Abstract
BACKGROUND AND PURPOSE: Sonography, CT, and MR imaging are commonly used to screen for neonatal intracranial ischemia and hemorrhage, yet few studies have attempted to determine which imaging technique is best suited for this purpose. The goals of this study were to compare sonography with CT and MR imaging prospectively for the detection of intracranial ischemia or hemorrhage and to determine the prognostic value(s) of neuroimaging in neonates suspected of having hypoxic-ischemic injury (HII).
METHODS: Forty-seven neonates underwent CT (n = 26) or MR imaging (n = 24) or both (n = 3) within the first month of life for suspected HII. Sonography was performed according to research protocol within an average of 14.4 ± 9.6 hours of CT or MR imaging. A kappa analysis of interobserver agreement was conducted using three independent observers. Infants underwent neurodevelopmental assessment at ages 2 months (n = 47) and 2 years (n = 26).
RESULTS: CT and MR imaging had significantly higher interobserver agreement (P < .001) for cortical HII and germinal matrix hemorrhage (GMH) (Grades I and II) compared with sonography. MR imaging and CT revealed 25 instances of HII compared with 13 identified by sonography. MR imaging and CT also revealed 10 instances of intraparenchymal hemorrhage (>1 cm, including Grade IV GMH) compared with sonography, which depicted five. The negative predictive values of neuroimaging, irrespective of technique used, were 53.3% and 58.8% at the 2-month and 2-year follow-up examinations, respectively.
CONCLUSION: CT and MR imaging have significantly better interobserver agreement for cortical HII and GMH/intraventricular hemorrhage and can reveal more instances of intraparenchymal hemorrhage compared with sonography. The absence of neuroimaging findings on sonograms, CT scans, or MR images does not rule out later neurologic dysfunction.
Sonography, CT, and MR imaging are all commonly used to screen for neonatal intracranial ischemia and hemorrhage (1−11). Few studies, however, address the issue of which imaging techniques and protocols are most appropriate for the diagnosis of neonatal intracranial hemorrhage and ischemia, both clinically and for research protocols (12−16). Sonography, although the most commonly used imaging technique in neonates, is less sensitive and less specific for the detection of intracranial ischemia and hemorrhage compared with CT or MR imaging (7, 17−19). It is unclear, however, whether the greater expense and logistical difficulties in performing CT and MR imaging in critically ill neonates are justified (20, 21). Furthermore, the lack of white matter myelination and patterns of ischemic and hemorrhagic injury are markedly different in premature infants compared with term infants and are additional complicating factors in any cost-benefit analysis of the neuroimaging of neonates with suspected hypoxic-ischemic injury (HII) (17).
Considering the progressive limitations of medical resources, imaging techniques should be judged primarily on their relative capabilities to depict the characteristics of intracranial abnormalities. An additional but equally important set of clinical considerations are those regarding the impact of specific imaging findings on short- and long-term care of neonates as well as prognosis (13−15, 20−25).
In a previous study, the interobserver variability of sonography was compared with that of CT and MR imaging for the detection of neonatal intracranial ischemia and hemorrhage (17). It was found that the sensitivity and interobserver agreement were significantly better for MR imaging and CT in the detection of cortical ischemia/infarction as compared with sonography. The study, however, was retrospective, and the time between the performance of sonography and CT or MR imaging ranged between 0 and 72 hours, with an average of 1.41 ± 0.99 days (33.8 ± 24.0 hours) between imaging studies. In addition, there was no long-term follow-up of neurodevelopment.
We herein report a prospective double-blinded imaging study of 47 neonates who were admitted consecutively to the neonatal intensive care unit (NICU) and who underwent sonography and CT or MR imaging within a 30-hour period at our institution. Our goal was to analyze prospectively the sensitivity and interobserver variability of sonography compared with CT or MR imaging in a group of preterm and term infants who underwent CT or MR imaging studies for the assessment of suspected HII as part of their clinical management in the NICU.
Methods
Patient Population
Forty-seven neonates for whom CT or MR imaging was ordered as part of the clinical care in the NICU were prospectively identified by the department of pediatric radiology and consecutively entered into the study between July 1995 and June 1996. There were 22 male and 25 female patients. Twelve patients were younger than 34 weeks. The remaining infants were term or near-term infants. Once identified, patients underwent portable sonography, which was performed by the pediatric radiology department, at no charge to the family, under the direction of the human subjects institutional review board.
Clinical data included birth weight, estimated gestational age, Apgar scores, age at imaging, and time between sonography and CT or MR imaging. These data are summarized in Table 1. The diagnoses at admission to the neonatal unit and clinical indications for ordering CT or MR imaging are summarized in Table 2. Neonates scheduled for CT or MR imaging who underwent sonography had their sonography study performed within 14.4 ± 9.6 hours of CT (n = 23) or MR imaging (n = 21). Three neonates underwent both CT and MR imaging within 9.5 ± 9.1 hours of sonography. Sonography was performed and interpreted without knowledge of CT or MR imaging findings. Patients with known congenital CNS malformations, infections, or tumors, or in whom sonography could not be performed within 30 hours of CT or MR imaging by the pediatric radiology department, were excluded from the study.
