Abstract
BACKGROUND AND PURPOSE: Hyperintense signals on diffusion-weighted MR images (DWIs) are believed to correspond accurately with cerebral ischemic events. Intraoperative transcranial Doppler sonography (TCD) can reveal hemodynamic and embolic events during carotid endarterectomy (CEA). Our purpose was to determine whether the occurrence of hyperintense signals on postoperative DWIs corresponds to intraoperative embolic or hemodynamic events.
METHODS: Seventy-seven CEAs were monitored intraoperatively with TCD to record blood flow velocity changes after cross clamping to ascertain the presence of adequate collateral flow and to record microembolic signals. DWI was used to classify the hemisphere ipsilateral to the CEA by type: 0, no lesions (n = 51); I, cortical lesions only (n = 2); II, subcortical white matter lesions only (n = 6); III, mixed type with cortical and subcortical lesions (n = 11); IV, large territorial infarcts (n = 6); and V, other types of lesions (n = 1).
RESULTS: Neither the five clinical events (one transient ischemic attack, two minor strokes, and two major strokes) nor any DWI type (I–V) showed a relationship to blood velocity decreases after cross clamping or, in patients who were selectively shunted, to total ischemic time necessary for shunt insertion and removal. Total microembolic signal count was significantly higher in the five CEAs with clinical events than in those without. It was also higher on the DWIs showing a hyperintense lesion as compared with DWIs showing no lesion.
CONCLUSION: Apart from lesions corresponding to clinical deficits, CEA is associated with a substantial number of small areas of brain tissue at risk for irreversible ischemia. The main cause of intraoperative stroke seems to be embolism, suggesting that microembolic signals in CEA are highly relevant events for brain tissue.
When performed appropriately, carotid endarterectomy (CEA) should have a perioperative morbidity and mortality of less than 7.0% in symptomatic stenosis (1, 2) and less than 3% in asymptomatic stenosis (3). Whether the procedure should include shunting to avoid cerebral ischemia during cross clamping is still under discussion, as reports of successful shunting are balanced by large series in which no shunting was used (4–8). Recent studies of CEA in which transcranial Doppler sonography (TCD) was used intraoperatively to detect microembolic signals have revealed a high prevalence of such signals but a low rate of perioperative clinical stroke (9–11), leading to the presumption that microemboli are of no or of only minor relevance (12). With the routine application of cranial MR imaging postoperatively, it has become obvious that new postoperative brain lesions occur but can remain clinically silent (9, 13). Diffusion-weighted MR imaging (DWI) in cerebral ischemia is sensitive for the detection of stroke-related hyperintense lesions. It is a matter of debate whether hyperintense signals seen after cerebral ischemia correspond exclusively to infarcted brain tissue or whether they may also indicate reversible ischemic tissue, in which the neurons are intact; such lesions may not be associated with clinical symptoms (14–16). On the other hand, there is evidence that the volume of a DWI hyperintense lesion (17–19) and the size of the embolic material (20, 21) are suggestive of the severity of clinical symptoms. We analyzed the frequency of hyperintense brain lesions on postoperatively performed DWI in patients who had undergone CEA to address two issues: first, to ascertain the relevance of intraoperative microembolic signals to brain tissue, and second, to determine whether this analysis can contribute to the controversial discussion as to whether a shunt should be routinely used in CEA.
Methods
Seventy-six consecutive patients (63 men and 13 women; mean age, 64 ± 8 years) underwent a total of 77 CEAs performed under normotensive, normocapnic, general anesthesia. Preoperatively, 40 stenoses were asymptomatic and 37 were symptomatic (three transient ischemic attacks [TIAs], 25 minor strokes, one major stroke, and eight episodes of amaurosis fugax). The mean degree of stenosis was 78% ± 15% according to the NASCET method (1). The contralateral ICA was normal or showed a stenosis of less than 50% in 51 cases, a moderate stenosis (>50% to <70%) in five cases, a severe stenosis (>70%) in 12 cases, and an occlusion in 12 cases. The mean time delay between symptoms and CEA was 5 weeks (range, 2 weeks to 6 months). Before CEA, the symptomatic patients received either acetylsalicylic acid or heparin intravenously. All patients had routine cranial CT examinations preoperatively. Preoperative DWI studies were performed only in some patients at the time of the clinical event if the CT scan had shown no corresponding brain lesion.
