Abstract
BACKGROUND AND PURPOSE: Pilomyxoid astrocytoma (PMA) is a recently described tumor that typically occurs in the chiasmatic-hypothalamic region in young children and has unique histopathologic and clinical characteristics. These tumors have been previously diagnosed as pilocytic astrocytoma (PA). PMA appears to have a higher rate of recurrence and CSF dissemination than typical PA.
METHODS: We analyzed MR findings in four patients with PMA and compared them with those of typical chiasmatic-hypothalamic PA.
RESULTS: MR findings of PMA were chiasmatic or hypothalamic enhancing solid tumor with hydrocephalus, highly homogeneous T2 signal intensity that extended into the deep white and gray matter, and CSF dissemination.
CONCLUSION: Larger series are needed before the MR imaging findings of chiasmatic or hypothalamic enhancing solid tumor with hydrocephalus, highly homogeneous T2 signal intensity extending into the deep white and gray matter, and CSF dissemination can be used in the differential diagnosis of such tumors.
Pilomyxoid astrocytoma (PMA) is a recently described astrocytic tumor with unique histopathologic and clinical characteristics (1). Some authors have defined these tumors as a pilocytic astrocytoma (PA) variant and named them an infantile type of PA, because they often occur very early in life (2). Others have described them as a separate entity or an atypical variant of PA (3, 4). The most pertinent features of PMA that differentiate it from typical PA are a higher rate of recurrence and CSF dissemination (1, 5). MR imaging has a major role in preoperative assessment of PMA for an appropriate therapeutic algorithm. In this article, we evaluate the MR imaging findings of PMA and compare them with the characteristic features of typical chiasmatic-hypothalamic PA.
Description of Cases
We analyzed the imaging findings in four patients with the diagnosis of PMA (three male, one female; age range, 5 months to 2 years [mean age at the presentation, 15.8 months]). Patients presented with failure to thrive (two cases), feeding difficulties, and developmental delay. MR imaging of the brain was performed in all patients, and two underwent MR imaging of the spine. All MR studies were performed on a 1.5-T GE Signa MR system (General Electric Medical Systems, Milwaukee, WI) by using the standard quadrature birdcage head and spine coil. In all four patients, gadopentetate dimeglumine (0.1 mmol/kg) was administered.
MR images were reviewed to localize lesions as well as to analyze composition (ie, solid versus cystic) and enhancement patterns of PMAs. Images were also reviewed to determine whether metastasis to surrounding tissues existed. Typical localization was determined in the areas of the optic chiasm and hypothalamus. The composition of PMAs was 100% solid in two patients, >90% solid in one patient, and >70% solid in one patient. Histopathologic analysis revealed that two of the solid tumors had small, central necrotic areas. Mass effect and encasement of surrounding vessels was extensive in two patients and moderate in two patients. Basilar and spinal meningeal enhancement was present in three and two patients, respectively. Extension of tumor to deep gray matter and white matter was found in two patients. Two patients had mild to moderate hydrocephalus. MR imaging findings and clinical features of our patients were compared with those of typical chiasmatic-hypothalamic PA (Table 1). A sample of our PMA case with spinal seeding via CSF is shown in Figure 1 .
