Abstract
Summary: We report unusual MR serial imaging and electron microscopy findings in a 3-year-old boy who had Leigh syndrome with cytochrome-c oxidase (cox) deficiency. The MR imaging findings included periventricular white matter involvement, posteroanterior progression, and extension through the corpus callosum and internal capsule; however, no basal ganglia or brain stem abnormality was found, which was suggestive of leukodystrophy. The most noteworthy findings were the cystic foci with contrast enhancement in the affected white matter.
Leigh syndrome, or subacute necrotizing encephalomyelopathy, is an inherited, progressive, neurodegenerative disease of infancy or early childhood with variable course and prognosis. (1, 2). The typical MR imaging findings include symmetrical putaminal involvement, which may be associated with the abnormality of the caudate nuclei, globus pallidi, thalami, brain stem, and, less frequently, the cerebral cortex (3). Few cases of white matter involvement have been reported (4). Pathologic examination of Leigh syndrome reveals spongiform degeneration, demyelination, astrocytosis, microglial reaction, vascular proliferation, and cystic cavitation of the cerebral white matter (5–7).
We report the unusual findings on serial MR images and histopathologic examinations in a 3-year-old boy with Leigh syndrome caused by cytochrome-c oxidase (cox) deficiency.
Case Report
A 3-year-old boy, who was healthy until 6 months earlier, when he presented with intermittent lateral deviation of the left eye and progressive mental and motor deterioration. He had no seizures.
He was the third living child of a sixth pregnancy from second-generation, consanguineous parents, and had brothers who died within their first week of life because of respiratory failure.
At admission, he was uncooperative and disoriented. His weight and height were below the third percentile, but his head circumference was normal. He had marked spasticity, having flexor posture on the upper and extensor posture on the lower extremities, with hyperactive deep tendon reflexes, bilateral positive extensor plantar response, and Clonus. Gag reflex and swallowing were absent. Eye movements were normal, with no nystagmus. The fundus examination was unremarkable.
Laboratory data included normal complete blood count, urinalysis, liver and renal functions, with normal triglyceride, cholesterol, and uric acid levels. He had mild metabolic acidosis, with normal anion gap. Serum amino acid screening, blood ammonia, lactate, and pyruvate levels were normal; however, urine amino acid assay showed increased glycine, alanine, and glutamine. CSF analysis was normal, including lactate and pyruvate levels, with lactate to pyruvate ratio measured at 1200:100 mmol/L. EEG showed slowing on background rhythm and no epileptic activity.
Brain MR imaging showed bilateral, symmetrical involvement of the parieto-occipital periventricular white matter, the posterior aspect of the posterior crus of the internal capsules, and abnormality of the corpus callosum at the genu and splenium, with T2 hyperintensity and T1 hypointensity (Fig 1A-B). There were cystic foci within the involved periventricular white matter. Foci of contrast enhancement, mostly located at the periphery of the abnormal areas, were present (Figure 1C). The basal ganglia, thalami, brain stem, and cerebellum were not involved, and the cortex appeared normal. There were no associated atrophic changes. Other tests, including nerve-conduction velocities, electro- and echocardiographies, and lysosomal enzyme and organic acid analysis, revealed no abnormality.
fig 1.
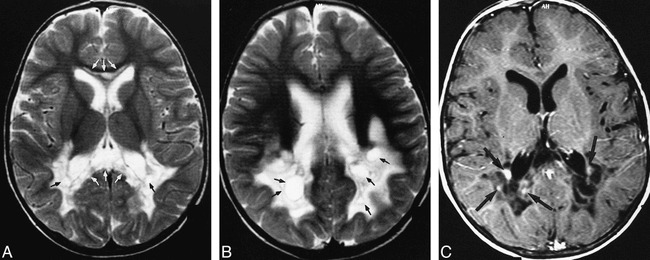
Initial MR examination.
A and B, T2-weighted (3500/98 [TR/TE]) axial turbo spin-echo images showing involvement of the posterior periventricular white matter, symmetrical bilaterally (A and B), with the abnormal signal intensity in the genu and splenium of the corpus callosum (white arrows, A). The hyperintensity extends to the posterior crus of the internal capsule (A). There are well-marginated cystic areas within the abnormal white matter (black arrows). Note the basal ganglia are not affected.