All patients were monitored by TCD intraoperatively with the machine set at previously published standard technical settings (Multi DopX4, DWL, Sipplingen, Germany; 2-MHz probe) (11, 22) for two purposes: first, to suggest (selective) shunting when the mean blood velocity in the middle cerebral artery (MCA) ipsilateral to the CEA fell, after cross clamping, below 70% of the level prior to clamping; and second, to detect microembolic signals during the period from skin incision to wound closure (Fig 1). In each CEA, the TCD recordings were saved on a digital audiotape (DAT) recorder for postoperative playback and analysis.
fig 1.
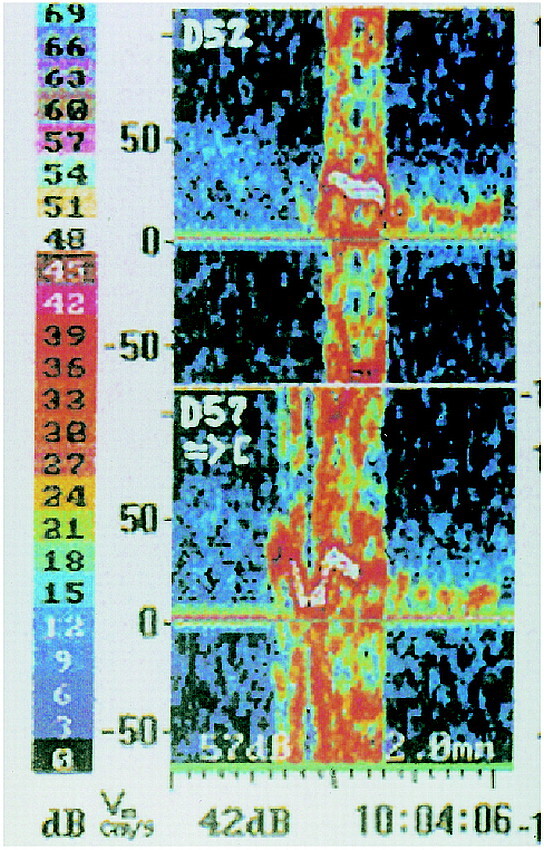
The TCD device provides two sample volumes for insonation of the MCA. An embolus streaming down the MCA will be recorded first in the proximal sample volume (depth of insonation, 57 mm [D57]) and with a time delay in the distal sample volume (depth of insonation, 52 mm [D52]). Next to the color-coded decibel scale, the Doppler velocity spectrum after fast Fourier transformation (FFT) of each sample volume is shown. The blood flow direction in both sample volumes is indicated in the proximal sample volume beneath D57 by an arrow directed toward the probe symbol ([), which means that the velocity spectrum of the blood flow in the MCA usually directed toward the probe is imaged above the zero line; the background velocity spectrum can be seen more clearly in the distal sample volume as a band with a signal intensity of 9 to 15 dB (colored slightly blue to green). The signal of the embolus after FFT is overloaded, as indicated by its bidirectional appearance
Microembolic signals are transient high-intensity signals that are usually unidirectional within the velocity spectrum, have a duration of less than 0.3 seconds, an intensity of more than 4 dB above the background velocity spectrum, and are accompanied by a characteristic chirping sound (23). When the signal intensity exceeds the decibel range of the Doppler instrument, a microembolic signal is “overloaded” and appears bidirectional. Such overloaded signals frequently occur after reclamping (10, 11).
To measure inter-rater agreement in detecting microembolic signals, we used the method suggested by Markus et al (24), which estimates the probability at which a second observer will record an abnormality if the first observer records such an abnormality. One observer played the DATs of five randomly selected CEA procedures and identified a total of 66 time points at which either an embolic signal or an artifact was recorded. Two other observers independently analyzed these time points and stated whether there was an artifact or an embolic signal.
The patients were examined postoperatively by a neurologist within 6 hours after CEA, and thereafter daily until discharge. A new postoperative neurologic deficit was considered a TIA when it lasted less than 24 hours, a stroke when it lasted longer than 24 hours. In the immediate postoperative period, blood pressure was kept below 170/85 mm Hg by using intravenous nitrates when necessary. The postoperative DWI studies were performed on day 2 or 3 after CEA. Thereafter, follow-up DWI studies were not performed. Informed consent was obtained for all examinations performed (CT, DWI, intraoperative TCD, and postoperative DWI).
