Abstract
Summary: We describe an unconventional endovascular approach to the treatment of traumatic carotid-cavernous fistulas. Four patients with large high-flow shunts have been successfully treated by trapping of the fistula by using a combination of proximal balloon occlusion and distal coil embolization. The latter was achieved following retrograde catheterization of the distal parent vessel via the contralateral carotid or ipsilateral vertebral artery.
Most direct carotid-cavernous fistulas (CCFs) are caused by trauma to the skull base. Most traumatic CCFs can be treated by transarterial balloon embolization or coil occlusion of the cavernous sinus with preservation of the parent artery, but in a small number of cases this approach is not possible. We describe high-flow CCFs with large venous compartments that could not be managed by conventional endovascular methods. In each instance, the fistula was successfully closed by a combination of proximal balloon occlusion and distal trapping by using coils advanced via a retrograde route through either the anterior or posterior communicating artery.
Illustrative Case Reports
Case 1
A 22-year-old male intravenous drug abuser was admitted with complex craniofacial injuries following a traffic accident. He suffered trauma to the left orbit that resulted in complete unilateral blindness. CT revealed multiple fractures affecting the skull vault, facial bones, nasoethmoidal complex, and left carotid canal. He made slow but steady progress with conservative treatment.
Four months later, he presented with progressive visual deterioration in his right eye (acuity 6/9) and slight left-sided proptosis. MR imaging showed a large expansile midline mass centered on the sphenoid sinus and posterior ethmoid air cells that was compressing the optic apparatus (Fig 1A). Conventional angiography demonstrated a left-sided high-flow fistula at the cavernous sinus with retrograde ophthalmic, petrosal, and cortical venous drainage. There was a massive venous pouch with no distal filling of the left internal carotid artery (ICA; Fig 1B and C). This large vascular structure accounted for part of the “mass” seen on MR images; the remainder of the lesion was believed to be a mucocele that had presumably developed secondary to obstruction of the sinuses either as a direct result of the injury or in response to the expanding fistula.
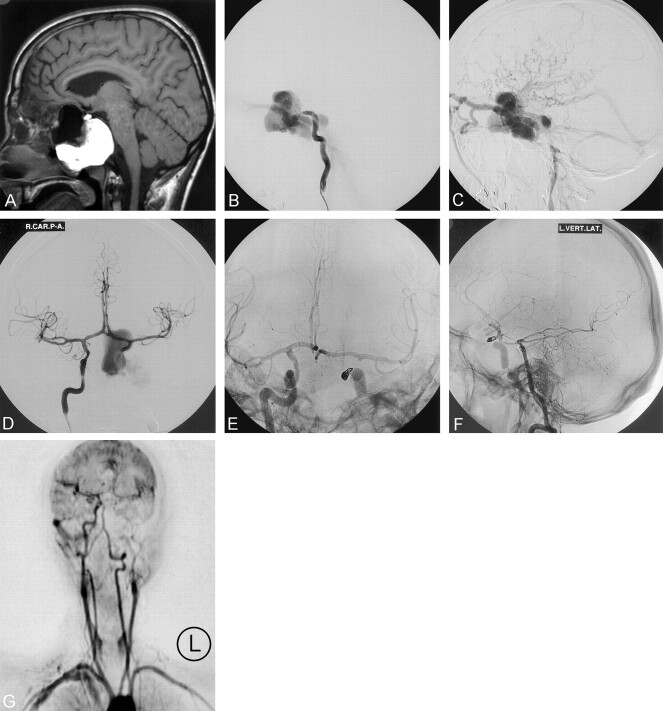
Fig 1.
A, Preoperative sagittal T1 MR image demonstrates a flow void corresponding to a large vascular compartment and a massive high signal intensity “mucocele.” Compare the black flow void with the lateral angiogram (B).
B, Preoperative lateral catheter arteriogram (left ICA). Early arterial phase shows the carotid artery terminating in an enlarged venous compartment.
C, Preoperative lateral catheter arteriogram (left ICA). Later arterial phase shows rapid arteriovenous shunt surgery into the ophthalmic, petrosal, and cortical veins
D, Frontal arteriogram (right ICA) following proximal balloon occlusion of the left carotid artery shows rapid filling of the fistula by retrograde flow.
