Abstract
BACKGROUND AND PURPOSE: Peripheral intracranial aneurysms can be difficult to treat with traditional surgical or embolization techniques that spare the parent vessel. We report the results of our use of coil occlusion of the parent vessel for the treatment of nine peripheral intracranial aneurysms.
METHODS: During approximately a 4-year period, nine patients (six men and three women, 27 to 68 years old; average age, 42 years) presented to our institution with peripheral intracranial aneurysms. The aneurysms were located on branches of the right posterior inferior cerebellar artery (n = 2), the right superior cerebellar artery (n = 1), the right anterior inferior cerebellar artery (n = 1), the right posterior cerebral artery (n = 3), the left middle cerebral artery (n = 1), and the left anterior cerebral artery (n = 1). Parent vessel occlusion was performed using microcoils after test injection with amobarbital (Amytal) in eight of the nine cases (one patient was comatose and could not be tested before occlusion).
RESULTS: Angiography immediately after the procedure showed aneurysmal occlusion in every patient. Follow-up arteriography, performed in six patients 2 to 12 months after treatment, documented continued aneurysmal occlusion in every case. Three patients exhibited mild, nondisabling neurologic deficits after coil placement; the rest had no new deficits, although one patient was severely disabled from the initial hemorrhage and one patient died of an unrelated cause.
CONCLUSION: Our results lend support to the use of parent vessel occlusion for peripheral aneurysms that are difficult to treat surgically or that are not amenable to intra-aneurysmal coil placement.
Surgical clipping of aneurysms is currently the most common treatment for cerebrovascular aneurysms. In some patients, however, the location of the aneurysm, its size, or its morphology (wall calcification, aneurysmal thrombosis, etc.) hinder surgical clipping. In these patients, endovascular embolization of the aneurysm is a viable alternative. The aneurysm can be packed with coils, a technique recently facilitated by the introduction of Guglielmi detachable coils (GDCs; Target Therapeutics, Fremont, CA) (1). On occasion, the diameter of the parent vessel may be too narrow to allow passage of a microcatheter to the aneurysm, the configuration of the aneurysm may not be amenable to coil placement, or conditions may be encountered that make occlusion of the parent vessel the preferred treatment. While parent vessel occlusion of the carotid and vertebral arteries is an accepted and frequently reported method of treatment for aneurysms of these vessels, endovascular occlusion of more peripheral vessels has been reported less frequently. We report our results in nine patients who underwent endovascular coil occlusion of peripheral vessels for the treatment of aneurysms.
Methods
From August 1993 to July 1997, nine patients presented to our institution with peripheral intracranial aneurysms that were treated with embolization of the parent vessel. The patients were 27 to 68 years old (average age, 42 years) at the time of treatment. Six patients were male and three were female. Four patients had subarachnoid hemorrhage (Hunt and Hess grades 1, 2, 2, and 5, respectively) (2), four patients had intracerebral hemorrhage, and one patient had intraventricular hemorrhage. In all nine patients, the aneurysms were located on peripheral intracranial branches either distal to the circle of Willis or within the posterior fossa. Aneurysms were located on the right (n = 1) and left (n = 1) posterior inferior cerebellar arteries (PICA), the right anterior inferior cerebellar artery (AICA) (n = 1), the right superior cerebellar artery (SCA) (n = 1), the right posterior cerebral artery (PCA) (n = 2), the posterior choroidal branch of the left PCA (n = 1), the parietal branch of the left middle cerebral artery (MCA) (n = 1), and the callosomarginal branch of the left anterior cerebral artery (ACA) (n = 1). Aneurysms were classified as small (<10 mm) in seven of the patients and large (>10 mm but <25 mm) in two patients. One aneurysm was believed to be mycotic, two were flow-related (feeding branch of an arteriovenous malformation), one was a pseudoaneurysm related to trauma, and the remaining five were associated with hemodynamically induced degenerative vascular injury (3).
All patients underwent parent vessel occlusion by coil embolization after giving informed consent. All the procedures were performed in the angiography suite using high-resolution digital imaging and roadmapping when necessary. Neuroleptic anesthesia was used in all patients. Anticoagulation was not used in the first six patients; however, patients received heparin during the last three procedures performed, as this had become part of our standard protocol when using GDCs. In these cases, 5000 U of heparin was administered at the start of the procedure, followed by 1000 U every hour until completion.