Clinical and Imaging Findings in Four Patients with the Diagnosis of PmA Compared with that of Chiasmatic/Hypothalamic PA
| MR Characteristics | PmA Patients |
Typical PA in Chiasmatic/Hypothalamic Region | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Composition solid versus cystic | >70% solid | >90% solid | 100% solid | 100% solid | Mostly solid possibly cystic |
| Mass effect and encasement of surrounding vessels at time of presentation | Moderate | Extensive | Extensive | Moderate | Mild to moderate |
| T1 signal intensity | Hypointense | Hypointense | Hypointense | Hypointense | Hypointense, cystic portion CSF isointense |
| T2 signal intensity | Hyperintense | Hyperintense | Hyperintense | Hyperintense | Hyperintense |
| Contrast enhancement of solid portion and cyst wall | Homogenous and thick peripheral enhancement | Homogenous | Homogenous | Homogenous | Variable, may not enhance |
| Basilar meningeal enhancement | No | Yes | Yes | Yes | No |
| Spinal meningial enhancement | n/a | Yes | Yes | n/a | No |
| Extension of T2 to deep gray/white matter | Yes | No | No | Yes | Yes |
| Location (optic chiasm/hypothalamus) | Optic chiasm/hypothalamus | Optic chiasm/hypothalamus | Optic chiasm/hypothalamus | Optic chiasm/hypothalamus | Optic chiasm/hypothalamus |
| Calcification, ossification | No | No | No | No | Yes (10%) |
| Hydrocephalus | Yes (mild to moderate) | None | Mild (slight prominence temp. horns) | None | Mild |
| Necrosis | No | No | Yes | Yes | No |
| Hemorrhage | No | No | No | No | No |
| Association with neurofibromatosis 1 | No | No | No | No | Yes |
| Age at presentation/sex | 2 years/boy | 5 months/boy | 2 years/girl | 10 months/boy | Childhood (2–6 years) |
| Clinical presentation | Failure to thrive | Feeding difficulties | Failure to thrive | Developmental delay | Nonspesific, similar to PMA |
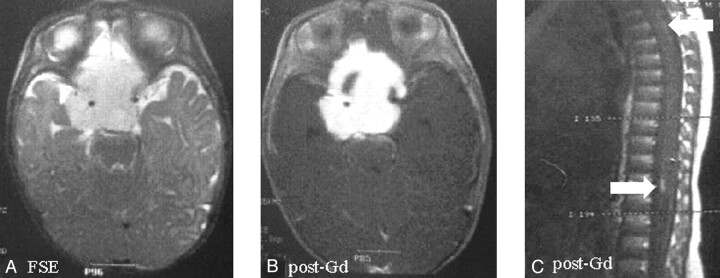
Fig 1.
Suprasellar pilomyxoid astrocytoma in a 5-month-old boy (patient 2). The mass, surrounding the circle of Willis, shows hyperintense signal on fast spin-echo images (A) and homogeneous enhancement after contrast material administration except for right anterior small cystic component (B). Peripheral pial enhancement of spinal cord on postcontrast sagittal T1 spin-echo images was detected (C).
Three of the patients reported in this study were included in a previously published report (1). All tumors consisted of monomorphic and piloid cells in a prominent myxoid background. All had a compact architecture with marked vascularity and an angiocentric pattern. The angiocentric arrangement of cells was reminiscent of perivascular pseudorosettes. There were no eosinophilic granular bodies or Rosenthal fibers. Tumor necrosis was seen in two patients. None of the specimens had glomeruloid vascular proliferation, calcification, or ossification. These histologic features mirror those described in the previous report (1).
All patients had undergone surgical excision of tumor. Two of the patients had rapid regrowth of the tumor, and each had undergone a decompressive reoperation. Chemotherapy was instituted for all four patients. None of the patients showed diencephalic syndrome. Two patients were alive with disease 30 and 36 months after initial surgery. One patient died of tumor after 61 months. The other patient was lost to follow-up 26 months after initial surgery.
Discussion
PMA can be defined as either a variant of PA or a separate entity with monomorphous pilomyxoid characteristics (1, 5). We have elsewhere demonstrated the monomorphous cellular composition and a higher rate of recurrence compared with typical PA (1). Progression-free survival at 1 year was 38.7% in PMA and 69.2% in PA (1).
PA is an indolent tumor typically located in the cerebellum, optic nerve, and hypothalamic-chiasmatic region. Although anaplastic evolution or CSF dissemination was reported for PA, this has been extremely rare (5, 6). The postoperative survival rate for PA is 86–100% at 5 years, 83% at 10 years, and 70% at 20 years (6). Survival rate with gross total tumor resection is 100%. Long-term survival is expected even in subtotally resected tumors. A small number of PAs tend to be aggressive (7).
There are definite histologic differences between PMA and PA. Eosinophilic granular bodies, Rosenthal fibers, plump “protoplasmic” cells, and a biphasic pattern are extremely rare in PMA but common in PA. PMA has a monomorphous myxoid background and angiocentric pattern, which are not common in PA. Both tumor types have abundant piloid cells (1).