E, Frontal arteriogram (right ICA) following coil embolization of the distal (L) carotid artery. The coils were deployed through a microcatheter that had been advanced from the right carotid artery into the left carotid via the anterior communicating artery.
F, Lateral arteriogram (left vertebral artery) following proximal balloon and distal coil trapping of the massive fistula. There is no compromise of the collateral circulation from the posterior circulation. Antegrade flow is restored within the left carotid artery via the posterior communicating artery.
G, Postoperative dynamic (1 frame/s) contrast-enhanced MR image shows isolation of the fistula and complete opacification of the left cerebral hemisphere. Six-month arteriogram (not shown) confirmed complete closure of the fistula.
Endovascular treatment was undertaken to salvage vision in the single functional eye and prevent intracranial hemorrhage. The venous compartment was felt to be too large to guarantee complete closure of the fistula with balloons or coils placed within the “cavernous sinus,” and a balloon could not be adequately positioned across the orifice of the fistula. A microcatheter could not be advanced beyond the fistula into the normal distal carotid. Therefore, the left ICA was sacrificed just proximal to the site of the fistula by using two detachable silicone balloons. This resulted in proximal occlusion of the carotid artery but prompt filling of the venous pouch continued by retrograde flow (Fig 1D). The distal left ICA was then approached from the right side via the anterior communicating artery. Using an Excel 14 microcatheter (Boston Scientific, Fremont, CA), the ICA was occluded immediately adjacent to the fistula by using three Guglielmi detachable coils (GDCs; 4 mm × 8 cm GDC-10 soft, 3 mm × 6 cm GDC-10 soft and a 3 mm × 6 cm GDC ultrasoft coil). This resulted in complete closure of the fistula with continued opacification of the left cerebral hemisphere by the collateral circulation (Fig 1E). A catheter placed in the left vertebral artery was used to assess the level of the posterior communicating and anterior choroidal arteries (Fig 1F). Follow-up dynamic contrast-enhanced MR angiography at one frame per second confirmed complete occlusion of the fistula at 6 months (Fig 1G).
Case 2
A 19-year-old man suffered multiple facial, skull base, and orbital fractures in a severe motorcycle accident. A contrast-enhanced CT scan showed large right frontal and temporal infarcts, subarachnoid blood, enhancement of the sphenoid sinus, and an enlarged right superior ophthalmic vein. He regained consciousness after 2 weeks and was blind in the right eye. Conventional angiography demonstrated a direct right-sided CCF with a large venous compartment that was confined by the right-sided sphenoid air cell (Fig 2A). The right ICA terminated in the fistula, and there was no filling of the distal carotid artery. Vertebral artery injection demonstrated retrograde filling of the intracranial portion of the right ICA from the posterior circulation via the posterior communicating artery with no onward flow to the brain.
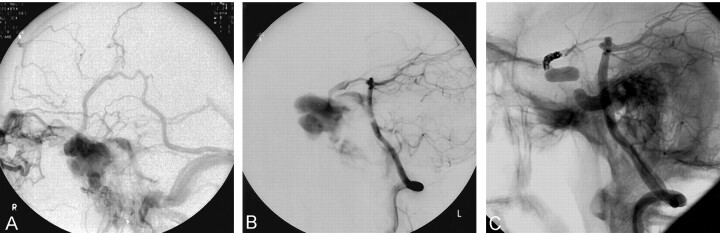
Fig 2.
A, Late phase of right internal carotid arteriogram. There is a large carotid-cavernous fistula with multidirectional venous outflow into the ophthalmic, cortical, petrosal, and pterygoid venous systems.
B, Lateral vertebral arteriogram following proximal balloon occlusion of the right ICA. There is rapid filling of the fistula via the posterior communicating artery.
C, Lateral vertebral arteriogram following proximal balloon occlusion and distal coil embolization of the right ICA, the latter performed via the posterior communicating artery. The fistula is closed.
The right anterior and middle cerebral arteries received blood from the left carotid territory via the anterior communicating artery.
