Abstract
BACKGROUND AND PURPOSE: The FLAIR (fluid-attenuated inversion-recovery) pulse sequence has been shown to be sensitive to abnormalities of the subarachnoid space. Our clinical experience led us to investigate whether intravenously injected contrast material can affect the appearance of the subarachnoid space on FLAIR MR images.
METHODS: After noting unexplained high signal in the subarachnoid space on FLAIR images in a patient, we studied two dogs with sequential FLAIR MR imaging after IV administration of contrast material. A third dog was studied with a 6-hour delayed FLAIR sequence after triple-dose (0.3 mmol/kg) IV contrast administration. CSF was obtained from two animals for measurement of gadolinium concentration. A phantom was developed to determine the lowest concentration at which the effects of gadolinium were evident on FLAIR images in vitro.
RESULTS: In all three animals, the appearance of the CSF in the ventricles or subarachnoid space was modified after administration of IV contrast. This was most evident on delayed images. The CSF samples showed a gadolinium concentration of 0.007 mmol/L in the dog who received the 0.1 mmol/kg dose and 0.02 mmol/L in the dog who received a triple dose. In our in vitro phantom experiments, gadolinium effects were evident on FLAIR images at a concentration four times lower than those on T1-weighted images.
CONCLUSION: IV contrast material can cross into the CSF in sufficient concentration to alter the appearance of the subarachnoid space on FLAIR images in normal dogs. Although we encountered two patients with CNS disease in whom enhancement of the CSF was seen on postcontrast FLAIR images, additional investigation is needed in humans to determine whether enhancement may occur at triple dose in healthy subjects.
Normal meninges and choroid plexus enhance after the administration of contrast material because they lack a blood-brain barrier; however, enhancement of the CSF has not been detected on conventional MR sequences in the absence of extensive meningeal or superficial brain disease. With the increased utility and availability of the fluid-attenuated inversion-recovery (FLAIR) sequence, subtle changes in the subarachnoid space due to hemorrhage or carcinomatous meningitis can now be routinely detected. After encountering a patient with unexplained hyperintensity of the CSF on postcontrast FLAIR images, we used an animal model to investigate the possibility that previously administered IV contrast material might also alter the CSF signal on this pulse sequence.
Case Reports
Case 1
A 76-year-old man was admitted after 3 weeks of progressive left-handed clumsiness. The patient had been a medical photographer for 20 years and had frequently handled brain sections without gloves. Physical examination revealed weakness of the left triceps, wrist, and finger extensors. An unenhanced CT scan showed age-appropriate atrophy. EEG on admission was unremarkable. Brain MR imaging showed high signal in several sulci over the convexities on FLAIR sequences (TR/TEeff/excitations = 10002/159/1, TI = 2200) (Fig 1). Just before the brain MR study, the patient underwent contrast-enhanced cervical MR imaging (gadodiamide, 0.1 mmol/kg). A coronal FLAIR sequence, which was recommended for confirmation of these subtle findings, was obtained 6 hours later. On this study, the CSF spaces appeared conspicuously bright over both hemispheres. Subarachnoid hemorrhage was suggested as a possible explanation for the findings, and a CT examination was performed 2 days after the MR study. The CSF obtained from a lumbar tap, also done 2 days after the MR examination, was clear and colorless with 1 WBC/hpf, 447 RBC/hpf, protein 39 mg/mL, and no xanthochromia. Findings on a repeat coronal FLAIR sequence, performed the day after the tap, were normal. Eight days after admission, the patient began to manifest bilateral upper extremity spasticity and required a long time to follow simple commands. The diagnosis of Creutzfeldt-Jacob disease was made on the basis of the clinical findings. The patient was discharged to a nursing care facility, where his dementia progressed rapidly. He died 3 months after discharge. An autopsy was not performed.