The radiologic appearance of chiasmatic hypothalamic PA is characteristic (7–9). In general, the tumors do not have peritumoral edema. They may have a solid nodular and cystic part. Contrast enhancement is variable. The walls of PA consist of nonneoplastic-compressed brain parenchyma that typically does not enhance, although occasionally some cases will show mural enhancement. Obstructive hydrocephalus occurs late (usually mild to moderate), and 10% of PAs have calcification (8). PA may be associated with neurofibromatosis and involve the optic pathways (10, 11). With this typical radiologic appearance and age of the patient, the diagnosis of typical PA can be made with confidence (8).
PMA is a relatively newly described entity; there are no reports on imaging findings of these unusual tumors. In our study, the most distinguishing radiologic features suggestive of PMA as opposed to a typical PA are predominantly solid tumor, homogeneous enhancement, hydrocephalus, extension of T2 signal intensity abnormality into the deep white mater and gray matter, and CSF dissemination.
Reports of atypical PA with more aggressive and worse clinical outcome have been clearly documented in the past. We have identified a substantial number of the PMA patients in our original study from such cases. It may be equally possible that the incidence of PMA may be much higher than that perceived among such aggressive tumors.
Treatment of PAs is mainly surgical debulking, after which the tumors remain stable for many years. Despite slow growth of the solid component, multiple cystic recurrences can be observed, and they can be treated by stereotactic surgical drainage, intracystic irradiation with P32, or craniotomy (5). By contrast, PMAs are more likely to recur and disseminate in CSF, and recurrence may be more solid than cystic. PMAs usually require adjuvant treatment (radiation therapy or chemotherapy) to control the disease. There is no specific adjuvant therapy protocol for PMA (1, 5).
Conclusion
The findings of a chiasmatic or hypothalamic enhancing solid tumor with hydrocephalus, highly homogeneous T2 signal intensity, extension of T2 signal intensity abnormality into the deep white and gray matter, and CSF dissemination characterize the radiologic features of PMA. Larger series are needed before these MR imaging findings can be used in the differential diagnosis from typical PAs.
References
- 1.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol 1999;58:1061–1068 [DOI] [PubMed] [Google Scholar]
- 2.Fuller CE, Frankel B, Smith M, et al. Suprasellar monomorphous pilomyxoid neoplasm: an ultrastructural analysis. Clin Neuropathol 2001;20:256–262 [PubMed] [Google Scholar]
- 3.Burger P. Pathology of brain stem astrocytomas. Pediatr Neurosurg 1996;24:35–40 [DOI] [PubMed] [Google Scholar]
- 4.Krieger MD, Gonzales-Gomez I, Levy ML, McComb JG. Recurrence patterns and anaplastic change in a long term study of pilocytic astrocytomas. Pediatr Neurosurg 1997;27:1–11 [DOI] [PubMed] [Google Scholar]
- 5.Burger PC, Cohen KJ, Rosenblum MK, Tihan T. Pathology of diencephalic astrocytomas. Pediatr Neurosurg 2000;32:214–219 [DOI] [PubMed] [Google Scholar]
- 6.Obana WG, Cogen PH, Davis RL, Edwards MSB. Metastatic juvenile pilocytic astrocytoma. J Neurosurg 1991;75:972–975 [DOI] [PubMed] [Google Scholar]
- 7.Strong JA, Hatten HP Jr, Brown MT, et al. Pilocytic astrocytoma: correlation between the initial imaging features and clinical aggressiveness. AJR Am J Roentgenol 1993;161:369–372 [DOI] [PubMed] [Google Scholar]
- 8.Lee YY, Van Tassel P, Bruner JM, et al. Juvenile pilocytic astrocytomas: CT and MR characteristics. AJR Am J Roentgenol 1989;152:1263–1270 [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni AV, Amstrong DC, Drake JM. MR characteristics of malignant spinal cord astrocytomas in children. Can J Neurol Sci 1999;26:290–293 [DOI] [PubMed] [Google Scholar]
- 10.Blah J, Jaffe R, Deutsch M, Adkins JC. Neurofibromatosis and childhood tumors. Cancer 1986;57:1225–1229 [DOI] [PubMed] [Google Scholar]
- 11.Jacoby CG, Go RT, Beren RA. Cranial CT of neurofibromatosis. AJR Am J Roentgenol 1980;135:553–557 [DOI] [PubMed] [Google Scholar]