In eight of the patients, a catheter was placed into the common femoral artery and then passed up into the aortic arch, and selective catheterization of either the vertebral or common carotid artery was performed. In one patient, the left axillary artery was used as an access site because the left vertebral artery was too torturous to allow access through the aortic arch. The diagnostic catheter was then exchanged for an introducer catheter, which was placed as distally as possible into either the internal carotid or vertebral artery. A microcatheter (Fastracker, Target Therapeutics) was passed in a coaxial fashion through the introducer catheter followed by selective catheterization of the artery with the aneurysm in each case. The tip of the catheter was placed proximal to the aneurysm, and 30 mg of amobarbital (Amytal) was injected through the microcatheter in eight of the nine patients (see Table). The patients then underwent neurologic evaluation, except for one patient, who was comatose and could not be examined.
Summary of results of provocative Amytal testing
In six of the patients, coils were placed just proximal to the aneurysm, and in three of the patients, the aneurysm was trapped with coils. GDCs were used in three patients and free coils were used in the remainder. Coils were selected on the basis of the size of the artery to be occluded. The smallest artery was occluded with five 2-mm straight coils. Four of the arteries were occluded with 2-mm diameter by 10-mm long complex helical coils; however, in one of these cases, occlusion was incomplete and 2-mm straight coils were placed within the conglomerate of the complex helical coils. In one patient, in whom the artery was slightly larger, Tornado coils (Cook, Inc, Bloomington, IN) were used. Initially, a 2 × 3-mm Tornado coil was placed, but because it slipped into the aneurysm during injection of contrast material, two 2 × 4-mm Tornados were placed. In three cases, 2 × 20-mm and 2 × 40-mm 0.010 GDCs were placed. In one of these cases, the coil packing was supplemented with 2-mm straight coils to complete the occlusion. Angiography was performed after coil placement to confirm occlusion of the parent vessel and aneurysm (Fig 1).
fig 1.
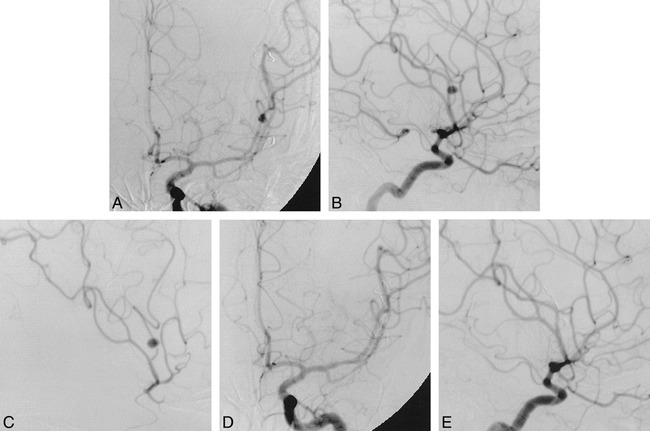
27-year-old man with hemiplegia and aphasia associated with a left parietal lobe hemorrhage who was found to have an aneurysm of the middle cerebral artery (MCA).
A and B, Anteroposterior (A) and lateral (B) views of a left internal carotid arteriogram show an aneurysm on the posterior parietal branch of the left MCA.
C, A selective MCA arteriogram (lateral view) better shows the aneurysm and the small branch from which it arises.
D and E, Anteroposterior (D) and lateral (E) views of a left internal carotid arteriogram after occlusion of the parent vessel no longer show the aneurysm. The aneurysm was trapped by using two 2 × 10-mm coils and 2-mm straight coils.
In eight patients, the postoperative course was uneventful; these patients were discharged, on average, by postoperative day 2 (range, day 1 through day 6). In one patient with a traumatic pseudoaneurysm of the callosomarginal branch of the left ACA, the postoperative course was protracted. This patient had preexisting methicillin-resistant Staphylococcus aureus and subsequently died as a result of sepsis. Clinical follow-up ranged from 1 to 12 months (average, 8 months). Six of the nine patients had follow-up angiography.
Results
Immediate Results
The procedure was technically successful in all cases. Coil occlusion was performed proximal to the aneurysm in six cases, and the aneurysm was trapped in three cases. In all cases, angiography performed immediately after the procedure showed occlusion of the parent vessel with no filling of the aneurysm. Two patients had retrograde flow into the peripheral branches of the occluded artery via leptomeningeal collaterals, but in no case was there flow to the aneurysm itself (Fig 2). We encountered no unanticipated complications related to the procedure.
fig 2.
