Abstract
Summary: Two cases of angiographically and surgically documented de novo intracranial aneurysm formation are reported. The first patient, a 32-year-old woman, developed two new aneurysms within a 6-month period, and the second patient, a 27-year-old woman, developed two new aneurysms within a 22-month period. In both patients, the new aneurysms were symptomatic, causing stroke and subarachnoid hemorrhage, respectively. The development of aneurysms de novo over such a short period of time has important implications for periodic imaging in patients considered to be at high risk for intracranial aneurysm formation.
De novo aneurysm formation is the occurrence of new aneurysms in locations previously shown to be normal by direct surgical exploration, angiography, or both. Although numerous cases of de novo aneurysms have been reported, little is known of the mechanisms underlying their development (1–10). Tonn et al (11) noted that cigarette smoking and a history of arterial hypertension were more frequently documented in patients with de novo aneurysms than in a control cohort. They also noted that patients with de novo aneurysms were younger and that more than a quarter of them presented with multiple aneurysms. Juvela et al (1) demonstrated women to be at greater risk for de novo aneurysm formation than men and also showed a positive, although not statistically significant, association between smoking and de novo aneurysm development. The annual risk of developing de novo aneurysms following previous aneurysm clipping has been estimated at 0.84–0.89% (1, 2). We report two cases of angiographically documented and surgically confirmed de novo aneurysm formation occurring over short periods of time (6 months and 22 months) and discuss the implications of such observations for aneurysm screening protocols.
Case Descriptions
Case 1
A 32-year-old woman with a history of renal insufficiency and arterial hypertension was investigated in May 1999 for acute headache. CT and lumbar puncture findings were negative for subarachnoid hemorrhage. MR imaging suggested the presence of a right middle cerebral artery (MCA) aneurysm, which was confirmed by digital subtraction angiography (DSA). The four-vessel angiogram was otherwise unremarkable, showing no other intracranial aneurysm. The right MCA aneurysm was clipped via a right pterional approach. Surgical exploration revealed no other lesion. An intraoperative angiogram confirmed adequate clipping of the aneurysm as well as patency of the MCA and its branches. Multiple images obtained during the intraoperative study again documented the absence of other distal right internal carotid artery (ICA) aneurysms (Fig 1A). The patient returned 6 months later to our clinic, in November 1999, with left facial paresis. Axial T2-weighted MR images revealed increased signal intensity in the right basal ganglia region consistent with an acute infarct. The right MCA was patent on DSA, without evidence of lumen narrowing or wall irregularity and without residual or recurrent MCA aneurysm. Two new aneurysms were, however, detected in the distal right ICA, in the region of the anterior choroidal artery and the posterior communicating artery (PComA), respectively (Figs 1B and C). An extensive workup failed to identify any other source of emboli, and no cause was found for her renal insuffiency. There was in particular no evidence of vasculitis and no history of illegal drug use. The patient underwent another right pterional craniotomy for clipping of the right PComA aneurysm and partial clipping and wrapping of the anterior choroidal region aneurysm.
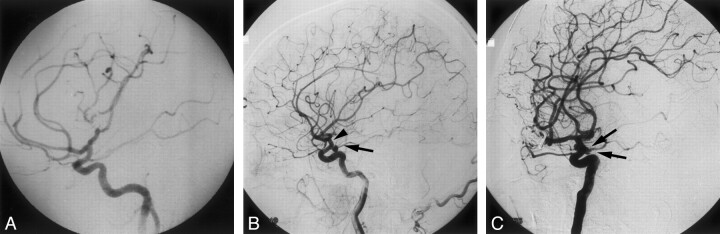
Fig 1.
Patient 1, a 32-year-old woman with two de novo aneurysms.
A, DSA, right carotid injection, lateral view, showing adequate clipping of a right MCA aneurysm and absence of additional aneurysms in the distal right ICA.
B, DSA, right carotid injection, lateral view, showing a new aneurysm in the right PComA region (arrow). A second new lesion appears as a double attenuation sign in the anterior choroidal region (arrowhead).
C, DSA, right carotid injection, contralateral oblique view, demonstrating both de novo aneurysm (arrows).
