Abstract
Summary: We report a case of leukoaraiosis that was studied for apoptosis. In the neuropil, the number of cells that showed DNA fragmentation was 2.5 times as great in the area of leukoaraiosis as in the adjacent white matter (P = .004) and 25 times as great as in the nearby cortex (P < .001). Our findings suggest that apoptosis, predominantly of oligodendrocytes, is involved in the pathogenesis of leukoaraiosis. Within the area of leukoaraiosis, we also found numerous small veins that were partially occluded by severe collagenous thickening of the vessel walls. This collagenosis may have contributed to or resulted from chronic ischemia in that area.
Leukoaraiosis, which appears as an area of hyperintense signal in the white matter on MR images, is an age-related neurodegenerative condition that, when severe, correlates with dementia (1). It is characterized histologically by demyelination, loss of glial cells, and spongiosis. The pathogenesis of leukoaraiosis is not yet established, but it is thought to be related to ischemia. Periventricular venous collagenosis, thickening of the vessel wall by multiple layers of collagen, has been reported to occur in aging brains and to be more severe in brains with leukoaraiosis (2). In postcapillary venules and small veins, the stenosis that results from severe periventricular venous collagenosis may be one contributing factor in chronic localized ischemia, with consequent cell injury and death. No obvious necrosis has been observed in leukoaraiosis lesions; therefore, we investigated the possibility that non-necrotic cell death (ie, apoptosis) (3, 4) occurs in such lesions.
Case Report
A 70-year-old man died of cardiac arrest 3 months after undergoing coronary artery bypass graft surgery of three vessels. His history included myocardial ischemia, peripheral vascular disease (atherosclerosis), hypercholesterolemia, hypertension, gout, insulin-dependent diabetes mellitus, 60 pack years of heavy smoking, and moderate pulmonary emphysema. Postoperatively, nonhealing ulcers developed at the vein harvest site in the patient's left lower extremity and progressive ischemia of the right foot was observed. The patient underwent a transmetatarsal amputation of the right foot 20 days before death and a left femoral-popliteal artery bypass 6 days before death. The autopsy report noted mild cerebral atrophy with no focal lesions such as infarcts, lacunes, or neoplastic lesions. Examination of the circle of Willis showed multiple mildly stenotic atherosclerotic lesions in the vertebral, basilar, middle, anterior, and internal carotid cerebral arteries. The carotid arteries in the neck exhibited considerable calcification and approximately 30% stenosis bilaterally. This patient was not reported to have dementia.
At autopsy, we obtained three coronal or coronal-oblique whole-brain slices, each 1.5-cm-thick. The postmortem time was 29 hours. The brain slices were refrigerated overnight in a solution of very weak buffered formalin (1% rather than 10%) and examined by means of MR imaging the next morning (5). A thin slice was taken from the face of each of the three blocks for standard (10%) formalin fixation for study of apoptosis. The original blocks were fixed in ethanol for enzyme histochemistry and immunohistochemistry studies. For apoptosis studies, the thin double-hemisphere slice was trimmed to make two blocks for paraffin embedding. Each of these blocks included much of the lateral ventricle, the entire centrum semiovale, and some of the superior cortex. Paraffin sections were cut 9 mm thick and were stained for apoptosis by using a modification of a technique originally described by Gavrieli et al (6), terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end-labeling (TUNEL). Briefly, sections were deparaffinized with xylene and ethanol, rehydrated in Tris-buffered saline, and made permeable with proteinase K or NeuroPore (Trevigen, Gaithersburg, MD). Endogenous peroxidase was inactivated with H2O2 in methanol/phosphate-buffered saline, and the sections were incubated for 1.5 hours at 37°C with Klenow enzyme and biotinylated deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (which becomes attached to the ends of DNA fragments). Sections were then incubated in a stop buffer, a blocking buffer, and avidin peroxidase (which conjugates to the biotin deoxynucleotidyl transferase-mediated deoxyuridine triphosphate). The color reaction was developed with either of two substrates for the conjugated peroxidase, diaminobenzidine, which is brown, or aminoethycarbazole, which is red. Sections stained with diaminobenzidine were counterstained with methyl green, and sections stained with aminoethycarbazole were counterstained with light hematoxylin. The red aminoethycarbazole stain was better for distinguishing apoptotic cells from the brown deposits of extracellular hemosiderin or intracellular lipofuscin.