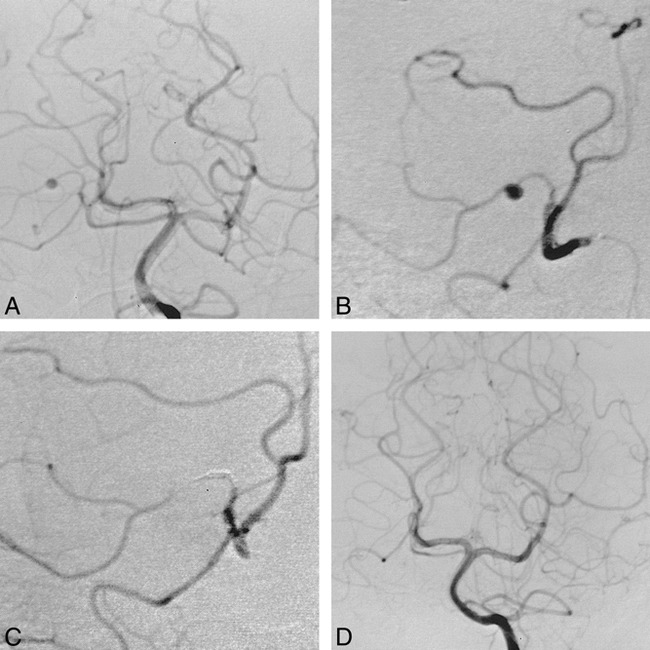
39-year-old man with sudden onset of severe headaches, nausea, and vomiting who was found to have subarachnoid hemorrhage and an aneurysm of the left superior cerebellar artery (SCA).
A, Left vertebral arteriogram (anteroposterior view) shows a small aneurysm on a hemispheric branch of the right SCA.
B, Selective right SCA arteriogram (anteroposterior view) better shows the aneurysm.
C, Postprocedural right SCA arteriogram (anteroposterior view) shows occlusion of the hemispheric branch and the aneurysm. Five 2-mm straight coils were used for occlusion. Note some retrograde filling of the occluded branch.
D, Left vertebral arteriogram (anteroposterior view) obtained 3 months after coil occlusion shows the aneurysm remains occluded.
Provocative Testing
Eight of the patients were tested with an Amytal injection before occlusion of the vessel (see Table). Four of the patients, all of whom had preexisting neurologic deficits from the initial hemorrhage, had no change in their neurologic status. In three patients, the Amytal test was overly predictive of the resultant deficit. In one patient, nausea, dysmetria, and nystagmus developed after injection of the Amytal into the SCA, but after occlusion of the parent vessel, only minimal nondisabling dysmetria remained. Another patient had mild dysmetria with Amytal injection into the PICA, but did not show any deficits after the procedure. A third patient experienced nystagmus, dysmetria, vomiting, dizziness, and decreased hearing during the Amytal test injection. This patient had a complex multilobulated aneurysm along the course of the AICA that was considered to be untreatable by surgery, and the vessel was sacrificed despite the Amytal test. After the procedure, the patient only had hearing loss on the ipsilateral side. In one patient, the Amytal test was correctly predictive of the deficit. This patient incurred partial loss of the left visual field and paresthesias on the left side of his body. Because it was believed that a neurosurgical approach would produce a similar deficit, occlusion of the vessel was performed despite the positive Amytal test. After occlusion, the patient continued to have the deficits predicted by the Amytal test, with some improvement in the deficits over time.
Angiographic and MR Imaging Results
Follow-up angiography was performed in six of the nine patients from 2 to 12 months after embolization. In each of the six patients, the angiogram showed no residual filling of the aneurysm.
Clinical Results
The period of clinical follow-up was 8 months on average (range, 1 to 12 months). One patient died 6 weeks after embolization as a result of preexisting S. aureus infection. Three of the remaining eight patients had deficits from the embolization procedure. One patient had mild right-sided dysmetria after coiling of a right SCA aneurysm. These deficits improved, and by 9 months had nearly resolved. One patient had left-sided paresthesias and a left visual field cut after embolization of a right PCA aneurysm. These deficits improved, and by 12 months the patient reported only minimal paresthesias on the left and minimal left-sided visual field deficit. Finally, one patient with a right AICA aneurysm had ipsilateral hearing loss after the procedure, which persisted. In the remaining five patients, the embolization procedure did not result in any new deficits.