MR imaging was performed on a 1.5-T whole-body unit. A standard circularly polarized head coil was used for radio frequency transmission and detection. In all patients, a T2-weighted turbo spin-echo sequence was performed for routine imaging (TR/TE/excitations = 4000/99/3; turbofactor = 11). For DWI, a highly diffusion-weighted single-shot echo-planar spin-echo sequence was applied. The machine has a resonant gradient set operating at 1000 Hz with a maximum gradient strength in the x-, y-, or z-direction of 25 mT/m and a gradient rise time of 250 μs. A diffusion gradient with a sensitivity (b value) of 1100 s/mm2 was applied in the slice direction. The DWI sequence parameters were adjusted to cover the whole brain: TEeff = 123, section thickness = 5 mm with no gap, number of sections = 25, matrix = 12 × 128, field of view = 240 mm, and acquisition time = 4 seconds. DWI was performed in the transverse, coronal, and sagittal planes. In highly diffusion-weighted images, all tissues and CSF have a low signal, and early ischemic lesions can be identified as areas of hyperintense signal. The high contrast allows a clear delineation of the lesions with good reader reproducibility (17).
In our series, two neuroradiologists independently analyzed the 77 DWIs (Figs 2-5). Neither was aware of the patients' vascular state or clinical outcome or of the results of the intraoperative TCD monitoring. With respect to common pathophysiologic concepts of the origin of stroke (embolic: territorial and small cortical infarctions; hemodynamic: watershed zones in the subcortical white matter), the findings on the DWIs were classified into the following types: 0, no new lesion; I, presence of cortical lesion(s) only (Figs 3B and 5C and D); II, presence of subcortical white matter lesion(s) only; III, mixed type with presence of both cortical and subcortical lesions (Fig 4B); IV, presence of large territorial infarctions (with or without accompanying small cortical or subcortical lesions; Figs 2D and 3D); and V, presence of other lesion types.
fig 2.
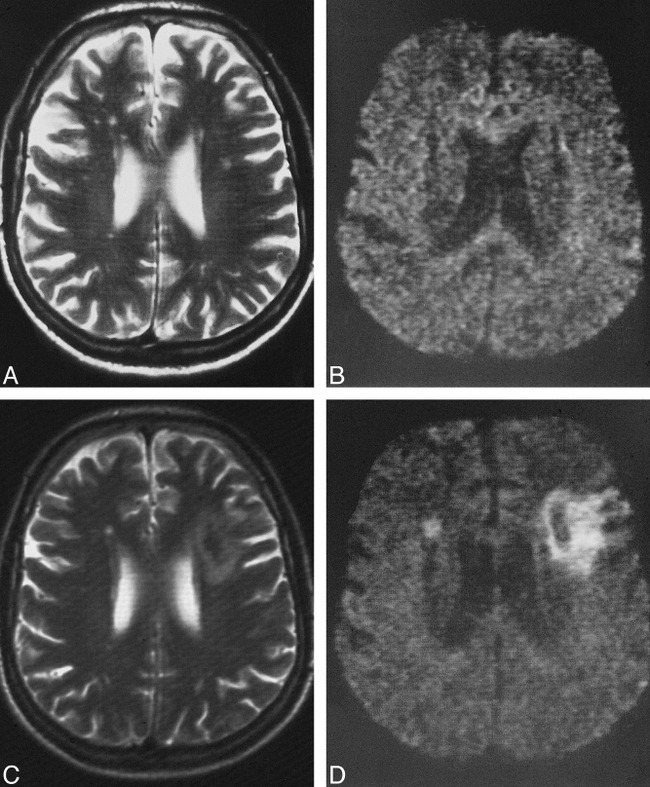
A–D, Pre- and postoperative T2-weighted images (A and C) and DWIs (B and D) of a patient in whom a territorial infarct was present on the postoperative images. The small white matter lesions on the preoperative T2-weighted (4000/99/3) image (A) are not present on the preoperative DWI (B) (episequence; 123/1 [TE/excitations]). The corresponding postoperative T2-weighted image (C) and DWI (D) show a medium-sized territorial infarct on the left side, and a small new lesion in the head of the caudate nucleus on the right side. This patient underwent surgery for a tight left-sided stenosis associated with occlusion of the contralateral internal carotid artery
fig 3.
