, together with stereotactic coordinates based on the brain atlas of Talairach and Tournoux (14). These results show areas of relative blood flow changes that emphasize differences between the two music-listening tasks and minimize the areas that are common to both. Significant increases in rCBF were found in the anterior portion of the bilateral temporal lobes, bilateral cingulate gyri, and the cerebellum (Fig 1). The soprano part–listening task produced a significantly greater activation compared with the harmony-listening task in the bilateral superior parietal lobules, bilateral occipital lobes, and right precuneus (Fig 2; Table 2).
TABLE 1:
Regions showing significant changes in rCBF during the harmony-listening task
| Anatomical Structure | Brodmann Area | Talairach Coordinate |
Z score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Anterior portion of temporal lobe: | 38/20 | ||||
| L | −46 | 21 | −25 | 3.61 | |
| −44 | −15 | −32 | 2.96 | ||
| R | 35 | 3 | −38 | 3.04 | |
| 48 | 1 | −28 | 3.41 | ||
| Cingulate gyrus: | 24/32 | ||||
| L | −3 | 5 | 36 | 3.12 | |
| −6 | 17 | 34 | 3.09 | ||
| R | 6 | 30 | 18 | 3.96 | |
| 3 | 10 | 40 | 3.25 | ||
| Cerebellum: | |||||
| L | −10 | −53 | −40 | 5.16 | |
| R | 10 | −62 | −25 | 4.18 | |
Note.—Coordinates x, y, and z are in millimeters, corresponding to the atlas of Talairach and Tournoux. The x coordinate refers to medial-lateral position relative to midline (negative = left); y coordinate anterior-posterior position relative to the anterior commissure (positive = anterior); z coordinate superior-inferior position relative to the anterior commissure-posterior commissure line.
Fig 1.
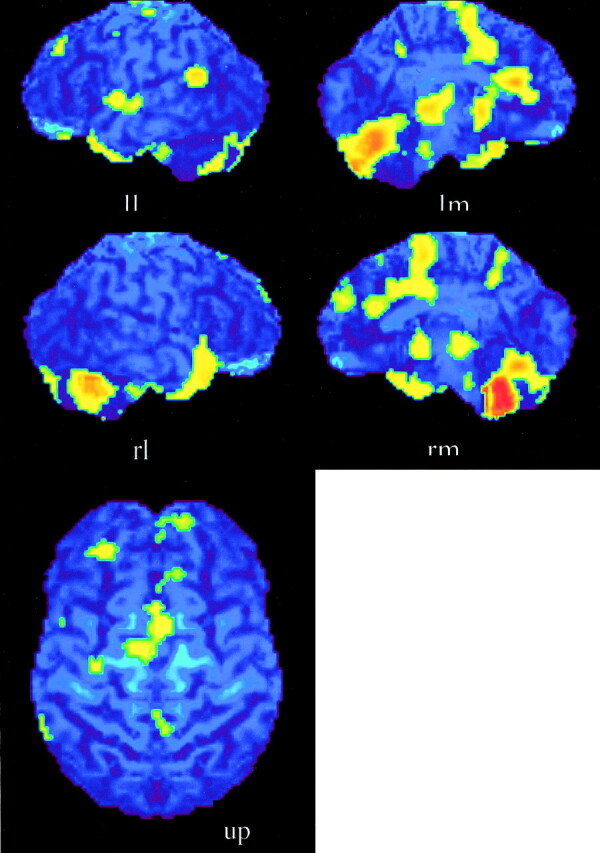
Activation maps for the subtraction of the harmony-listening versus the soprano part–listening tasks. Areas of significant activation (P < .01) are superimposed onto the surface maps of the averaged MR imaging of the brains of eleven subjects. Anterior portions of temporal lobes, anterior cingulate gyri, and the cerebellum are bilaterally activated. Lateral surface of left hemisphere (ll); medial surface of left hemisphere (lm); lateral surface of right hemisphere (rl); medial surface of right hemisphere (rm); upper surface (up). The left side of the bottom-image shows the left side of the brain.
Discussion
Anterior Portion of the Bilateral Temporal Lobes Participates in Music Perception
During the harmony-listening task, the anterior portion of the bilateral temporal lobes, the bilateral cingulate gyri, and the cerebellum were significantly activated. As for the activation of the latter two regions, we speculate that, as shown in our previous PET activation study (10), the bilateral cingulate gyri and the cerebellum were activated in relation to mental processes involved in trying to identify the musical stimuli and the anticipation of a new learned task, respectively. In a PET study of functional neuroanatomy in nonmusicians, Platel and colleagues (15) reported that the left cingulate gyrus was activated during a familiar task that required subjects to answer whether the tonal sequence brought to mind something that they knew. In our experiment, our subjects might also have tried to judge whether they had ever listened to that musical piece. Several reports have suggested that the cerebellum participates in the organization of higher cognitive functions, especially in the performance of difficult, newly learned tasks (16, 17). We propose that, in the present study, the cerebellum might also have been activated by the anticipation of the unfamiliar musical task that required our subjects to deal with musical pieces that were unknown to them.