Discussion
Some aneurysms may be difficult to treat with traditional surgical techniques or by placement of intra-aneurysmal coils because of their location, size, or morphology. For some of these aneurysms, an alternative treatment is parent vessel occlusion. This technique has been frequently reported in the literature for treatment of aneurysms of the cavernous or paraclinoid internal carotid artery (4–7) and for aneurysms involving the vertebrobasilar junction (8–10). The technique is successful in these aneurysms because the distal circulation usually has adequate collateral blood flow, primarily from the circle of Willis. Occlusion of peripheral intracranial branches (ie, those that do not have collateral branches from the circle of Willis to bypass the occlusion) has been reported less frequently (11–13).
Hodes et al (11) described 16 patients who were treated for giant intracranial aneurysms that were not amenable to treatment with clips. Eight of the aneurysms treated were peripheral in location (four in the MCA, one in the ACA, two in the PCA, and one in the PICA), similar to those in our series. All the patients in this group who were successfully treated had thrombosis of the aneurysm and minimal or no deficits after vessel occlusion. One patient in this group, in whom test occlusion failed, was treated surgically but subsequently died of MCA thrombosis; a second patient had successful treatment of an MCA aneurysm but suffered a fatal hemorrhage from an untreated aneurysm in the carotid terminus. Platinum microcoils were used in only one of the patients; the rest were treated either with autologous clot or detachable balloons. One of the MCA aneurysms initially treated with a detachable balloon was retreated 4 years later with microcoils, presumably after the vessel recanalized from balloon deflation.
Peripheral vessel occlusion for mycotic aneurysms has also been reported previously (12). These aneurysms are often located distally, making them difficult to isolate at surgery. Additionally, they are very fragile or lack a true wall, which makes it hard to salvage the parent vessel, even at surgery. Thus, treatment via endovascular parent vessel occlusion is well suited to this type of aneurysm. Khayata et al (12) reported on three patients with mycotic aneurysms. Two of these aneurysms (one in the PCA and one in the MCA) were treated with parent vessel occlusion. Embolization was performed with N-butyl cyanoacrylate glue in one case (the PCA) and with autologous clot in the other (the MCA). The third case was treated by placing a detachable balloon within the MCA aneurysm. A 3-month follow-up arteriogram in the patient treated with the detachable balloon revealed aneurysmal recurrence caused by partial balloon deflation, which was successfully treated with microcoils. Teitelbaum et al (13) also reported on the treatment of a mycotic PICA aneurysm using parent vessel occlusion with microcoils.
Treatment of the aneurysms with parent vessel occlusion was planned in most of our cases. Early in our experience, GDCs were not yet available, and filling the aneurysm with coils while preserving the parent vessel was not considered an option. However, had GDCs been available, it is likely that they would have been used in only a few of our patients. In one patient, the aneurysm was mycotic, and another patient had a pseudoaneurysm from trauma. Since these aneurysms lack a stable wall, we would not ordinarily consider them candidates for GDCs. In two cases, the aneurysms were either extremely peripheral or there were excessively tortuous vessels that did not allow access to the aneurysm with the catheter, and thus parent vessel occlusion was performed. In two cases, there was lobulated aneurysmal dilatation along a segment of the vessel that would not have allowed for preservation of the parent vessel, even with GDCs.
In our series of nine patients with peripheral intracranial aneurysms treated by parent vessel occlusion, excellent results were obtained without significant complications. In contrast to most cases previously reported, in which glue embolization or detachable balloons were used, all nine patients in this series were treated by using coils. It is our belief that coils, as compared with balloons, are more likely to create a permanent occlusion if the vessel is well packed. Balloons may deflate (11, 12), resulting in recanalization of the aneurysm. Moreover, in our series, balloons would have been more difficult or even impossible to navigate into position in many of the peripheral branches we occluded. As compared with glue and autologous clot, coils can be placed more precisely, with less chance of distal embolization, which would block possible collateral branches. There is also a chance that autologous clot could recanalize before thrombosis of the aneurysm.