C, Contrast-enhanced T1-weighted (600/14 [TR/TE]) axial image, corresponding to figure A, showing the cystic nature of the periventricular white matter with foci of contrast enhancement mostly at the periphery of the abnormal area (black arrows).
His symptoms were progressively increasing during his clinical course. Follow-up brain MR imaging at 3 months revealed markedly increased periventricular white matter abnormality, with lesions extending anteriorly and involving the entire periventricular white matter, including the frontal and temporal regions. The U-fibers remained unaffected (Figure 2A). Again noted were the cystic changes located particularly in the posterior periventricular white matter. Foci of contrast enhancement were still present at the periphery of the abnormal white matter. The external capsule was partially involved. The basal ganglia, thalami, and the brain stem remained unaffected. On the other hand, the involvement of the corpus callosum was striking (Figure 2B). New lesions appeared in the cerebellar hemispheres. Mild atrophy developed during this interval.
fig 2.
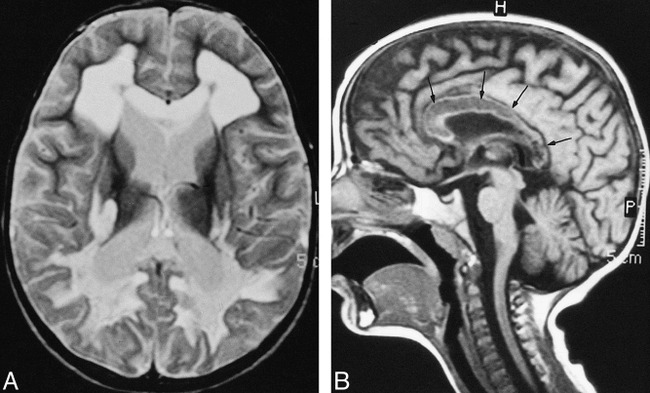
Repeat MR examination at 3 months
A, T2-weighted (2340/90 [TR/TE]) axial turbo spin-echo image through the same level as 1A, showing the progression of periventricular white matter abnormality. Corpus callosum appear swollen and hyperintensity extends further through the internal capsule as well as in the external capsule. Note that the U-fibers are spared.
B, Unenhanced T1-weighted (550/15 [TR/TE]) midsagital image showing diffuse involvement of the corpus callosum, with decreased signal intensity (arrows).
Histochemical investigation of the muscle biopsy specimen revealed no abnormalities except for the absence of cox activity. Mitochondrial DNA analysis, performed by polymerase chain reaction technique, did not show any known mutation.
Histopathologic examination of the brain biopsy specimen revealed thickening of leptomeninges and prominent spongiosis, nonspecific macrophage infiltration, and variable degrees of astrogliosis, where the astrocytes appeared swollen within the white matter. Cerebral cortex had relatively preserved layering. Electron microscopic examination revealed diffusely scattered foam cells within the cerebral white matter (Fig 3).
fig 3.
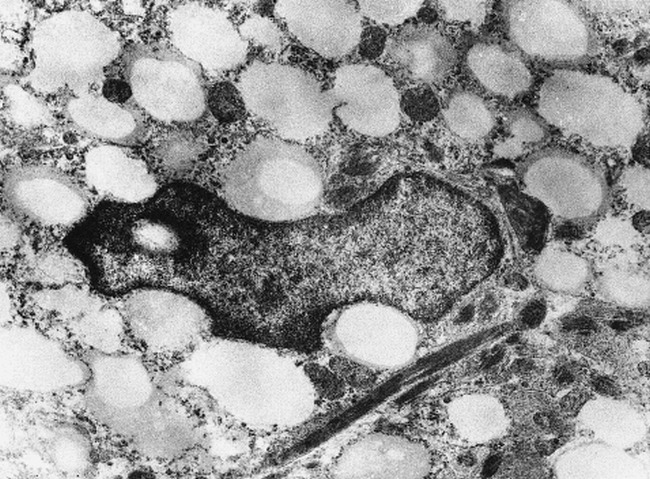
Electron microscopic findings of the brain tissue showing foam cells within the cerebral white matter
The patient was diagnosed with Leigh syndrome resulting from cox deficiency based on clinical presentation and laboratory findings. Mitochondrial cocktail therapy, including coenzyme Q, thiamine, riboflavin, and vitamin C, was started. He showed mild improvement at 6-month follow-up, with spasticity mildly reduced, swallowing function regained, and normal background activity re-established on EEG.