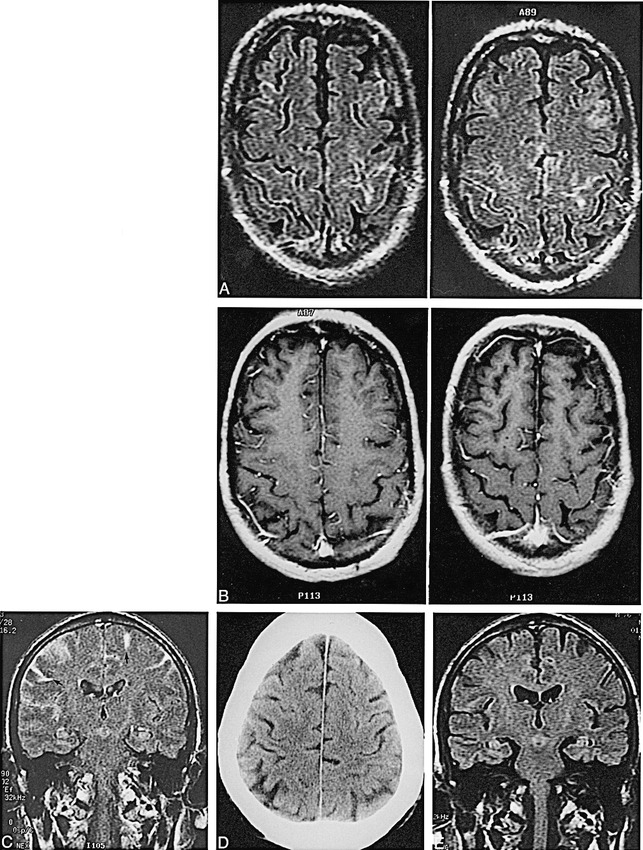
fig. 1. A–E, Axial contrast-enhanced FLAIR images (A) (TR/TEeff/excitations = 10,002/158.8/1, TI = 2200; 5-mm-thick sections) show linear increased signal in several sulci over both convexities. Corresponding T1-weighted images (B) show no analogous abnormality. Coronal FLAIR image (C) (10,002/158.8/1, TI = 2200) obtained 6 hours after the axial image shows hyperintense CSF in sulci over both hemispheres (arrows). An unenhanced CT scan (D) was normal. Follow-up FLAIR image 2 days later (E) was also normal
Case 2
A 54-year-old man was admitted with right upper quadrant tenderness and fever following cholecystectomy and choledochojejunostomy. Blood studies revealed a low sodium content, which was attributed to the syndrome of inappropriate antidiuretic hormone, and the patient's water intake was restricted. Additional studies, including MR imaging of the pituitary, were ordered in search of a tumor as the explanation for this syndrome. The MR images revealed several subtle areas of enhancement in the brain. The patient returned for a more complete evaluation of the brain, this time with triple-dose contrast material in an attempt to maximize the visualization of any small metastatic lesions. This study revealed several areas of enhancement with abnormal signal on diffusion images, most consistent with acute infarct. During this triple-dose examination, FLAIR images were obtained both before and after contrast injection. The postcontrast images revealed high signal in multiple frontal cortical sulci that was not evident on the preinjection FLAIR studies (Fig 2).

fig. 2. A and B, Noncontrast FLAIR image (A) shows the expected dark cortical sulci over the frontal convexity. Contrast-enhanced FLAIR image (B) 15 minutes after injection of a triple dose of contrast material shows high signal in multiple sulci, most consistent with contrast effects
Methods
Because we suspected that contrast material was responsible for the CSF appearance in case 1, we studied three dogs with MR imaging after administration of IV gadopentetate dimeglumine. Dogs 1 and 2 were given the usual dose of contrast agent (0.1 mmol/kg) but dog 3 was given a triple dose (0.3 mmol/kg). All three dogs were subjects in a separate imaging study of the prostate. Each animal had undergone a laparoscopic prostate procedure 30 days or more before imaging but were otherwise completely normal. The dogs were anesthetized and both brain and prostate were imaged during the same session. After induction with telazol (13.2 mg/kg) the dogs were intubated and maintained on isoflurane gas anesthesia at 2% to 4%. The dogs were ventilated on room air during the scans. Contrast agent was injected through a cephalic vein. In animal 2, CSF was drawn from the cervicomedullary cistern with a 22-gauge spinal needle 1 hour after injection of contrast material. In animal 3, a precontrast CSF sample was drawn, and a second sample was obtained 6 hours after contrast injection.
In dogs 1 and 2, coronal FLAIR images of the entire brain were obtained before contrast injection on our 1.5-T scanner. FLAIR images (10007/189/1, TI = 2200) were then obtained at 1, 5, 10, 20, 30, and 60 minutes after injection (0.1 mmol/kg). Dog 2 had additional sagittal FLAIR images (10002/156/1, TI = 2200) at 1, 10, and 30 minutes. In dog 3, contrast material (0.3 mmol/kg) was administered 6 hours before imaging. FLAIR (100002/156/1, TI = 2200) and T1-weighted (500/16/1, 4-mm-thick sections) images were obtained in coronal and sagittal planes.