In eight of our patients, neurologic status was monitored before final placement of coils by an Amytal injection. Amytal was correctly predictive of the resultant neurologic status in five of these cases and was overly predictive of deficit in three. We believe that Amytal testing is overly predictive of deficits owing to its deep penetration into all of the peripheral vessels. When the vessel is actually occluded, collaterals may partially bypass the occlusion, which accounts for the discrepancy. We performed the Amytal testing to get an idea of the deficit the patient might expect when the artery was occluded. We discussed with our neurosurgical colleagues all the patients in whom a new deficit developed before the vessel was occluded. The only patient in whom a severe, possibly disabling deficit (nystagmus, dysmetria, vomiting, dizziness, and decreased hearing) developed had a complex multilobulated aneurysm along the course of the AICA, and it was believed that even surgery would require occlusion of the vessel. Fortunately, this patient experienced only decreased hearing on the ipsilateral side.
Conclusion
Until recently, there has been little evidence that parent vessel occlusion of peripheral aneurysms was an efficacious or safe alternative for the treatment of aneurysms. In our series, excellent results were obtained, with successful occlusion of the parent vessel in all nine patients. Follow-up arteriograms, which were available in most of our patients, confirmed continued aneurysmal occlusion. In three patients, new deficits developed after the procedure, but all were mild and nondisabling, and improved over time. With the exception of one death that was unrelated to the procedure, all the patients had uneventful hospital courses with discharge only a few days after the procedure (day 2, on average). We attribute the low complication rate to leptomeningeal collateral blood flow in some cases, to occlusion of nonelegant branches in other cases, and to occlusion of branches that supply portions of brain damaged by previous hemorrhage in other cases. We believe our results lend support to the use of parent vessel occlusion for aneurysms peripheral to the circle of Willis in cases in which direct embolization of the aneurysm is not possible.
Footnotes
Presented in part at the annual meeting of the Congress of Neurological Surgeons, New Orleans, September 1997.
Address reprint requests to Donald Eckard, MD, Department of Diagnostic Radiology, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS 66160.
References
- 1.Martin D, Rodesch G, Alvarez H, Lasjaunias P. Preliminary results of embolization of nonsurgical intracranial aneurysms with GD coils: the 1st year of their use. Neuroradiology 1996;38:S142-S150 [DOI] [PubMed] [Google Scholar]
- 2.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14-20 [DOI] [PubMed] [Google Scholar]
- 3.Stehbens WE. Etiology of I.C. berry aneurysms. J Neurosurg 1989;70:823-831 [DOI] [PubMed] [Google Scholar]
- 4.Berenstein A, Ransohoff J, Kupersmith M, Flamm E, Graeb D. Transvascular treatment of giant aneurysms of the cavernous carotid and vertebral arteries. Surg Neurol 1984;21:3-12 [DOI] [PubMed] [Google Scholar]
- 5.Fox AJ, Vinuela F, Pelz DM, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg 1987;66:40-46 [DOI] [PubMed] [Google Scholar]
- 6.Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg 1990;72:857-863 [DOI] [PubMed] [Google Scholar]
- 7.Nichols DA, Meyer FB, Piepgras DG, Smith PL. Endovascular treatment of intracranial aneurysms. Mayo Clin Proc 1994;69:272-285 [DOI] [PubMed] [Google Scholar]
- 8.Aymard A, Gobin YP, Hodes JE, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg 1991;74:393-398 [DOI] [PubMed] [Google Scholar]
- 9.Higashida RT, Halbach VV, Cahan LD, Hieshima GB, Konisha Y. Detachable balloon embolization therapy of posterior circulation intracranial aneurysms. J Neurosurg 1989;71:512-519 [DOI] [PubMed] [Google Scholar]
- 10.Picard L, Bracard S, Lehericy S, et al. Endovascular occlusion of intracranial aneurysms of the posterior circulation: comparison of balloons, free coils, and detachable coils in 38 patients. Neuroradiology 1996;38:S133-S141 [DOI] [PubMed] [Google Scholar]
- 11.Hodes JE, Aymard A, Gobin YP, et al. Endovascular occlusion of intracranial vessels for curative treatment of unclippable aneurysms: report of 16 cases. J Neurosurg 1991;75:694-701 [DOI] [PubMed] [Google Scholar]
- 12.Khayata MH, Aymard A, Casasco A, Herbreteau D, Woimant F, Merland JJ. Selective endovascular techniques in the treatment of cerebral mycotic aneurysms. J Neurosurg 1993;78:661-665 [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum GP, Dowd CF, Larsen DW, et al. Endovascular management of biopsy-related posterior inferior cerebellar artery pseudoaneurysm. Surg Neurol 1995;43:357-359 [DOI] [PubMed] [Google Scholar]