Specimen MR imaging, the technique of which is described elsewhere (2, 5), showed an area of hyperintensity in the centrum semiovale in the left frontal brain slice (Fig 1). Paraffin-embedded sections from this area, stained for Luxol fast blue (Fig 2), exhibited a pattern of demyelination corresponding to that seen in the hyperintense area on MR images. Trichrome-stained sections revealed severe periventricular venous collagenosis in the leukoaraiosis area (Fig 3). TUNEL staining showed scattered labeled brain parenchymal cells, as well as many labeled cells in vessel walls (Fig 4). The labeled cells appeared in all layers of the vessel wall, from endothelial cells to pericytes. From an informal survey, it was concluded that vessels of all sizes had labeled cells and those with excessive collagen did not have more of them. A moderate number of amyloid plaques were found in the overlying cortex (Fig 5), but very few were present in the hippocampus.
fig 1.

Spin-echo MR image (2600/20/1 [TR/TE/excitations]) of a 1.5-cm-thick coronal slice from the frontal region of the brain. Only the left hemisphere is shown. The open arrows indicate an area of leukoaraiosis (white matter hyperintensity) that corresponds to the area that was investigated for apoptosis (see fig 2). C, cortex of the sulcus identified in fig 2
fig 2.
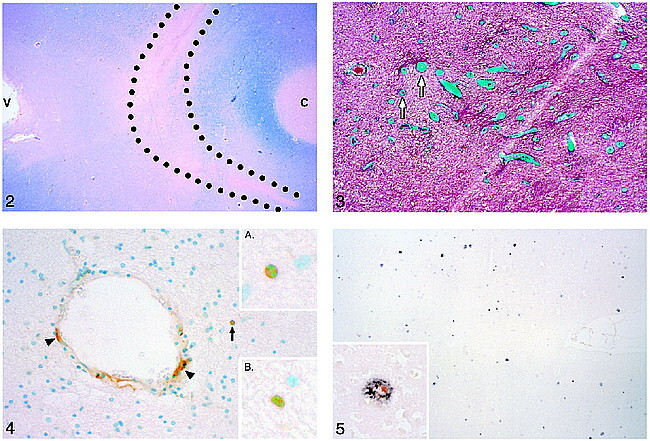
Luxol fast blue–stained section from the brain slice imaged in figure 1. The outlined area corresponds to the area of leukoaraiosis in the MR image in figure 1. Reduced Luxol fast blue staining in this area indicates demyelination. Note the darker blue staining (indicating intact myelin) of the U fibers adjacent to the cortex (C) at the bottom of a sulcus. V, lateral ventricle.fig 3. Trichrome staining shows excessively thick collagen layers (green) in the walls of small veins and venules (arrows) in the area of demyelination, where these thick-walled veins were most numerous. They are usually more numerous near the angle of the lateral ventricle.fig 4. TUNEL staining in the area of demyelination shows positive cells (brown stain) in the wall of a blood vessel (arrowheads) and in the brain parenchyma (arrow). Inset A, The brown, TUNEL-stained cell in the parenchyma appears to be apoptotic histologically in that its blue-stained nucleus is condensed and split into two segments. Inset B, Another TUNEL-stained cell in the lesional white matter with nuclear fragmentation.fig 5. A moderate number of amyloid plaques are shown in the area of cortex that was counted. Inset, Double stained for β-amyloid (black) and interleukin-1 (red) (the latter stains activated microglia and macrophages)
Each of five sections stained for apoptosis was positioned over the section stained for Luxol fast blue, and a marking pen was used to trace the pattern of the demyelinated area, the lateral ventricle, and the cortex. TUNEL-positive brain parenchymal cells (but not vascular cells) were counted in an outlined area of the lesion, in an outlined area of white matter without demyelination between the lesion and the cortex, and in an outlined area of adjacent cortex. The area of each of these counted regions was determined by means of computerized image analysis. The average cell counts for the five sections were 97.1 cells/cm2 in the leukoaraiosis lesion, 38.0 cells/cm2 in the adjacent white matter, and 3.8 cells/cm2 in the nearby cortex (Table). Statistical analysis using Student's t test indicated that apoptosis counts were significantly greater in the leukoaraiosis lesion than in the adjacent white matter (P = .004) or in the nearby cortex (P < .001).