The CSF samples were refrigerated and then sent out in a cooled container for measurement of contrast concentration (Mayo Medical Laboratories, Rochester, MN). This was determined with the use of an inductively coupled plasma mass spectrometer, which employs an alternating current to ignite argon gas (6000° K). The CSF sample, diluted with 1% nitric acid, is injected into this hot gas, in which it dissociates into constituent atoms. The sample is then focused with electronic lenses into a mass counter, which has been calibrated with known standard samples of the atom of interest (gadolinium).
In order to define the lowest concentration of contrast material that would produce a significant signal on FLAIR as compared with T1-weighted images, we developed a phantom in which deionized water was used to dilute the MR (gadodiamide) progressively and radiographic (iopamidol) contrast agents in 10-mL plastic test tubes. These were imaged in a standard laboratory plastic test tube rack in the same 1.5-T scanner using FLAIR (10002/154/1, TI = 1630) and T1-weighted (400/16/1) sequences. Two reference water tubes were included in the rack. A section thickness of 4 mm was used. The first tube in the series contained a gadolinium concentration of 0.5 mmol/L and subsequent tubes contained serial 1:1 dilutions. The TI value (1630) differed from that used for patient CSF imaging (2200) in order to suppress the signal from the water. Even the deionized water and the tap water appeared different at this TI = 1630 value, most likely reflecting the variation of T1 relaxation caused by the higher concentrations of metal ions in tap water. The tubes were partially submerged in a water bath containing deionized water to decrease susceptibility effects of the fluid within the tubes with the surrounding air. Using the Windows workstation, we measured the numerically averaged intensity of each tube using the functional tools menu. These data were then plotted as signal magnitude versus gadolinium concentration. All signal data on the y-axis were background-corrected. The background signal value was determined in each experiment as the mean signal intensity of at least two tubes containing deionized water only. The threshold concentration of imaging agent defined as producing a signal was estimated by one-way analysis of variance (ANOVA) of the signal magnitude at the lower concentrations of contrast tested.
Five patients who were being examined with enhanced MR imaging to assess clinical symptoms were imaged with the FLAIR sequence immediately after the contrast-enhanced sequence. All patients received a single dose of gadodiamide (0.1 mmol/kg). We specifically looked for increased signal in the subarachnoid spaces on the postcontrast FLAIR images of these patients.
Results
In dogs 1 and 2 (who received 0.1 mmol/kg of contrast agent), the enhanced images showed a change in the signal of the CSF in the fourth ventricle 30 minutes after injection such that it became isointense with surrounding brain (Fig 3). Signal abnormalities were more evident in animal 3 (who received 0.3 mmol/kg of contrast material). The CSF in the midline sulci was hyperintense relative to surrounding brain, as was the premedullary cistern on the FLAIR images (Fig 4).

fig. 3. A and B, Sagittal FLAIR image (A) (10,007/189, TI = 2200; 5-mm-thick sections) in dog 2 immediately after injection of contrast agent (0.1 mmol/kg) reveals the expected low signal intensity of CSF in the fourth ventricle (arrow). Sagittal FLAIR image 30 minutes after contrast administration (B) shows that the CSF in the fourth ventricle is now isointense with surrounding brain (arrow)
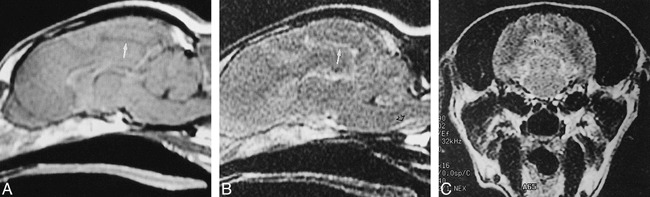
fig. 4. A–C, Sagittal T1-weighted image (A) (550/15/1) obtained 6 hours after IV contrast administration (0.3 mmol/kg) shows the expected low-signal CSF in the cingulate sulcus (arrow). Sagittal FLAIR image (B), also obtained 6 hours after triple-dose contrast injection, shows abnormal high signal in the premedullary cistern (black arrow) as well as the cingulate sulcus (white arrow). The abnormal CSF signal on the coronal FLAIR image (C) is striking in the occipital sulci (arrows)
In dog 3, the only animal in whom pre- and postcontrast levels were obtained, gadolinium concentration in the CSF showed a marked change from baseline, from less than 1 ng/mL to 3375 ng/mL (0.02 mmol/L). In animal 2, in whom only the postinjection level was available, the CSF concentration was 1153 ng/mL (0.007 mmol/L).