Case 2
A 27-year-old woman with a history of arterial hypertension of unknown cause presented with acute headache followed by nausea and vomiting. Emergent CT was consistent with diffuse subarachnoid hemorrhage. Four-vessel DSA demonstrated a right PComA region aneurysm (Fig 2A), which was clipped via a right pterional craniotomy. The surgical exploration showed no other lesion. The patient reported no history of illegal drug use, and further medical evaluation revealed no evidence of systemic disease. Follow-up was unremarkable. The patient presented 22 months later with a new episode of acute headache followed by nausea and vomiting. CT was consistent with subarachnoid hemorrhage. DSA demonstrated a new aneurysm in the right PComA region as well as a large, complex aneurysm at the termination of the right ICA, partially fusiform and partially saccular (Figs 2B and C). A right pterional craniotomy was performed, the PComA region aneurysm was clipped, and the aneurysm at the termination of the ICA was partially clipped and wrapped. The latter had an irregular appearance at surgery, with a superior secondary extension (bleb) that was considered the source of the subarachnoid hemorrhage.
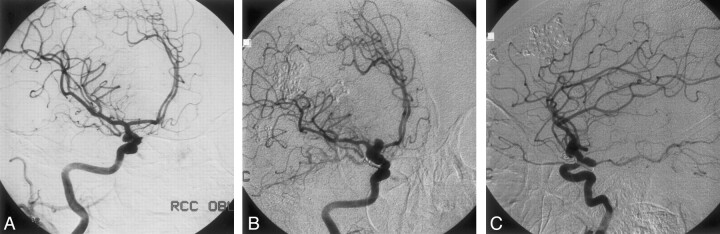
Fig 2.
Patient 2, a 27-year-old woman with two de novo aneurysms.
A, DSA, right carotid injection, oblique view, revealing a right PComA aneurysm (arrowhead). Note the normal appearance of the carotid termination.
B, DSA, right carotid injection, oblique view, showing a complex de novo aneurysm at the carotid termination, with combined saccular and fusiform characteristics (arrowheads). A second lesion is seen above the clip placed 22 months before on the initial PComA aneurysm.
C, DSA, right carotid injection, lateral view, confirming the presence of two separate new aneurysms, one located at the carotid termination (arrowhead), and the other in the PComA region (arrow).
Discussion
We report two observations of de novo aneurysm formation occurring in two young patients (32 and 27 years old) with a history of arterial hypertension. These two factors (ie, young age and hypertension) have been associated with an increased risk of de novo aneurysm development (11). Furthermore, both patients had multiple lesions (two new aneurysms each), a finding also previously reported in patients who develop de novo aneurysms (12). The unusual characteristic of our two cases lies in the very short period of time separating the initial clinical presentation from the detection of the new aneurysms (ie, 6 months and 22 months, respectively). Although “pseudo”–de novo aneurysms may certainly be related to inadequate initial documentation of preexisting lesions, the absence of additional aneurysms was confirmed in both cases by multiple angiographic incidences as well as direct surgical exploration. In our second patient, however, one of the new aneurysms occurred in the PComA region, where an aneurysm had previously been diagnosed and treated. Therefore, despite an initial intraoperative exploration that indicated successful aneurysm clipping, it is not possible to exclude completely the possibility of a residual or recurrent lesion resulting from an incomplete initial clipping. The other aneurysm found in this patient, located at the ICA termination, was, by contrast, clearly absent both angiographically and during surgical exploration. In each patient, one of the new aneurysms showed an atypical anatomic configuration, with dysplastic, ill-defined necks that rendered surgical treatment difficult. These aneurysms could only be partially clipped and wrapped. This tendency to assume complex and irregular shapes might represent another characteristic of de novo aneurysms, possibly linked to their rapid growth rate. Extensive medical evaluations of both patients failed to reveal any systemic disease, such as a vasculitis, that could explain their renal insufficiency, arterial hypertension, or both. In the absence of another detectable embolic source, the right basal ganglia stroke documented by MR imaging in the first patient was attributed to an embolus originating from one of the distal right ICA aneurysms.
Although neither of our patients had a history of illegal drug use, it is worth mentioning that such agents have been shown to predispose individuals with aneurysms to aneurysmal rupture (13, 14). Patients using cocaine present with ruptured aneurysms earlier in life and with aneurysms that are smaller in size at the time of rupture. Illicit drug use has, however, not been shown to be a risk factor for the development of multiple intracranial aneurysms (15).