Discussion
The imaging findings of Leigh syndrome have been reported in a number of publications and typical imaging findings have been considered to be the diagnostic hallmark (8, 9). Putaminal involvement has been reported to be the constant feature, and several reports have supported this statement (10, 11). Putaminal lesions have showed increased T2 signal intensity that might be associated with swelling in the acute-subacute phase or shrinkage in the late stage. The other abnormalities were foci of increased T2 signal involving the medulla, substantia nigra, cerebral peduncles, decussation of superior cerebellar peduncles, brachium of inferior colliculi, subthalamic nuclei, thalami, periaquaductal region, globus pallidus, and caudate nuclei. Cortical involvement might also accompany the basal ganglia and brain stem lesions. Involvement of cerebral white matter has only been reported in a few cases (12).
In the presented case, Leigh disease has found to be resulting from cox deficiency. The initial MR examination, obtained at 3 years of age, revealed an appearance of leukodystrophy with increased T2 signal intensity confined to periventricular white matter, particulary at the posterior region, internal capsule, and corpus callosum. The basal ganglia and brain stem were intact. There were well-defined cystic areas within the abnormal white matter and foci of contrast enhancement. The parenchyma appeared swollen with mild mass effect on the ventricles and sulcal effacement. The MR imaging findings were suggestive of primary white matter disease with a “leukodystrophy pattern” rather than gray and white matter involvement. The patient showed rapid deterioration within 3 months. The follow-up MR examination showed significant extension of the white matter abnormality, involving the entire periventricular region and associated parenchymal loss, which was evident by ventricular dilation and, to a lesser degree, by a widened cortical sulci. The sparing of U-fibers was noteworthy. The brain stem and basal ganglia remained intact. Additional involvement of cerebellar white matter, however, was noted. There was still some contrast enhancement, seemingly at the periphery of the abnormal regions.
The diffuse involvement of white matter in the absence of basal ganglia abnormality is uncommon in Leigh syndrome. Harpey et al (12) reported a similar case in which cox deficiency was detected and their patient also presented with isolated diffuse leukodystrophy; however, the entire cerebral white matter was affected, unlike our case, in whom the peripheral white matter was spared. Zafeiriou and colleagues (4) reported two siblings with Leigh disease with clinical and radiologic features suggestive of leukodystrophy. Interestingly, deficient enzyme again was cox. These two patients, however, had pons involvement in addition to diffuse abnormality of the cerebral white matter, and dorsal pons involvement has been previously reported in the cox deficiency. In both reports, the white matter was affected both anteriorly and posteriorly. In our patient, however, the initial examination provided a clue that the white matter involvement preferably started posteriorly, then extended to result in diffuse white matter involvement and the U-fibers remained spared, at least at the onset. In the series of Barkovich et al (11), one patient in a group of seven, had diffuse white matter involvement, but that particular patient also had putaminal and caudate lesions. In the group of Valanne et al (10), three patients had white matter abnormality. One also had cyst-like changes, with involved parenchyma appearing swollen. Frontotemporal white matter was preferentially affected in this patient, unlike the posterior involvement in ours. The remaining patients had diffuse white matter signal intensity change. Moreover, our patient showed prominent symmetrical lesions in the cerebellar white matter, and this has not been reported in any of these previous publications.
