Abstract
Summary: We herein describe a 12-year-old male patient with a germinoma of the basal ganglia who presented with progressive hemiparesis. MR imaging showed ipsilateral cerebral hemiatrophy predominantly in the left basal ganglia, whereas no mass or enhancement was depicted. Single photon emission CT revealed no significant uptake of thallium, whereas 11C-methionine positron emission tomography showed clearly discernible uptake in the left putamen. Stereotactic biopsy, referencing the results of 11C-methionine positron emission tomography, was performed, allowing histologic verification of germinoma to be established. 11C-methionine positron emission tomography was the only technique that indicated the precise localization of the tumor in our patient and enabled biopsy-based final diagnosis of the basal ganglia germinoma without any overt mass formation.
Approximately 5% to 10% of intracranial germinomas arise from the basal ganglia. Early diagnosis of this type of tumor is often difficult because its progression is very slow and it shows only subtle signal intensity change or no mass effect on MR images obtained during the early course of the disease (1, 2). Nonetheless, early diagnosis is crucial because germinoma is highly sensitive to radiation therapy and chemotherapy and delayed treatment can result in more severe neurologic deficits (3, 4). Recently, single photon emission CT and positron emission tomography (PET) have been used to detect brain tumors. In particular, 11C-methionine PET (MET-PET) has been reported to be beneficial for the evaluation of the metabolic activity of malignant brain tumors (5). However, the usefulness of MET-PET in infiltrative germinoma remains unclear. We studied a case of basal ganglia germinoma that showed only brain hemiatrophy but no mass formation and found that MET-PET was very useful for diagnosis and precise localization of the tumor.
Case Report
A 12-year-old, previously healthy and developmentally normal, left-handed Japanese male patient developed slow progressive right-sided hemiparesis, which had originated in the right hand and gradually spread to the right leg during 1.5 years. He was admitted to a hospital because of intermittent vomiting. Brain hemiatrophy was identified by using MR imaging, but a brain tumor was not suspected at that time. The patient was referred to our hospital because he was suspected of having a degenerative disorder. At admission, he could walk but was found to have right spastic hemiparesis with hyperreflexia and dystonic posture of the right hand. Right Babinski sign was positive. His mental status examination was within the normal range, but his school performance had declined.
Laboratory examination revealed mildly increased serum α-fetoprotein (560 ng/mL; reference amount, <100 ng/mL). β-human chorionic gonadotropin was not detected in the serum but was increased in CSF (1.0 mIU/mL; reference amount, <0.5 mIU/mL).
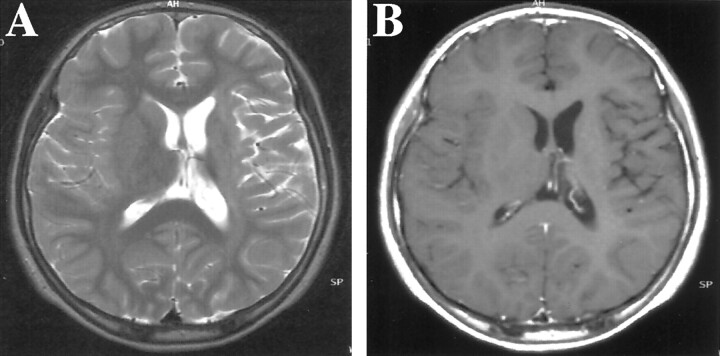
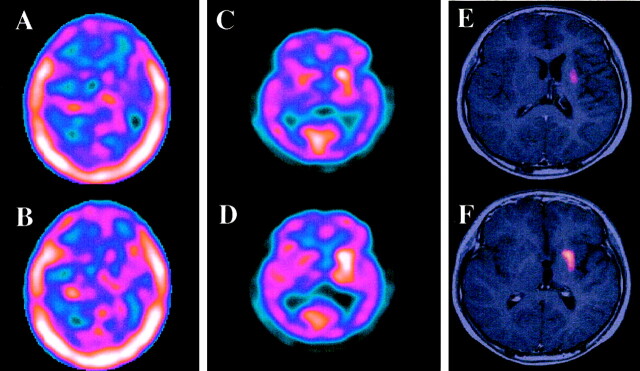
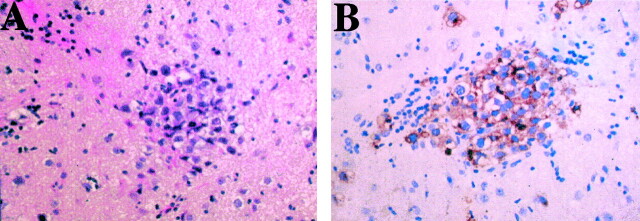
CT and MR imaging of the brain revealed left cerebral hemiatrophy, predominantly in the left basal ganglia and thalamus, without mass formation. Subtle high intensity was shown within the internal capsule on T2-weighted images (Fig 1A), but no enhancement was seen (Fig 1B). Slow growing germinoma arising from the basal ganglia was suspected, and single photon emission CT and PET studies were performed to define possible tumor localizations. Single photon emission CT with thallium-201 identified no significant uptake, whereas the MET-PET study clearly showed discernible uptake in the left putamen (Fig 2). MET-PET findings allowed