The phantom tests revealed a striking difference in the sensitivity of T1-weighted and FLAIR imaging with respect to contrast effects (Fig 5). The FLAIR data showed a threshold contrast signal at 0.000061 mmol/L as compared with T1-weighted data at 0.00024 mmol/L, at least a fourfold increase in sensitivity (Fig 6). Little difference was evident, however, on the graphic depiction of the intensity values of iodinated contrast material on the MR images.

fig. 5. A and B, Images of the phantom obtained with FLAIR (10,000/154, TI = 1630) (A) and T1-weighted (400/16/1) (B) techniques. The lower tubes contain increasing dilutions of gadodiamide. There are more bright tubes in the lower two rows (lowest concentrations of contrast material) on the FLAIR image than on the T1-weighted image.
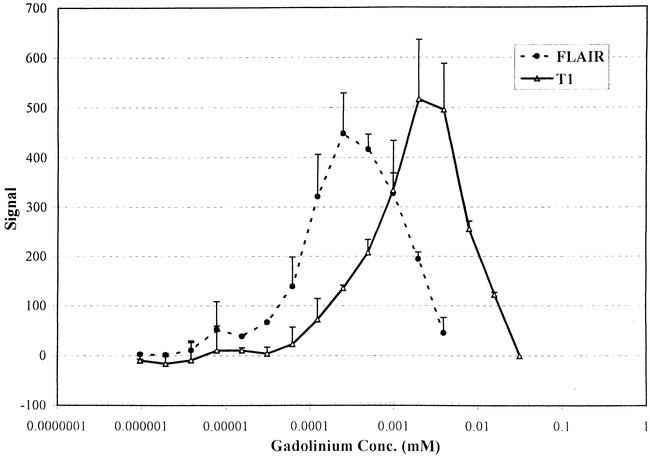
fig. 6. Graph shows the effect of varying the concentrations of contrast material (range, 0.000001 to 0.5 mM) on the mean FLAIR and T1 signal intensity in the test tube phantom. The error bars represent +ISD and the data are from two separate experiments.
Among the five patients in whom FLAIR sequences were obtained after administration of a single dose of contrast agent, none had evidence of abnormal signal in the subarachnoid space on FLAIR images.
Discussion
The concept that radiographic contrast material might enter the CSF after IV injection is not novel. There are several previous reports of iodinated contrast material entering the subarachnoid space (1–3). A study by Knutzon et al (4) of CSF samples taken from dogs after IV injection of a gadolinium contrast agent showed a T1 shortening effect in vitro. Visualization of this effect on routine MR images has been reported in humans but only in the setting of extensive disease of the meninges or brain. One report described an 8-year-old patient with a large enhancing brain tumor in whom diffuse high signal intensity of the CSF was apparent on T1-weighted MR images (5). CSF measurement of gadolinium concentration in that case was reported as 24,405 ng/mL (155.4 mmol/L). A report of a patient with diffuse meningeal fibrosis from VP shunting as well as cryptococcal meningitis described enhancement of the CSF (6). A third article described four patients with carcinomatous meningitis who all had evidence of CSF enhancement on T1-weighted images that became more evident on delayed T1-weighted images (7). Intravenously administered contrast material has also been reported to modify the appearance of the fluid within pineal cysts on delayed T1-weighted images (8). The mechanism in all cases is presumably the result of extravasation of the contrast agent into the CSF from large surfaces with absent blood-brain barriers.