The appearance of the abnormal white matter at the periventricular region was peculiar in that there were cystic foci with well-defined margins and small nodular contrast enhancement, primarily at the periphery of the involved parenchyma. This appearance can easily be attributed to characteristic pathologic findings in Leigh syndrome; ie, cystic cavitation and spongiform degeneration in addition to demyelination, astrocystosis, and microglial reaction. The vascular proliferation may possibly be accounted for the contrast enhancement. The electron microscopic finding of diffusely scattered foam cells within the cerebral white matter detected in our patient has not been reported previously. This finding may also have contributed to the appearance of milimetric cystic foci present within the cerebral white matter, in addition to the above-mentioned light microscopy findings.
In conclusion, absence of putaminal involvement does not rule out the diagnosis of Leigh syndrome. Diffuse white matter abnormality, with the appearance of leukodystrophy, may occur in the absence of basal ganglia lesions, the hallmark of the disease. Cox deficiency may present with symmetrical periventricular posteroanterior white matter abnormality, with cystic changes, contrast enhancement, and diffuse corpus callosum involvement on MR images.
Footnotes
Address reprint requests to Prof. Dr. Meral Topçu, Hacettepe University Department of Child Neurology, 06100 Ankara Turkey.
This study was supported by the Turkish Child Neurology Association.
References
- 1.DiMaurio S, Servidei S, Zeivani M, et al. Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol 1987;22:498-506 [DOI] [PubMed] [Google Scholar]
- 2.Santorelli FM, Mak SC, Vazquez-Memije ME, et al. Clinical heterogenity associated with the mitochondrial DNA T8993C mutation. Pediatr Res 1996;39:914-917 [DOI] [PubMed] [Google Scholar]
- 3.Medina L, Chi TL, DeVivo DC, Hilal SK. MR findings in patients with subacute necrotizing encephalomyelopathy (Leigh disease): correlation with biochemical defect. AJNR Am J Neuroradiol 1990;11:379-384 [PMC free article] [PubMed] [Google Scholar]
- 4.Zafeiriou DI, Koletzko B, Mueller-Felber W, Paetzke I, Kueffer G, Jensen M. Deficiency in complex IV (cytochrome c oxidase) of the respiratory chain, presenting as a leukodystrophy in two siblings with Leigh syndrome. Brain Dev 1995;17:117-121 [DOI] [PubMed] [Google Scholar]
- 5.Walter G, Brucher J, Martin J, Ceuterick C, Pilz P, Freund M. Leigh disease-several nosologic entities with an identical histopathological complex. Neuropathol Appl Neurobiol 1986;12:95-107 [DOI] [PubMed] [Google Scholar]
- 6.Leigh D. Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry 1951;14:216-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willems JL, Monnens LAH, Trijbels JMF, et al. Leigh's encephalomyelopathy in a patient with cytochrome c oxidase deficiency. J Inher Metab Dis 1983;6:121-1226321854 [Google Scholar]
- 8.Koch TK, Yee MHC, Hutchinson HT, Berg BO. Magnetic resonance imaging in subacute necrotizing encephalomyelopathy (Leigh disease). Ann Neurol 1986;19:605-607 [DOI] [PubMed] [Google Scholar]
- 9.Greenberg SB, Faerber EN, Riviello JJ, De Leon G, Capitanio MA. Subacute necrotizing encephalomyelopathy (Leigh disease): CT and MRI appearances. Pediatr Radiol 1990;21:5-8 [DOI] [PubMed] [Google Scholar]
- 10.Valanne L, Ketonen L, Majander A, Suomalainen A, Pihko H. Neuroradiological findings in children with mitochondrial disorders. AJNR Am J Neuroradiol 1998;19:369-377 [PMC free article] [PubMed] [Google Scholar]
- 11.Barkovich AJ, Good WV, Koch TK, Berg BO. Mitochondrial disorders: analysis of their clinical and imaging characteristics. AJNR Am J Neuroradiol 1993;14:1119-1137 [PMC free article] [PubMed] [Google Scholar]
- 12.Harpey JP, Heron D, Prudent M, et al. Diffuse leukodystrophy in an infant with cytochrome-c oxidase deficiency. J Inher Metab Dis 1998;21:748-752 [DOI] [PubMed] [Google Scholar]