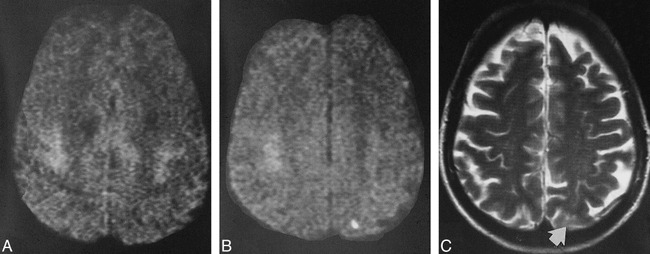
A–C, Pre- and postoperative images of a patient who had had an episode of amaurosis fugax. The preoperative DWI (A) shows no signal abnormality. The postoperative DWI (B) shows a small cortical lesion in the distribution of the posterior branches of the MCA; on the postoperative T2-weighted image (C), this lesion (arrow) can be seen only in light of the findings on the DWI
fig 4.
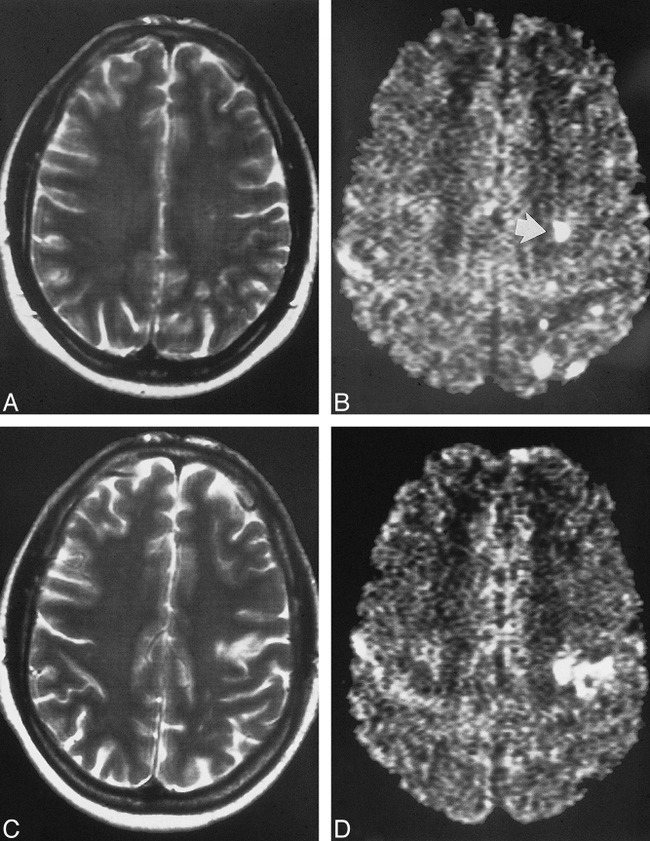
A–D, Pre- and postoperative images of a patient who underwent carotid endarterectomy 14 days after the clinical event. The preoperative T2-weighted (A) and DWI (B) studies show four small cortical lesions and one subcortical lesion (arrow, B). The cortical lesions cannot be detected on the corresponding postoperative DWI (D). The subcortical lesion is still present, but there is a new, smaller territorial infarction nearby as shown on the T2-weighted (C) and DWI (D) studies. These findings may suggest that cortical lesions disappear sooner than subcortical lesions, probably because of the better collateral blood flow in cortical regions as compared with subcortical regions
Statistical Analysis
The number of microembolic signals is given as the median and the interquartile range. The nonparametric Kruskal-Wallis test and the Mann-Whitney U test were used to compare groups with respect to microembolic signal count. A χ2-test was used to compare frequencies of clinical events and hyperintense lesions.
Results
Observer Agreement
Concerning reader reproducibility of TCD signals at the defined time points, the combined probability of observers 2 and 3 for identifying the artifacts or microembolic signals distinguished by observer 1 was 0.97 and 0.87, respectively, indicating good agreement.
Concerning reproducibility between the two neuroradiologists, both observers found one or more new lesions in the hemisphere ipsilateral to the CEA on 26 (34%) of the 77 DWIs. Each observer identified one new lesion that the other did not find. Thus, agreement between the two neuroradiologists as to the occurrence of a new lesion was excellent (chance-corrected κ value, .94) (25). Each observer detected 42 hyperintense lesions. Observer 1 classified 13 of the 42 hyperintense lesions as cortical, 22 as subcortical, five as large territorial, and two as other type (small hyperintense zone around an old infarcted brain tissue). Observer 2 classified 16 of the 42 lesions as cortical, 19 as subcortical, six as large territorial, and one as other type. Apart from territorial lesions, most of the hyperintense signals were as small as those shown in Figures 3B and 4B. Surprisingly, there was no new lesion in the area of the basal ganglia.