The most characteristic finding was the activation of the anterior portion of the bilateral temporal lobes, including the bilateral temporal poles, which were the same regions as those identified in our previous PET study (10). We suppose that these regions might participate in the discrimination of chords, because, in the harmony-listening task in this and our previous PET study, nonmusicians and musicians were required to listen to the harmony as a whole, and to judge whether the sound was dissonant or minor, respectively. Additional support for the participation of the anterior portion of the bilateral temporal lobes in music perception comes from our recent lesion study (18). Following bilateral infarctions in the anterior portion of the temporal lobes, our patient manifested the following deficits in the perception of music: 1) the recognition and discrimination of familiar melodies, 2) the discrimination of unfamiliar melodies, and 3) the discrimination of chords. In the task of the discrimination and recognition of familiar melodies, 30 familiar nursery songs were chosen and, in 20 of these songs, a part of the melody was altered. The patient had to give a “familiar-unfamiliar” and “correct-incorrect” response. For the latter response, the patient was required to compare the target melody that was emitted by the examiner with the melody that was stored in his or her long-term memory and to judge whether the target melody was correct. In the task of the discrimination of unfamiliar melodies, that is, the tonal memory test in Seashore measures of musical talents (19), the patient was presented with two serial unfamiliar tonal sequences and asked which tone was altered in the second one. In the task of the discrimination of chords, a pair of chords was serially presented, and the patient made a “same-different” discrimination. In these two tasks, it was necessary for the patient to compare the second tonal sequence or chord with the first one and to judge whether the first and second tonal sequence or chord were identical. We speculated that these three tasks might commonly contain the mental processing of discrimination of melodies and chords. We may, therefore, reasonably conclude that the anterior portion of the bilateral temporal lobes participates in music perception, especially in the discrimination of melodies and chords.
Regions Activated during the Soprano Part–Listening Task and the Functional Difference in Music Perception between Musicians and Nonmusicians
In the past few decades, several articles have been devoted to the study of hemispheric lateralization in listening to music. Some authors had suggested the dominance of the left hemisphere in musicians dealing with musical stimuli and of the right hemisphere in nonmusicians (20–22). During the soprano part–listening task in the present study, the bilateral superior parietal lobules, bilateral occipital lobes, and right precuneus were significantly activated, which was not in agreement with the above-mentioned hypothesis in the literature (20, 21, 22). Comparing this finding with that found during the alto part–listening task for the musicians in our previous PET study, we can summarize the activated brain regions as follows: 1) regions commonly activated, 2) regions activated only in musicians, and 3) regions activated only in nonmusicians.
First, both in nonmusicians and musicians, the bilateral superior parietal lobules and the right precuneus were commonly activated. We thought that these regions participated in auditory selective attention. The superior parietal lobules belong to the posterior attention network and are thought to be involved in selective attention (23). Le and colleagues (24) reported that shifting visual attention produced an activation of the bilateral superior parietal lobules and the cuneus or precuneus or both. In the present study, our subjects listened to the soprano part within harmony in which three vocal parts were sounding simultaneously. So, for the alto part–listening task of the previous study, the superior parietal lobules and right precuneus might be activated by auditory selective attention during the soprano part–listening task.
Second, for the brain regions activated only in musicians, we can identify the left precuneus and the bilateral frontal areas. We supposed that the activation of the left precuneus in musicians was derived from imagery processing of a mental score. This hypothesis is supported by the reported literature and our previous PET study. Platel and colleagues (15) reported that, during a pitch task, the left cuneus or precuneus was activated. They thought that this region might participate in a mental imagery strategy employed to perform the pitch task, namely, in writing the tones on a mental score. In our previous experiment, musicians listened to the alto part of the harmony and were required to make a sign when they listened to a dominant or tonic tone. The subjects had to write the notes of the alto part on a mental score to analyze the pitch of each tone. By contrast, in the present study, nonmusicians were required only to respond to the end of phrases, so they did not need to perform the pitch analysis. Therefore, we suppose that, only in the musicians, the imagery processing for writing notes on a mental score resulted in the activation of the left precuneus. With regard to the activation of the frontal regions in musicians, we conclude that the association of the sound of musical tones and the notes on a mental score participated in the activation of these regions. Some authors have suggested that the prefrontal regions had a relationship with the formation of the association between tones and visual events (25) and of the verbal-tonal association (26). In the alto part–listening task of the previous study, musicians had to name each tone of the alto part. Although the subjects did not read the scores visually, they needed to form and read mental images of the scores. They integrated the information of the sound of the musical tone with that of the note on a mental score. Therefore, we speculate that the tonal-verbal association might function only in musicians and that the demands of integration between auditory and visual or verbal information resulted in the activation of the frontal regions in the previous study.