The identity of the cell type or types in the white matter parenchyma that were TUNEL-positive was not fully determined. Because very few neurons are found deep in the white matter, those TUNEL-positive cells would likely be glial cells. Double staining for TUNEL and the astrocyte marker glial fibrillary acidic protein showed a few double-stained cells in the cortex, but none of the numerous TUNEL-positive cells in the white matter showed glial fibrillary acidic protein staining. We also stained for interleukin-1, a marker for activated microglia and macrophages, and found few positive cells in the white matter or cortex. By a process of elimination, we determined that the TUNEL-positive cells in the white matter are likely to be predominantly oligodendrocytes. This fits with the histologic finding that leukoaraiosis lesions in general, and this lesion in particular, show demyelination (Fig 2) with a profound loss of oligodendrocytes (Fig 6).
Discussion
The occurrence of apoptosis in the adult brain neuropil is abnormal. With TUNEL staining, brain parenchymal cells showing DNA fragmentation were observed to be 2.5 times as numerous in the leukoaraiosis lesion as in the adjacent white matter. In addition, some of the parenchymal cells that were labeled for DNA fragmentation showed the histologic appearance of cells undergoing apoptosis. Therefore, apoptosis seems to account for at least some of the cell loss observed in this leukoaraiosis lesion. Additional evidence suggests that the apoptotic cells in the lesion may be primarily oligodendrocytes. The significance of finding apoptosis in these lesions is that apoptosis is a delayed cell death that can be inhibited by interruption of the cell death program, whereas necrosis is rapid and irreversible.
Because very few interleukin-1-positive (ie, activated) microglia or macrophages were present in the area of the leukoaraiosis lesion, we can conclude that little or no inflammatory reaction was present in this lesion at the time of the patient's death. This conclusion weighs against the possibility that necrosis was significantly involved in the lesion at the time of death. The TUNEL-positive cells were most likely apoptotic, and the apoptosis was not related to an ongoing inflammatory reaction. This likelihood has therapeutic implications; if there were a significant inflammatory component in the development of leukoaraiosis, anti-inflammatory drugs might be of benefit (as has been proposed for the treatment of Alzheimer's disease). The possibility remains that this lesion could have had an earlier phase with inflammation and necrosis, but the preponderance of evidence points to chronic ischemia with apoptosis, not necrosis, as the mechanism of cell loss.
The TUNEL staining technique has been established as a means of detecting cells undergoing apoptosis, although some investigators have suggested that cells undergoing necrosis or DNA repair might contain enough DNA fragments to yield false-positive results with TUNEL staining (7). Because neither the technique itself nor the interpretation of the staining is easy or straightforward, additional caution is warranted before drawing conclusions regarding TUNEL studies. Nevertheless, the majority of studies of TUNEL staining in human brain have shown the technique to be reliable and reproducible. Several antibodies have been developed recently for detecting apoptosis in tissue sections, such as the so-called apoptosis specific protein (7), single-stranded DNA (8), the cleaved fragment of caspase-3 (Pharmingen, San Diego, CA), and the cleaved fragment of poly (adenosine 5′-diphosphate-ribose) polymerase (Promega, Madison, WI). Previous studies of apoptosis in neonatal brain tissues (data not shown), used both TUNEL and antibodies to single-stranded DNA and showed similar numbers of labeled parenchymal cells with the two methods, as well as comparable staining of cells in the walls of vessels. Thus, these preliminary studies confirmed the specificity of TUNEL staining.
It has also been suggested that postmortem processes could result in artifactual false-positive TUNEL-stained cells, but the likely result would be a generalized occurrence, not one confined to a specific area of the brain, as in this case. Furthermore, Petito and Roberts (9) showed that TUNEL staining is not influenced by postmortem delays of up to 72 hours in rat brain and intestine. Other studies have shown TUNEL-positive cells in human brains with various types of disease, such as HIV infection (10) and Creutzfeldt-Jakob disease (11), but not in the control brains. We were concerned about finding even a few TUNEL-positive cells in the parenchyma of the cortex in this case, but we subsequently discovered amyloid plaques in the cortex that might have been responsible for causing some apoptosis there.
The occurrence of TUNEL-positive cells in the walls of blood vessels in human brains has been previously reported (10−12). Why brain blood vessels contain so many TUNEL-positive cells is not known, but they do provide a convenient positive internal control, indicating that the staining process is operating appropriately. The presence of these numerous TUNEL-positive vascular cells, however, can greatly increase the difficulty of counting labeled parenchymal cells because it is not always clear whether a labeled cell is associated with a vessel. Further studies of apoptosis in vessels in leukoaraiosis and Alzheimer's disease may help to define the role, if any, of vascular involvement in these neurodegenerative diseases.