Regarding the classification of each scan as to the five types of lesion distribution, the number of agreements/disagreements between the two observers was as follows: in type 0, 50/2; in type I, 1/0; in type II, 3/7; in type III, 6/2; in type IV, 5/0, and in type V, 1/1, resulting in an overall chance-corrected κ value of .73 (indicating good agreement). The observers reanalyzed the DWIs in which there was disagreement and reached a final decision by consensus. Thus, the hemispheres ipsilateral to the CEA were ultimately classified as follows: type 0, n = 51; type I, n = 2; type II, n = 6; type III, n = 11; type IV, n = 6; and type V, n = 1.
In the hemispheres contralateral to the CEA, both observers agreed that there were no lesions except in two cases (in one, in the territory of the anterior cerebral artery, and, in the second, a few lesions in the territory of the MCA). In both these patients the new lesions remained clinically silent, and each was treated surgically for a tight stenosis associated with occlusion of the contralateral internal carotid artery. Because we did not monitor the contralateral MCA intraoperatively, the DWI results of the contralateral hemispheres are not included in our analysis.
After the final DWI classification, the two observers compared the DWIs with the corresponding T2-weighted images. In light of the DWI findings, they agreed that all hyperintense lesions were also present as abnormalities on the T2-weighted sequences (Fig 3C).
Clinical Events
All carotid arteries treated by endarterectomy were patent and without restenosis or occlusion at color-coded duplex examination 6 to 7 days after CEA. In the few patients in whom DWI was performed when the clinical event had occurred, the postoperative DWI studies, except for the case depicted in Figure 4, showed no hyperintense signal that was also hyperintense on their preoperative images.
Clinically, there was one TIA, two minor strokes (2.5%), and two major strokes (2.5%). All deficits were present at the examination 6 hours after surgery and affected the body side contralateral to the CEA. Further deficits did not occur. The TIA was associated with DWI type II (adequately allocated subcortical lesion, clinically), each of the minor strokes with type IV (presence of a larger territorial lesion), one of the major strokes with type II, and the other with type IV. Four clinical events (one TIA and three strokes) occurred in the group of 58 CEAs in which a shunt was not used, one clinical event (major stroke) occurred in the group of 19 CEAs in which shunting was used (no significant difference by χ2 analysis). The five patients in whom a clinical event occurred had a significantly higher total microembolic signal count (n = 36; range, 30–88) than did the postoperatively asymptomatic patients (n = 11; range, 5–24; P = .002).
DWI Type versus Microembolic Signals
Microembolic signals were present in all but three CEAs. The total microembolic signal count was significantly lower in DWI type 0 (n = 9; range, 4–17) as compared with all DWIs with a hyperintense signal (n = 26; range, 12–39; P < .001). The total microembolic signal count in DWI type 0 was nine (range, 4–17); in I, 26 (range, 14–39); in II, 36 (range, 22–69); in III, 15 (range, 6–28); in IV, 33 (range, 25–38); and in type V, 9 (Kruskal-Wallis test: P < .005). When DWI types I, III, and IV are considered as a group with all lesions embolic in origin (combined total microembolic signal count, 24; range, 10–38), this group did not differ from DWI type II, and both groups had significantly more microembolic signals than did DWI type 0 (P < .005).
DWI Type versus Shunting or No Shunting
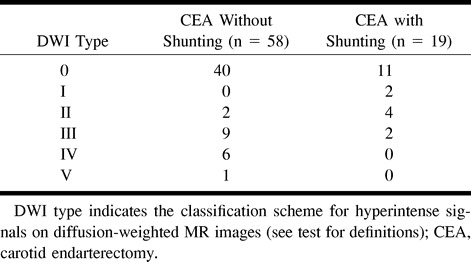
The patients in whom shunting was and was not performed did not differ as to the total number of hyperintense lesions. The DWI type distribution in the CEAs with and without shunting is reported in the Table 1. Concerning the proportion of DWIs containing hyperintense signals, no difference was found between the CEAs with and without shunting. For the CEAs without shunting, in no DWI type was the occurrence of a hyperintense signal related to the duration of cross clamping. For the procedures with shunting, the time needed for shunt insertion and shunt removal was slightly but not significantly longer (14 ± 7 minutes) for the patients with any kind of hyperintense DWI signal than for those without a hyperintense signal (9 ± 3 minutes). In all CEAs with shunting, the total microembolic signal count was significantly higher (n = 24; range, 11–39) than in CEAs without shunting (n = 10; range, 5–21; P = .01), and the total microembolic signal count was higher (by trend) in the shunted patients who had hyperintense DWI signals (n = 34; range, 15–76) than in the shunted patients who had no hyperintense signals (n = 17; range, 6–33; P = .07).