Conclusion
Periodic cerebrovascular screening has been recommended for individuals considered to be at high risk of de novo aneurysm formation (2, 16–19). They include first-degree relatives of patients with intracranial aneurysms (17, 18) and patients previously treated for an intracranial aneurysm (2, 16, 19). Defining a reasonable screening program that takes into consideration the potential benefit for the patient, as well as the constraint imposed upon the health system, however, remains a difficult task. In both of our patients, the new lesions presented with aneurysm-related life-threatening conditions (ie, stroke in one case and subarachnoid hemorrhage in the other). Our observations demonstrate that de novo aneurysms can develop over very limited periods of time and therefore suggest that short screening intervals may be necessary, at least under certain circumstances, which include young age and history of arterial hypertension.
References
- 1.Juvela S, Poussa K, Porras M. Factors affecting the growth of intracranial aneurysms. Stroke 2001;32:485–491 [DOI] [PubMed] [Google Scholar]
- 2.Tsutsumi K, Ueki K, Morita A. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms. Stroke 2001;32:1191–1194 [DOI] [PubMed] [Google Scholar]
- 3.David AD, Vishten AG, Spetzler RF, et al. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg 1999;91:396–401 [DOI] [PubMed] [Google Scholar]
- 4.De Witte O, Noterman J, Oulad Ben Taib N, et al. Multiple and de novo aneurysms in Ehlers-Danlos syndrome. Neurochirurgie 1997;43:250–254 [PubMed] [Google Scholar]
- 5.Ikeda H, Izumiyama H, Hirota N, et al. Angiographic documentation of de novo aneurysm. Neurol Med Chir (Tokyo) 1998;38:730–732 [DOI] [PubMed] [Google Scholar]
- 6.Lebland R. De novo formation of familial cerebral aneurysms: case report. Neurosurgery 1999;44:871–877 [DOI] [PubMed] [Google Scholar]
- 7.Maiuri F, Spaziante R, Iaconetta G, et al. De novo aneurysm formation: a report of two cases. Clin Neurol Neurosurg 1995;97:233–238 [DOI] [PubMed] [Google Scholar]
- 8.Miller CA, Hill SA, Hunt WE. “De novo” aneurysms: a clinical review. Surg Neurol 1985;24:173–180 [DOI] [PubMed] [Google Scholar]
- 9.Parakh HC, Prabhu SS, Keogh AJ. De novo development of saccular aneurysms: a report of two cases. Br J Neurosurg 1995. :695– 698 [DOI] [PubMed]
- 10.Rinne JK, Hernesniemi JA. De novo aneurysms: special multiple intracranial aneurysms. Neurosurgery 1993;33:981–985 [DOI] [PubMed] [Google Scholar]
- 11.Tonn J, Hofmann E, Schlake HP, et al. “De novo” formation of intracranial aneurysms: who is at risk? Neuroradiology 1999;41:674–679 [DOI] [PubMed] [Google Scholar]
- 12.Briganti F, Cirillo S, Caranci F, et al. Development of “de novo” aneurysms following endovascular procedures. Neuroradiology 2002;44:604–609 [DOI] [PubMed] [Google Scholar]
- 13.Nanda A, Vannemreddy P, Polin R, Willis B. Intracranial aneurysms and cocaine abuse: analysis of prognostic indicators. Neurosurgery 2000;46:1063–1069 [DOI] [PubMed] [Google Scholar]
- 14.Fessler RD, Esshaki CM, Stankewitz RC, et al. The neurovascular complications of cocaine. Surg Neurol 1997;47:339–345 [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AI, Suarez JI, Parekh PD, et al. Risk factors for multiple intracranial aneurysms. Neurosurgery 1998;43:22–27 [DOI] [PubMed] [Google Scholar]
- 16.David CA, Vishteh AG, Spetzler RF, et al. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg 1999;3:396–401 [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Hashi K, Kurokawa Y, Yamamura A. Family history of subarachnoid hemorrhage and the incidence of asymptomatic, unruptured cerebral aneurysms. J Neurosurg 1999;91:391–395 [DOI] [PubMed] [Google Scholar]
- 18.Ronkainen A, Miettinen H, Karkola K, et al. Risk of harboring an unruptured intracranial aneurysm. Stroke 1998;29:359–362 [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumi K, Ueki K, Usui M, et al. Risk of subarachnoid hemorrhage after surgical treatment of cerebral aneurysms. Stroke 1999;30:1181–1184 [DOI] [PubMed] [Google Scholar]