There is, however, little evidence of this phenomenon in healthy subjects. On the contrary, it has been suggested that gadolinium in the CSF may be used as a marker for disruption of the blood-brain barrier in animals (9). In the report by Knutzon et al (4), however, there was clear evidence that gadopentetate dimeglumine was appearing in the CSF of normal dogs after IV injection. Altered signal was apparent on T1-weighted images of CSF in vitro and was most evident at 60 minutes. Extrapolating from a study by Brasch et al (10) that studied the effects of titrations of fluid with gadolinium-based contrast material, T1 shortening was evident in vitro at concentrations in fluid above 0.01 mmol/L. The maximum effect, however, was not observed until concentrations of 1.5 mmol/L were reached. An interruption of the blood-brain barrier would be expected to increase the passage of IV gadolinium contrast into the CSF, and Luzzani et al (9) studied this in rats. They found an elevated CSF concentration of gadolinium, which they expressed as approximately 60 nmol/g. They reported neurologic effects in rats at CSF concentrations of 0.01 mmol/kg. We found that the measured concentration of gadolinium in the CSF of our animals followed the IV dose (ie, a threefold increase in the CSF concentration with a triple dose).
These animal experiments illustrate the extreme sensitivity of FLAIR imaging to changes in the T1 relaxation of CSF. Changes in the appearance of the CSF were apparent even at gadolinium concentrations of 0.007 mmol/L. The FLAIR imaging technique uses an inverting pulse to null the signal of CSF as these spins pass through the zero point in their relaxation trajectory. Any shortening of the CSF T1 relaxation time would then make it appear bright with respect to the usual dark CSF. The extreme sensitivity of FLAIR imaging can be best appreciated by the fact that there is evidence to suggest that breathing 95% oxygen can change the relaxation time of CSF sufficiently to modify its appearance on FLAIR images (11). This effect can be used to good advantage in cases of epidermoid tumors, subarachnoid hemorrhage, and carcinomatous meningitis (12). In such cases, findings on all the other routine MR images may be normal, including enhanced T1-weighted images. The in vitro phantom experimental data provide additional evidence that the FLAIR sequence can reveal changes in CSF that might not be evident on T1-weighted studies. Similar results were reported by Mathews et al (13) in their in vitro experiments. This sensitivity might account for the fact that this effect was not evident on conventional MR images prior to the advent of FLAIR imaging.
The results of this study can be cautiously applied in clinical practice. The two cases we report on yielded abnormal findings. The CSF analysis of case 1, while not completely normal, argues against a missed subarachnoid hemorrhage or meningitis, either infectious or carcinomatous. In instances of a late tap after a subarachnoid hemorrhage, there should be some change in color of the fluid, xanthochromia, elevation of protein, a low glucose level, or elevation of the WBC count. None of these were present. The RBC count of 448, in the absence of these other findings, most likely reflects a traumatic tap. The normal protein and the WBC count do not support meningitis as the cause of the abnormal MR findings. Cell cytology was obtained and was normal in the CSF as well. The normal CT findings, and the completely normal FLAIR imaging findings 3 days later, are additional evidence against subarachnoid hemorrhage or carcinomatous meningitis as the cause of the patient's initially abnormal scans. The clinical course and history of case 1 were consistent with Creutzfeldt-Jacob disease. His fairly unusual occupational exposure to brains provides a potential source of exposure to prions, the proposed infectious agent in this disease. The MR images did not show any abnormal signal in the basal ganglia, which has been reported in some cases of this disease, and there was no autopsy or biopsy, so the diagnosis cannot be offered as indisputable. Although contrast effects in the CSF might be accentuated by renal disease, since the plasma concentration would stay elevated longer, there was no evidence of renal dysfunction by serum markers (BUN/Cr).
We have performed pre- and postcontrast FLAIR imaging in five other patients who were being imaged for a variety of neurologic complaints. In none of these patients did we see significant enhancement of the CSF on postcontrast FLAIR images. These patients all received gadodiamide at 0.1 mmol/kg. In a report of a series of more than 100 patients who were examined with FLAIR imaging sequences both before and after contrast administration, no mention was made of abnormal subarachnoid signal (13). The authors did indicate, however, an increased sensitivity of postcontrast FLAIR imaging to meningeal enhancement.
Although we did not see contrast effects after a single dose, the postcontrast FLAIR images in case 2 revealed abnormal high signal in cortical sulci that most likely represented contrast enhancement. We predicted this effect on the basis of the nearby blood-brain barrier breakdown in combination with the higher dose of contrast material. Further experience with triple-dose FLAIR imaging in humans may show that this effect is commonplace, and, given our experience with animals, that it could even occur in healthy subjects.