Proximal balloon occlusion was performed by using two detachable silicone balloons, and the distal end of the fistula was occluded with coils. On this occasion, the distal carotid artery was approached from the left vertebral artery via the basilar, right posterior cerebral, and posterior communicating arteries (Fig 2B). The fistula was closed completely with seven GDCs (2 and 3 mm) placed in the supraclinoid carotid artery (Fig 2C).
Discussion
Most direct communications between the ICA and the cavernous sinus occur following skull base trauma or aneurysm rupture (1–3). The symptoms and sequelae of CCFs are caused by arterialization of the venous outflow from the cavernous sinus and include proptosis, ophthalmoplegia, impaired visual acuity, and intracranial hemorrhage (4).
Many traumatic CCFs can be successfully treated with preservation of the carotid artery. This is usually achieved by placing detachable balloons in the cavernous sinus by a transarterial route (1, 2). There are occasions, however, when this method is not feasible. Technical failure may occur, for example, if the rent in the vessel wall is too small to allow passage of a balloon or if sharp bony fragments or foreign bodies rupture the balloon during inflation (5). In these circumstances, the fistula may be treated with platinum coils placed in the cavernous sinus, either by a transarterial (6) or transvenous approach (5). In some patients, it may not be possible to achieve complete closure of the fistula, but subtotal occlusion may be sufficient to alleviate the patient’s symptoms. When using either balloons or coils, however, it is important not to redirect or increase venous drainage into the ophthalmic or cortical veins, because this may aggravate ocular symptoms or precipitate intracranial hemorrhage.
Certain fistulas present further difficulties and cannot be completely occluded without parent vessel sacrifice. We have encountered four such cases, two of which are described above. In each instance, we encountered a torrential arteriovenous shunt into a markedly enlarged “cavernous sinus” through a large rent in the arterial wall. As a result of the skull base fractures, the venous compartments receiving the arterialized blood had massively enlarged in three cases and were partially confined by the bony margins of the sphenoid sinus. It was felt that the fistulas could not be completely closed without sacrifice of the parent vessel. It was impossible to place a balloon(s) in a stable position across the orifice of the arteriovenous shunt, and we were unable to navigate either a balloon or microcatheter beyond the fistula to allow distal control. The procedures could have been simplified if the microcatheter could have been positioned distal to the fistula via the ipsilateral artery by using temporary balloon inflation to produce flow arrest, but it was agreed that this would probably have failed because of exaggeration of the retrograde flow to the fistula. Therefore, we elected to occlude the carotid artery, proximal to the fistula, with detachable balloons. Then, with the aid of marked retrograde flow to the fistula, a microcatheter was navigated into the supraclinoid segment of the carotid artery via the anterior communicating (three cases) or posterior communicating arteries (one case). Using a combination of soft and ultrasoft GDCs it was possible to completely occlude the “distal” carotid artery over a relatively short segment in three cases without occluding either the posterior communicating or anterior choroidal artery.
Distal trapping with coils proved a relatively straightforward procedure, and this is largely due to high-quality braided hydrophilic microcatheters and ultrasoft coils that are now available. In one case, however, the retrograde shunt into the fistula was so great that the parent vessel could not be closed with bare platinum coils, because the coil mass was unstable and kept migrating into the venous pouch. The vessel was finally occluded by using retrievable fibered coils that are a somewhat more difficult to deploy but are much more thrombogenic than are conventional GDCs. To deploy the fibered coils, it was necessary to use a larger microcatheter (Excelsior 1018, Boston Scientific). The increased size of this microcatheter, however, resulted in nearly complete flow arrest of the right middle cerebral artery. The coiling was therefore performed rapidly under systemic heparinization (three times normal) and induced hypertension (phenylephrine). In less than 6 minutes, three fiberd coils were deployed and the microcatheter was withdrawn. Normal flow spontaneously returned to the right cerebral hemisphere. Immediately on waking he experienced flaccid monoplegia (left arm) that totally resolved within 45 minutes. If it had proved impossible to gain access to the distal carotid via the collateral circulation, surgical ligation would have been required.
Retrograde catheterization of the carotid artery by using the circle of Willis has been described elsewhere (7–9), usually when an ipsilateral approach was contraindicated because of proximal vessel occlusion. Use of the communicating arteries is not without risk, however, because these routes may form part of crucial collateral pathways to brain tissue. Wilms (9) reports transient occlusion of a middle cerebral artery when placing a microcatheter across the anterior communicating artery, possibly because of vasospasm or thrombosis.