Finally, the lateral surface of the bilateral occipital lobes (Broadmann area 19) was activated only in nonmusicians. Zatorre et al (27) reported that, when subjects listened to novel tonal sequences, the right posterior cortex (area 19) was activated. Although its exact function remains unclear, the posterior cortex might be involved in the perception of unfamiliar melodies.
Conclusion
During the harmony-listening task, activated brain regions were almost the same in musicians and nonmusicians. The most characteristic finding was the activation of the anterior portion of the bilateral temporal lobes, which we suggest are vital in the discrimination of melodies and chords. By contrast, the activated brain regions were different in musicians and nonmusicians during their listening to a certain vocal part of music; namely, the alto and soprano parts. The frontal regions were activated only in musicians, because they used the verbal-tonal association for pitch analysis. The results of our present and previous PET studies lead to the conclusion that the differences in activated brain regions between musicians and nonmusicians are caused by differences in the involvement of the neural networks in performing the musical task. Further studies are needed to clarify whether there are specific brain regions that function only in musicians.
Fig 2.
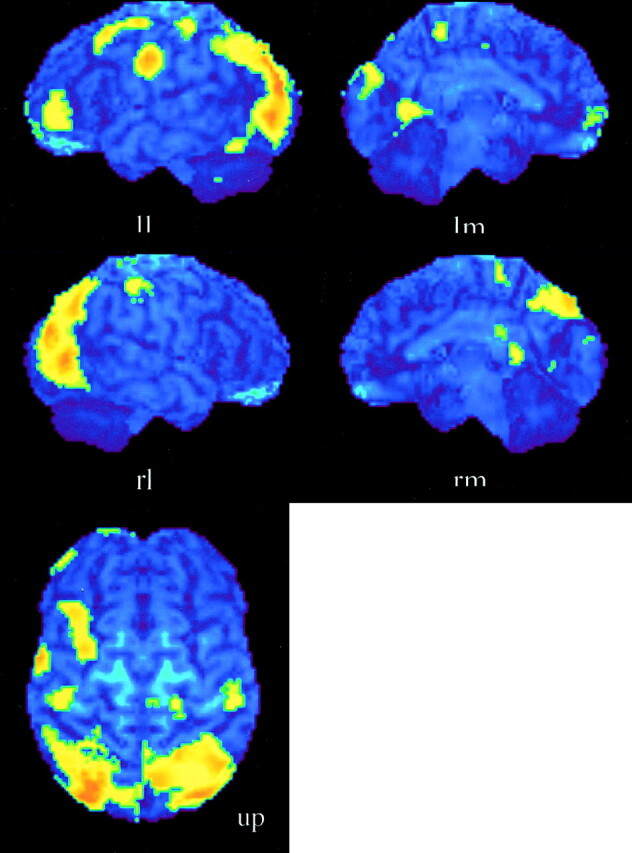
Activation maps for the subtraction of the soprano part–listening minus versus the harmony-listening conditions. Areas of significant activation (P < .01) are superimposed onto the surface maps of the mean formatted averaged MR images of the brains of the eleven subjects. Bilateral superior parietal lobules occipital lobes, and right precuneus were remarkably activated. Areas in bilateral premotor and orbital frontal cortices were also activated. The left side of the bottom image shows the left side of the brain.
TABLE 2:
Regions showing significant changes in rCBF during the soprano part–listening task
| Anatomical Structure | Brodmann Area | Talairach Coordinate |
Z score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Superior parietal: | 7 | ||||
| L | −21 | −55 | 45 | 3.79 | |
| R | 24 | −49 | 43 | 3.62 | |
| Occipital lobe: | 18/19 | ||||
| L | −28 | −78 | 20 | 4.04 | |
| −42 | −69 | 4 | 3.98 | ||
| R | 26 | −69 | 27 | 4.21 | |
| 30 | −82 | 18 | 4.19 | ||
| Precuneus: | 7 | ||||
| R | 10 | 73 | 40 | 3.73 | |
Note.—For explanation of coordinates, see Table 1.