Conclusion
This study has shown the high sensitivity of FLAIR imaging to alterations of fluid relaxation times and has demonstrated that IV gadolinium-based contrast material crosses into the CSF of dogs in sufficient concentrations to alter the appearance of the subarachnoid spaces. While this effect is negligible on immediate postcontrast FLAIR images in humans after administration of conventional doses of contrast material, it seems likely that changes will be evident on delayed images of patients with meningeal or cortical disease. CSF analysis shows that the CSF concentration of intravenously administered contrast material is roughly proportional to the IV dose, so that these changes should be more evident at a triple dose (0.3 mmol/kg). While abnormal cortical sulci were seen in normal dogs after a triple dose of contrast agent, the effects of this dose in humans will require further investigation. We suggest that in cases in which there is unexplained high signal in the subarachnoid space on FLAIR images of patients who have had a previous enhanced MR study, it is reasonable to regard the high signal as a contrast effect.
Footnotes
Address reprint requests to Alexander C. Mamourian, MD, Department of Radiology, Dartmouth-Hitchcock Medical Center, One Medical Center Dr, Lebanon, NH 03756.
References
- 1.McClennan BL, Becker JA. Cerebrospinal fluid transfer of contrast material at urography. AJR Am J Roentgenol 1971;113:427-432 [DOI] [PubMed] [Google Scholar]
- 2.Coin CG, Keranen VJ, Pennink M, Ahmad WD. Evidence of CSF enhancement in the spinal subarachnoid space after intravenous contrast medium administration: is intravenous computer assisted myelography possible? J Comput Assist Tomogr 1979;3:267-269 [DOI] [PubMed] [Google Scholar]
- 3.Harnish PP, Northington FK, Samuel KA. Diatrizoate levels in cerebrospinal fluid following intravenous administration: role of fluid production rate. Invest Radiol 1988;23:377-380 [DOI] [PubMed] [Google Scholar]
- 4.Knutzon RK, Poirier VC, Gerscovich EO, Brock JM, Buonocore M. The effect of intravenous gadolinium on the magnetic resonance appearance of cerebrospinal fluid. Invest Radiol 1991;26:671-673 [DOI] [PubMed] [Google Scholar]
- 5.Naul LG, Finkenstaedt M. Extensive cerebrospinal fluid enhancement with gadopentetate dimeglumine in a primitive neuroectodermal tumor. AJNR Am J Neuroradiol 1997;18:1709-1711 [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto S, Kitagaki H, Ishii K, Yamaji S, Ikejiri Mori E. Gadolinium enhancement of the cerebrospinal fluid in a patient with meningeal fibrosis and cryptococcal infection. Neuroradiology 1997;39:504-505 [DOI] [PubMed] [Google Scholar]
- 7.Pui MHW, Langston JW, Arai Y. Gd-DTPA enhancement of CSF in meningeal carcinomatosis. J Comput Assist Tomogr 1993;17:940-944 [DOI] [PubMed] [Google Scholar]
- 8.Mamourian AC, Yarnell T. Enhancement of pineal cysts in MR. AJNR Am J Neuroradiol 1991;12:773-774 [PMC free article] [PubMed] [Google Scholar]
- 9.Luzzani F, Cipolla P, Pelaprat ML, et al. Brain penetration and neurological effects of gadobenate dimeglumine in the rat. Acta Radiol 1997;38:268-272 [DOI] [PubMed] [Google Scholar]
- 10.Brasch RC, Weinmann H-J, Wesberg GE. Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR Am J Roentgenol 1984;142:625-630 [DOI] [PubMed] [Google Scholar]
- 11.Ketonen K, Hendrick E, Huynh K, Guinto FC, Swischuk L. Effect of anesthesia on vascular enhancement and CSF signal on fast fluid-attenuated inversion recovery brain images. In: Proceedings: ASNR, ASHNR, ASPNR, ASITN, ASSR, Joint Meetings, San Diego, 1999. Oak Brook, IL: American Society of Neuroradiology; 1999: 322–323 (Poster #152)
- 12.Singer MB, Atlas SW, Drayer BP. Subarachnoid space disease: diagnosis with fluid-attenuated inversion-recovery MR imaging and comparison with gadolinium-enhanced spin-echo MR imaging: blinded reader study. Radiology 1998;208:417-422 [DOI] [PubMed] [Google Scholar]
- 13.Mathews VP, Caldemeyer KS, Lowe MJ, Greenspan SL, Weber DM, Ulmer JL. Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 1999;211:257-263 [DOI] [PubMed] [Google Scholar]