us to perform CT-guided biopsy targeting the high uptake lesion. A histopathologic examination revealed the two-cell pattern of large tumor cells and small lymphocytes (Fig 3A). The tumor cells were positive on immunohistochemical staining with placental alkaline phosphatase (Fig 3B). The diagnosis of germinoma was verified. Radiation therapy of 24 Gy to a whole ventricle field and a 6-Gy boost to the left basal ganglia was administered after four courses of the induction of chemotherapy with ifosfamide, cisplatin, and etoposide. One year after treatment, the patient remained tumor free and his clinical condition had gradually improved.
Fig 1.
MR imaging findings in the case of a 12-year-old, previously healthy and developmentally normal, left-handed Japanese male patient who developed slow progressive right-sided hemiparesis, which had originated in the right hand and gradually spread to the right leg during 1.5 years.
A, Axial view T2-weighted MR image (4000/96.0 [TR/TE]) shows a high intensity area in the left internal capsule, with volume loss of the left basal ganglia and thalamus. The lateral ventricle, sulci, and sylvian fissure are dilated on the left side.
B, Contrast-enhanced T1-weighted MR image (600/14) shows no enhancement in the lesion.
Fig 2.
Comparison of Tl-weighted single photon emission CT and MET-PET findings in the same case as that shown in Figure 1.
A and B, Axial sections of thallium-201 single photon emission CT (Tl-weighted single photon emission CT) show no abnormal uptake.
C and D, Axial sections of MET-PET, obtained at the same level, show definite high uptake in the left basal ganglia.
E and F, MET-PET scans superimposed over MR images clearly show that the high uptake portion (standardized uptake value ≥1.6) on the MET-PET scans is in the left putamen.
Fig 3.
Histopathologic examination of a specimen obtained from the left putamen by stereotactic biopsy.
A, Hematoxylin and eosin staining shows two different types of cells: large atypical cells (tumor cells) and infiltrating lymphocytes (original magnification, ×200).
B, Placental alkaline phosphatase staining shows positive tumor cells (original magnification, × 200)
Discussion
Ipsilateral hemiatrophy is reported to occur in approximately 20% of germinomas originating in the basal ganglia and thalamus (2, 6). Diagnosis of germinoma in these sites is often delayed because of a slow progressive clinical course and subtle findings without space-occupying lesions shown on CT scans or MR images (1–3, 6). A male patient younger than 25 years with gradually progressive hemiparesis and cerebral hemiatrophy involving the basal ganglia should be suspected of having germinoma, even in the absence of any overt mass lesion (3). For our patient, CT, MR imaging (plain and enhanced), and thallium single photon emission CT failed to detect specific locations of the tumor. Nevertheless, MET-PET readily detected the lesion of the hot spot. This finding was critical to locating the precise biopsy target lesion, which ensured successful treatment. This is a rare report in which only MET-PET clearly defined the localization of an intracranial brain tumor.
Imaging that uses amino acid metabolism, such as MET-PET, has been adopted, particularly for imaging of malignant tumors, because amino acid transport is generally increased in malignant tumors and because this imaging modality is less influenced by inflammation in comparison with fluorine-18-fluorodeoxyglucose-PET (5). In contrast to fluorine-18-fluorodeoxyglucose, background uptake of MET in normal brain tissue is very low; therefore, MET-PET provides excellent contrast, particularly of brain tumors from the background (5, 7).