The leukoaraiosis lesion examined in this case is somewhat unusual in that it is quite distant from the lateral ventricle. Similarly, the most severe venous collagenosis was in the leukoaraiosis lesion, rather than nearer the lateral ventricle, as is generally true (2). This case was studied after those cited in a previous report (2). Because the collagenosis “followed” the lesion away from the ventricle, we can assume a mechanistic link between leukoaraiosis and venous collagenosis, rather than an incidental finding that both happen to occur near the ventricle. Although we do not know what that link is, we think that further studies of venous collagenosis may shed light on the pathogenesis of leukoaraiosis.
In conclusion, the studies described in this case report demonstrate the feasibility and potential value of investigating apoptosis in leukoaraiosis. Additional studies of both apoptosis and venous collagenosis in a series of cases of leukoaraiosis may provide confirmation of the present findings as well as further information regarding the pathogenesis of leukoaraiosis lesions. If apoptosis is found to be a prominent feature of leukoaraiosis, the fact that apoptosis is potentially inhibitable may have therapeutic implications.
TABLE: Apoptotic cell counts/cm2 in three areas in five sections
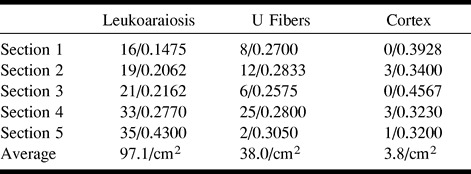
fig 6.

Comparison of the leukoaraiosis lesion (A) and an unaffected area in nearby white matter (B). Note that in the leukoaraiosis lesion, oligodendrocytes (arrows) appear to be preferentially less numerous than astrocytes, which have nuclei that are slightly larger and less densely stained (arrowheads) (hematoxylin and eosin)
Acknowledgments
We thank Patricia Wood for technical assistance and Donna S. Garrison, PhD, for editorial assistance.
Footnotes
Supported by National Institutes of Health Grant NS-20618.
Address reprint requests to Dixon M. Moody, MD, Department of Radiology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1088.
References
- 1.Kobari M, Meyer JS, Ichijo M, Oravez WT. Leukoaraiosis. Correlation of MR and CT findings with blood flow, atrophy, and cognition. AJNR Am J Neuroradiol 1990;11:273-281 [PMC free article] [PubMed] [Google Scholar]
- 2.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis. Association with leukoaraiosis. Radiology 1995;194:469-476 [DOI] [PubMed] [Google Scholar]
- 3.Kerr JFR, Wyllie AH, Currie AR. Apoptosis. A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings MC, Winterford CM, Walker NI. Apoptosis. Am J Surg Pathol 1997;21:88-101 [DOI] [PubMed] [Google Scholar]
- 5.Brown WR, Moody DM, Mathews VP. Brain slice holder for MR. AJNR Am J Neuroradiol 1995;16:1446-1448 [PMC free article] [PubMed] [Google Scholar]
- 6.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992;119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadelmann C, Bruck W, Bancher C, Jellinger K, Lassmann H. Alzheimer disease. DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol 1998;57:456-464 [DOI] [PubMed] [Google Scholar]
- 8.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L. Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res 1996;226:387-397 [DOI] [PubMed] [Google Scholar]
- 9.Petito CK, Roberts B. Effect of postmortem interval on in situ end-labeling of DNA oligonucleosomes. J Neuropathol Exp Neurol 1995;54:761-765 [DOI] [PubMed] [Google Scholar]
- 10.Adle-Biassette H, Levy Y, Colombel M, et al. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol 1995;21:218-227 [DOI] [PubMed] [Google Scholar]
- 11.Gray F, Chrétien F, Adle-Biassette H, et al. Neuronal apoptosis in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol 1999;58:321-328 [DOI] [PubMed] [Google Scholar]
- 12.Lucassen PJ, Chung WCJ, Kamphorst W, Swaab DF. DNA damage distribution in the human brain as shown by in situ end labeling. Area-specific differences in aging and Alzheimer disease in the absence of apoptotic morphology. J Neuropathol Exp Neurol 1997;56:887-900 [DOI] [PubMed] [Google Scholar]