Types of hyperintense signal distribution on postoperative diffusion-weighted images in patients who had endarterectomies performed without and with shunting
Discussion
From a clinical viewpoint, CEA has proved to be a safe procedure in preventing future strokes (1–3), providing the perioperative rate of clinically relevant complications is low. When clinical events are used to analyze the cause of intraoperative stroke, a large number of patients has to be studied, because, in many CEAs, microembolisms occur that are not followed by a neurologic deficit, a result leading to doubt about the relevance of microembolic signals as a potential indicator of intraoperative stroke. The increasing experience with MR imaging and the advances made in its technological development have shown that ischemic brain lesions corresponding to clinical syndromes are detected by DWI with both high sensitivity (up to 94%) and specificity (up to 100%) (26–29). Thus, the presence of hyperintense signals on postoperative DWIs most probably indicates new ischemic lesions. We therefore used DWI to investigate the relevance of intraoperative microemboli to cerebral ischemia in patients undergoing CEA.
Our clinical results are within the range of reported perioperative morbidity rates associated with CEA (30, 31). The clinical viewpoint, however, seems only half the truth. The 33% rate of new brain lesions that occurred in patients after CEA is astonishingly high, and do confirm previous studies (9, 13). Pathophysiologically, our data provide evidence that nearly all lesions arise from an embolic origin, even if they appear to be hemodynamic in origin. Therefore, microemboli in CEA seem to be highly relevant events for brain tissue, and the infarct type occurring in association with CEA may not correspond to the mechanism proposed by infarct topology (32).
There are several reasons why microemboli cause no lesion or lesions that remain clinically silent. First, there is evidence that stroke severity correlates with DWI lesion size (17–19). As is known from CT studies, even large brain infarcts can remain clinically silent, which explains why three of six territorial hyperintense lesions in our patients remained silent. Second, stroke is associated with lesion location. Among all small lesions in our series, only two were accompanied by symptoms. Both were small subcortical lesions and associated in one case with a TIA and in the other with a major stroke. We therefore assume that the small subcortical lesion causing the major stroke represented an infarction in a strategic area. For all the other small lesions, it is beyond the scope of our study to state which part of the brain tissue (glial cells or neurons) was affected by ischemic edema causing no or only a transient neurologic deficit (with rapid functional or cellular recovery). Third, clinical deficit is associated with the size of an embolus (20, 21). Since, in the majority of our patients, microembolic signals were not accompanied by hyperintense signals on postoperative DWIs, it is likely that the cerebrovascular system is able to deal sufficiently with sudden arterial occlusions (eg, by thrombolysis of small emboli) (20). If such mechanisms fail to reestablish perfusion (eg, when large emboli are present), ischemia with neurologic symptoms can occur (21). Finally, the total number of microembolic signals in each procedure may be relevant. It has been reported (11) that the risk of a neurologic deficit increases when more than 10 microembolic signals occur during a procedure. Reclassifying our CEAs into those with a total microembolic signal count of 10 or less (n = 32) and those with more than 10 (n = 45) places the relative risk at 2.75 (95% CI, 1.20–6.30) that a new lesion will be present on postoperative DWIs in the CEAs with more than 10 microembolic signals (five of 32 CEAs with less than 10 microembolic signals versus 21 of 45 CEAs with more than 10 microembolic signals; χ2-test, P = .004).
We found no significant difference between the shunted and nonshunted patients in terms of the frequency of strokes and the frequency of hyperintense lesions. The number of clinical events in our study was too small to allow conclusive results regarding the clinical events; however, when hyperintense lesions are considered indicative of brain tissue at risk for irreversible ischemia, then shunting does not prove to prevent brain ischemia during CEA (hyperintense lesions in 31% of the nonshunted patients versus 42% of the shunted patients). The pathophysiologic background of shunting (namely, to prevent hemodynamically caused severe ischemia while cross clamping) is counterbalanced by the increased number of microembolic signals. Fifteen of 19 CEAs performed with shunting exhibited more than 10 microembolic signals, whereas among the nonshunted CEAs, 28 of 58 had more than 10 microembolic signals. The relative risk that more than 10 microembolic signals will occur when a shunt is used is 2.29 (95% CI, 0.92–5.70; χ2-test, P = .03).