MET-PET has been reported to be superior to other modalities in detecting the extent or staging of gliomas (7–11). Sasaki et al (7) investigated the extent of tracer uptake in 23 patients with astrocytic tumors and showed that increased MET-PET uptake was greater than that of thallium single photon emission CT or fluorine-18-fluorodeoxyglucose-PET in most cases. Moreover, MET uptake of malignant astrocytoma was significantly greater than that of benign astrocytoma. Sato et al (9) found that a positive correlation was present between the proliferative capacity of the brain tumor and MET-PET uptake and that MET-PET uptake was significantly greater in high grade gliomas than in low grade gliomas. However, few reports of MET-PET imaging of lesions other than other brain tumors, including germinomas, have been presented. With germinomas, abnormalities may not be detected by CT or MR imaging during the early stage of the tumor, although the tumor is later seen as a space-occupying lesion with mass effect (3). Therefore, we speculate that MET-PET is also worthwhile for imaging germinomas, particularly in cases without space-occupying lesions. Nonetheless, we should keep in mind that MET uptake has been observed also in inflammatory processes (5) and cerebrovascular diseases (12).
Conclusion
This case report suggests the usefulness of MET-PET in the detection of basal ganglia germinoma without overt mass formation. MET-PET should be considered for pediatric patients with progressive hemiparesis and the MR imaging finding of only cerebral hemiatrophy.
References
- 1.Higano S, Takahashi S, Ishii K, Matsumoto K, Ikeda H, Sakamoto K. Germinoma originating in the basal ganglia and thalamus: MR and CT evaluation. AJNR Am J Neuroradiol 1994;15:1435–1441 [PMC free article] [PubMed] [Google Scholar]
- 2.Kim DI, Yoon PH, Ryu YH, Jeon P, Hwang GJ. MRI of germinomas arising from the basal ganglia and thalamus. Neuroradiology 1998;40:507–511 [DOI] [PubMed] [Google Scholar]
- 3.Sawamura Y, Shirato H, Nicholas de Tribolet. Intracranial Germ Cell Tumor. New York: Springer-Verlag;1998. :104–106
- 4.Sawamura Y, Shirato H, Ikeda J, et al. Induction chemotherapy followed by reduced-volume radiation therapy for newly diagnosed central nervous system germinoma. J Neurosurg 1998. :66–72 [DOI] [PubMed]
- 5.Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–445 [PubMed] [Google Scholar]
- 6.Soejima T, Takeshita I, Yamamoto H, Tsukamoto Y, Fukui M, Matsuoka S. Computed tomography of germinomas in basal ganglia and thalamus. Neuroradiology 1987;29:366–370 [DOI] [PubMed] [Google Scholar]
- 7.Sasaki M, Kuwabara Y, Yoshida T, et al. A comparative study of thallium-201 SPET, carbon-11 methionine PET and fluorine-18 fluorodeoxyglucose PET for the differentiation of astrocytic tumours. Eur J Nucl Med 1998;25:1261–1269 [DOI] [PubMed] [Google Scholar]
- 8.Voges J, Herholz K, Holzer T, et al. 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg 1997;69:129–135 [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Suzuki M, Kuwata N, et al. Evaluation of the malignancy of glioma using 11C-methionine positron emission tomography and proliferating cell nuclear antigen staining. Neurosurg Rev 1999;22:210–214 [DOI] [PubMed] [Google Scholar]
- 10.Derlon JM, Chapon F, Noel MH, et al. Non-invasive grading of oligodendrogliomas correlations between in vivo metabolic pattern and histopathology. Eur J Nucl Med 2000;27:778–787 [DOI] [PubMed] [Google Scholar]
- 11.Ribom D, Eriksson A, Hartman M, et al. Positron emission tomography (11)C-methionine and survival in patients with low-grade gliomas. Cancer 2001;92:1541–1549 [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, Kuwabara Y, Sasaki M, et al. 11C-methionine uptake in cerebrovascular disease: a comparison with 18F-fDG PET and 99mTc-HMPAO SPECT. Ann Nucl Med 2002;16:207–211 [DOI] [PubMed] [Google Scholar]