References
- 1.Loring DW, eds. INS dictionary of Neuropsychology. New York: Oxford University Press;1999
- 2.Lechevalier B, Eustache F, Rossa Y. Les troubles de la perception de la musique d’origine neurologique, Masson, Parigi,1985
- 3.Mazzucchi A, Marchini C, Budai R, Parma M. A case of receptive amusia with prominent timbre perception defect. J Neurol Neurosurg Psychiatry 1982;45:644–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peretz I, Kolinsky R, Tramo M, et al. Functional dissociations following bilateral lesions of auditory cortex. Brain 1994;117:1283–1301 [DOI] [PubMed] [Google Scholar]
- 5.Milner B. Laterality effects in audition. In: Mountcastle VB, ed. Interhemispheric Relations and Cerebral Dominance. Baltimore: Johns Hopkins University Press;1962. :177–195
- 6.Shankweiler D. Effects of temporal-lobe damage on perception of dichotically presented melodies. J Comp Physiol Psychol 1966;62:115–119 [DOI] [PubMed] [Google Scholar]
- 7.Zatorre RJ. Discrimination and recognition of tonal melodies after unilateral cerebral excisions. Neuropsychologia 1985;23:31–41 [DOI] [PubMed] [Google Scholar]
- 8.Samson S, Zatorre RJ. Learning and retension of melodic and verbal information after unilateral temporal lobectomy. Neuropsychologia 1992;30:815–826 [DOI] [PubMed] [Google Scholar]
- 9.Liegeois-Chauvel C, Peretz I, Babai M, et al. Contribution of different cortical areas in the temporal lobes to musica processing. Brain 1998;121:1853–1867 [DOI] [PubMed] [Google Scholar]
- 10.Satoh M, Takeda K, Nagata K, et al. Activated brain regions in musicians during an ensemble: a PET study. Cogn Brain Res 2001;12:101–108 [DOI] [PubMed] [Google Scholar]
- 11.Sadie S. The Grove Concise Dictionary of Music. London: Macmillan;1994
- 12.Kanno I, Iida H, Miura S, et al. A system for cerebral blood flow measurement using an H215O autoradiographic method and positron emission tomography. J Cereb Blood Flow Metab 1987;7:143–153 [DOI] [PubMed] [Google Scholar]
- 13.Minoshima S, Koeppe RA, Fessler JA, et al. Integrated and automated data analysis method for neuronal activation studies using [O-15] water PET. In: Uemura K, Lasen NA, Jones T, Kanno I, eds. Quantification of Brain Function, Tracer Kinetics and Image Analysis in Brain PET. Amsterdam: Excerpta Medica/Elsevier;1993;409–417
- 14.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme;1988
- 15.Platel H, Price C, Baron JC, et al. The structural components of music perception: a functional anatomical study. Brain 1997;120:229–243 [DOI] [PubMed] [Google Scholar]
- 16.Barinaga M. The cerebellum: movement coordinator or much more? Science 1996;272:482–483 [DOI] [PubMed] [Google Scholar]
- 17.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain 1998;121:561–579 [DOI] [PubMed] [Google Scholar]
- 18.Satoh M, Takeda K, Murakami Y, et al. A case of amusia caused by the infarction of anterior portion of bilateral temporal lobes Cortex (in press) [DOI] [PubMed]
- 19.Seashore CE, Lewis DL, Saetveit JG. Seashore Measures of Musical Talents. New York: Psychological Corporation;1960
- 20.Bever TG, Chiarello RJ. Cerebral dominance in musicians and nonmusicians. Science 1974;185:137–139 [DOI] [PubMed] [Google Scholar]
- 21.Mazziotta JC, Phelps ME, Carson RE, et al. Tomographic mapping of human cerebral metabolism: auditory stimulation. Neurology 1982;32:921–937 [DOI] [PubMed] [Google Scholar]
- 22.Evers S, Dannert J, Roedding D, Roetter G, Ringelstein EB. The cerebral haemodynamics of music perception: a transcranial Doppler sonography study. Brain 1999;122:75–85 [DOI] [PubMed] [Google Scholar]
- 23.Benedict RHB, Lockwood AH, Shucard JL, et al. Functional neuroimaging of attention in the auditory modality. Neuroreport 1998;9:121–126 [DOI] [PubMed] [Google Scholar]
- 24.Le TH, Pardo JV, Hu X. 4T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol 1998;79:1535–1548 [DOI] [PubMed] [Google Scholar]
- 25.McIntosh AR, Cabeza RE, Lobaugh NJ. Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. J Neurophysiol 1998;80:2790–2796 [DOI] [PubMed] [Google Scholar]
- 26.Zatorre RJ, Perry DW, Beckett CA, et al. Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci U S A 1998;95:3172–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zatorre RJ, Evans AC, Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 1994;14:1908–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]