Our study has limitations. On occasion, a hyperintense signal on DWI can remain hyperintense for about 2 weeks (33), as shown in Figure 4 for the subcortical lesion. This leads to the possibility that some of our postoperative hyperintense signals may have persisted from the original clinical event. Our clinical experience with DWI in stroke patients (33) corresponds to that of Burdette et al (26), who reported that hyperintense signals after stroke remain stable for 5 days and decrease progressively during the following 10 days, and that they usually disappear completely 14 days after the clinical event, as demonstrated in the small cortical lesions depicted in Figure 4. Although we cannot say for sure, it seems unlikely that in our symptomatic patients, in whom CEAs were performed with a mean time delay of 5 weeks after the clinical event, a signal would still be hyperintense. To have resolved such considerations, a DWI study performed within a few days before CEA would have been of help.
Conclusion
Our data suggest that, apart from lesions corresponding to clinical deficits, CEA is accompanied by a substantial number of small areas of brain tissue at risk for irreversible ischemia. The main cause of intraoperative stroke seems to be embolism, even when the lesions appear to be hemodynamic in origin. There is evidence that microembolic signals are highly relevant events for brain tissue. The proposed benefit of shunting (namely, to prevent ischemia during cross clamping) seems counterbalanced by an increased number of microembolic signals.
fig 5.
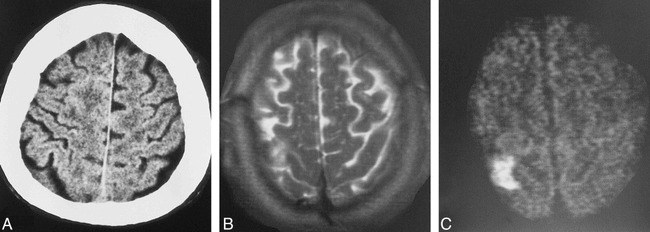
A–C, Preoperative CT scan (A) of a patient with an asymptomatic tight stenosis of the right internal carotid artery. In the right parasagittal area, the slight leukoencephalopathy is one aspect of the coincident microvascular disease, which is more clearly present on the other CT sections (not shown). Postoperatively, an infarct in the right precentral gyrus is seen on the axial T2-weighted (B) and DWI (C) studies
Footnotes
Address reprint requests to Martin Müller, MD, Department of Neurology, University Hospital of the Saarland, Kirrberger Str, D-66421 Homburg/Saar, Germany.
References
- 1.Barnett HJM, Taylor DW, Eliasziw M, et al. (for The North American Symptomatic Carotid Endarterectomy Trial Collaborators) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339:1415-1425 [DOI] [PubMed] [Google Scholar]
- 2. European Carotid Surgery Trialists' Collaborative Group Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379-1387 [PubMed] [Google Scholar]
- 3. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;237:1421-1428 [PubMed] [Google Scholar]
- 4.Allen GS, Preziosi TJ. Carotid endarterectomy: a prospective study of its efficacy and safety. Medicine 1981;60:298-309 [PubMed] [Google Scholar]
- 5.Bland JE, Lazar ML. Carotid endarterectomy without shunt. Neurosurgery 1981;8:153-157 [DOI] [PubMed] [Google Scholar]
- 6.Whitney DG, Kahn EM, Estes JW, Jones CE. Carotid artery surgery without a temporary indwelling shunt. Arch Surg 1980;115:1393-1399 [DOI] [PubMed] [Google Scholar]
- 7.Javid H, Julian OC, Dye WS, et al. Seventeen-year experience with routine shunting in carotid artery surgery. World J Surg 1979;3:167-177 [DOI] [PubMed] [Google Scholar]
- 8.Giannotta SL, Dicks RE, Kindt GW. Carotid endarterectomy: technical improvements. Neurosurgery 1980;7:309-312 [DOI] [PubMed] [Google Scholar]
- 9.Jansen C, Ramos LMP, van Heesewijk JPM, Moll FL, van Gijn J, Ackerstaff RGA. Impact of microembolism and hemodynamic changes in the brain during carotid endarterectomy. Stroke 1994;25:992-997 [DOI] [PubMed] [Google Scholar]
- 10.Gaunt ME, Martin PJ, Smith JL, et al. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy. Br J Surg 1994;81:1435-1439 [DOI] [PubMed] [Google Scholar]
- 11.Müller M, Behnke S, Walter P, Omlor G, Schimrigk K. Microembolic signals and intraoperative stroke in carotid endarterectomy. Acta Neurol Scand 1998;97:110-117 [DOI] [PubMed] [Google Scholar]