There are other alternatives to the procedure we have described, such as Onyx (MicroTherapeutics, Inc, Irvine, CA) a liquid embolic agent that has been used with success for the treatment of large or giant aneurysms (10) and arteriovenous malformations. Using a stent placed across the orifice of the fistula may prove safe to occlude the cavernous sinus with this novel agent. Also, there is likely to be increasing use of intracranial stents for a number of clinical applications including CCF (11, 12). The current generation of covered coronary stents, however, are rather stiff and difficult to navigate in tortuous vessels. Both these techniques should allow preservation of the carotid artery but are still somewhat experimental and are probably associated with greater risks than the procedure we describe. Islak et al have recently described an elegant technique of deploying a short, covered coronary stent within a longer open stent for the treatment of large aneurysms (13). To our knowledge, this technique has not yet been attempted with traumatic CCF, but it seems likely that it would succeed if the origin of the fistula remains below the carotid siphon.
Conclusion
A small number of traumatic CCFs will be incurable without parent vessel sacrifice. We have treated four such fistulas, all of which were closed by a technique of endovascular trapping by using proximal balloon occlusion and distal coiling of the parent vessel.
References
- 1.Debrun GM, Vinuela F, Fox A, et al. Indications for treatment and classification of 132 carotid-cavernous fistulas. Neurosurgery 1988;22:285–289 [DOI] [PubMed] [Google Scholar]
- 2.Higashida RT, Halbach VV, Tsai FY, et al. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases. AJR Am J Roentgenol 1989;153:577–582 [DOI] [PubMed] [Google Scholar]
- 3.Phatouros CC, Meyers PM, Dowd CF, et al. Carotid artery cavernous fistulas. Neurosurg Clin North Am 2000;11:67–84 [PubMed] [Google Scholar]
- 4.Lasjaunias P, Ming C, Brugge KT, Atul T. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 1986;64:724–730 [DOI] [PubMed] [Google Scholar]
- 5.Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of direct carotid cavernous fistulas. AJNR Am J Neuroradiol 1988;9:741–747 [PMC free article] [PubMed] [Google Scholar]
- 6.Halbach VV, Higashida RT, Barnwell SL, et al. Transarterial platinum coil embolization of carotid-cavernous fistulas. AJNR Am J Neuroradiol 1991;12:429–433 [PMC free article] [PubMed] [Google Scholar]
- 7.Debrun GM, Ausman JI, Charbel FT, et al. Access to the cavernous sinus through the vertebral artery: technical case report. Neurosurgery 1995;37:144–147 [DOI] [PubMed] [Google Scholar]
- 8.Luo CB, Chong FC, Teng MM, et al. Endovascular embolization of carotid-cavernous fistula using the posterior communicating artery approach: a case report. Kaohsiung J Med Sci 2001;17:112–115 [PubMed] [Google Scholar]
- 9.Wilms G, Demaerel P, Lagae L, et al. Direct caroticocavernous fistula and traumatic dissection of the ipsilateral internal carotid artery: endovascular treatment. Neuroradiology 2000;42:62–65 [DOI] [PubMed] [Google Scholar]
- 10.Mawad ME, Cekirge S, Ciceri E, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg 2002;96:474–482 [DOI] [PubMed] [Google Scholar]
- 11.Kocer N, Kizilkilic O, Albayram S, et al. Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. AJNR Am J Neuroradiol 2002;23:442–446 [PMC free article] [PubMed] [Google Scholar]
- 12.Weber W, Henkes H, Berg-Dammer E, et al. Cure of a direct carotid cavernous fistula by endovascular stent deployment. Cerebrovasc Dis 2001;12:272–275 [DOI] [PubMed] [Google Scholar]
- 13.Islak C, Kocer N, Albayram S, et al. Bare stent-graft technique: a new method of endoluminal vascular reconstruction for the treatment of giant and fusiform aneurysms. AJNR Am J Neuroradiol 2002;23:1589–1595 [PMC free article] [PubMed] [Google Scholar]