- 12.Finocchi C, Gandolfo C, Carissimi T, Del Sette M, Bertoglio C. Role of transcranial Doppler and stump pressure during carotid endarterectomy. Stroke 1997;28:2448-2452 [DOI] [PubMed] [Google Scholar]
- 13.Cantelmo NL, Babikian VL, Samaraweera RN, Gordon JK, Pochay VE, Winter MR. Cerebral microembolism and ischemic changes associated with carotid endarterectomy. J Vasc Surg 1998;27:1024-1030 [DOI] [PubMed] [Google Scholar]
- 14.Minematsu K, Li L, Sotak CH, Davis MA, Fisher M. Reversible focal ischemic injury demonstrated by diffusion-weighted magnetic resonance imaging in rats. Stroke 1992;23:1304-1311 [DOI] [PubMed] [Google Scholar]
- 15.Bryan RN. Diffusion-weighted imaging of stroke: a brief follow-up. AJNR Am J Neuroradiol 1998;19:1003-1004 [PMC free article] [PubMed] [Google Scholar]
- 16.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423-429 [PMC free article] [PubMed] [Google Scholar]
- 17.Lövblad KO, Baird AE, Schlaug G, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol 1997;42:164-170 [DOI] [PubMed] [Google Scholar]
- 18.Lövblad KO, Jakob PM, Chen Q, et al. Turbo spin-echo diffusion-weighted MR of ischemic stroke. AJNR Am J Neuroradiol 1998;19:201-208 [PMC free article] [PubMed] [Google Scholar]
- 19.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol 1997;41:581-589 [DOI] [PubMed] [Google Scholar]
- 20.Kessler C, Kelly AB, Suggs WD, et al. Introduction of transient neurological dysfunction in baboons by platelet microemboli. Stroke 1992;23:697-702 [DOI] [PubMed] [Google Scholar]
- 21.Moody DM, Bell MA, Challa VR, Johnston WE, Prough DS. Brain microemboli during cardiac surgery or aortography. Ann Neurol 1990;28:477-486 [DOI] [PubMed] [Google Scholar]
- 22.Müller M, Pan X, Walter P, Schimrigk K. Variability of velocity and duration of microembolic signals detected by bigated transcranial Doppler sonography in carotid endarterectomy. Eur J Ultrasound 1998;8:1-6 [DOI] [PubMed] [Google Scholar]
- 23. Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium Basic identification criteria of Doppler microembolic signals. Stroke 1995;26:1123. [PubMed] [Google Scholar]
- 24.Markus H, Bland JM, Rose G, Sitzer M, Siebler M. How good is intercenter agreement in the identification of embolic signals in carotid artery disease? Stroke 1996;27:1249-1252 [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-174 [PubMed] [Google Scholar]
- 26.Burdette JH, Ricci PE, Petitti N, Elster AD. Cerebral infarction: time course of signal intensity changes on diffusion-weighted MR images. AJR Am J Roentgenol 1998;171:791-795 [DOI] [PubMed] [Google Scholar]
- 27.Lövblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 1998;19:1061-1066 [PMC free article] [PubMed] [Google Scholar]
- 28.van Everdingen KJ, van der Grond J, Kapelle LJ, Ramos LM, Mali WP. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke 1998;29:1783-1790 [DOI] [PubMed] [Google Scholar]
- 29.Singer MB, Chong J, Lu D, Schonewille WJ, Tuhrim S, Atlas SW. Diffusion-weighted MRI in acute subcortical infarction. Stroke 1998;29:133-136 [DOI] [PubMed] [Google Scholar]
- 30.Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the risks of stroke and death due to carotid endarterectomy for symptomatic and asymptomatic stenosis. Stroke 1996;27:266-269 [DOI] [PubMed] [Google Scholar]
- 31.Rothwell PM, Slattery J, Warlow CP. A systematic review of the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis. Stroke 1996;27:260-265 [DOI] [PubMed] [Google Scholar]
- 32.Hennerici M, Daffershofer M, Jakobs L. Failure to identify cerebral infarct mechanisms from topography of vascular territory lesions. AJNR Am J Neuroradiol 1998;19:1067-1074 [PMC free article] [PubMed] [Google Scholar]
- 33.Bartylla K, Hagen T, Glöbel H, Jost V, Schneider G. Diffusion-weighted magnetic resonance imaging in the diagnosis of cerebral infarct. Radiologe 1997;37:859-864 [DOI] [PubMed] [Google Scholar]