TABLE 1:
Patient characteristics (n = 47)
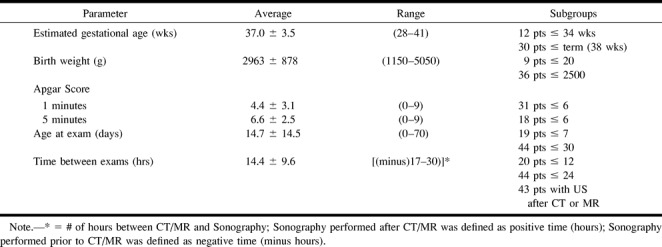
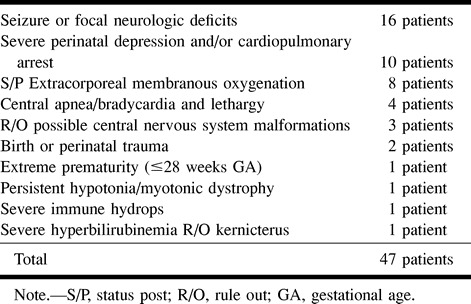
TABLE 2:
Associated diagnoses in neonates undergoing CT or MR for suspected HII
Imaging Studies
Sonography was performed with an Acuson 128 XP/10 unit (Mountain View, CA) using a V7 (7 MHz) electronic sector probe. Standard images in sagittal and coronal planes were obtained through the anterior fontanelle. Posterior sagittal views were also obtained from each patient via the posterior fontanelle.
Unenhanced CT was performed on 23 neonates with a GE 9800 helical scanner (GE Medical Systems, Milwaukee, WI) using the single-section mode at 120 keV, 200 mA, and 2-second scan time per section; 5 × 5-mm contiguous axial sections were obtained. Three studies were performed on an Imatron 12.35 electron beam scanner (So. San Francisco, CA) in cone-beam mode at 130 keV, 650 mA, and 1-second scan time per section; 6 × 6-mm contiguous axial sections were obtained.
MR imaging was performed with a 1.5-T Signa unit (GE Medical Systems) using a quadrature head coil. T1-weighted sagittal images consisted of 5-mm-thick sections with 1-mm section separation, 600–800/16–20 /1 (TR/TE/excitations), and a 256 × 256 matrix. Axial images were obtained as T1-weighted single-echo and T2-weighted dual-echo sequences. A 5-mm section thickness with 2- to 2.5-mm section separation was used. T1-weighted series were obtained with 600−800/16–20/1 and a 256 × 256 matrix. T2-weighted dual-echo sequences were obtained with 3000/30 and 120/1, flow compensation, and a 256 × 256 matrix.
Image Analysis
All sonograms were reviewed by three senior neuroradiologists (A.M.N., B.L., D.R.E.) who are responsible for the routine joint clinical reading of sonograms of the neonatal head on a daily basis with the pediatric radiology department. Sonography was prospectively performed by or under the direction of a pediatric radiologist (F.G.B.). The neuroradiologists were blinded to interpretations, patient history, and previous radiologic reports. The corresponding CT scans and MR images were evaluated by the same three neuroradiologists, blinded to the reviews of other investigators and to their own sonography scores. All CT scans and MR images were graded after the sonograms had been scored, regardless of the chronologic order of the imaging studies.
Using the grading system originally proposed by Papile et al (26), images were assessed for the following abnormalities: germinal matrix hemorrhage (GMH) Grades I through IV, including intraventricular hemorrhage (IVH) and parenchymal extension (PE) ; non-matrix-related hemorrhage, including intraparenchymal hemorrhage (IPH) and extra-axial (subdural/epidural/subarachnoid) hemorrhage; HII of the cortex, basal ganglia, brain stem, thalamus, or cerebellum; and periventricular leukomalacia (PVL). Each abnormality was scored on a scale of 0 to 4 (0 = definitely not present, 1 = probably not present, 2 = indeterminate, 3 = probably present, 4 = definitely present). An abnormality was considered present if at least two observers assigned a particular finding a minimum score of three.
Neurologic Assessment
Neurodevelopmental outcome and neurologic disabilities were graded by a senior neonatologist (W.D.R.) without knowledge of the neuroimaging scores. The neonatologist reviewed all clinical records, with the exclusion of radiology reports. Overall neurologic assessments were grouped into three categories: normal, suspect, and abnormal. Abnormal findings included major tone or motor abnormalities at discharge and a history of seizures during hospitalization or a moderate to severely abnormal EEG. Suspect findings included a mildly abnormal EEG, mild tone, or general movement (motor) abnormalities. Infants were then followed up for up to 2 years of age by the department of pediatrics or the patient's primary pediatrician or both. Twenty-one infants were lost to follow-up after the age of 2 months. Normal outcomes excluded all of the above-mentioned neurologic findings.
Statistical Analysis: Interobserver Agreement
For each technique, possible findings from the examinations of the 47 participants were rated as one of five categories (0 = definitely not present, 1 = probably not present, 2 = indeterminate, 3 = probably present, 4 = definitely present) by the three observers (A.M.N., B.L., D.R.E.). The kappa statistic for multiple ratings per participant was used to measure intergrader agreement (27). Kappa statistics of less than 0.40 indicate poor agreement beyond chance, 0.41 to 0.75 indicates fair to good agreement, and greater than 0.75 indicates excellent agreement. Because kappa statistics depend on category prevalence (ie, low kappa values exist for rare events), the number of events (number of imaging findings) is presented with each finding's kappa (28). Kappa statistical analyses can be applied to situations such as neonatal neuroimaging when an abnormality cannot be assessed directly (ie, pathologic confirmation). In these circumstances, the reliability of that imaging technique can be regarded as the upper bound for accuracy, according to Fleiss (27).
Hypothesis tests were evaluated as statistically significant when P values were less than .05. The individual and overall kappa statistics, their associated standard errors, and the one-tailed Student's t test for null hypotheses ratings are independent and were calculated as described by Fleiss (27). Comparisons of kappa statistics between imaging techniqes were conducted using the χ2 test with one degree of freedom. When two techniques were compared, the technique with the statistically higher kappa value was considered to be more accurate for the detection of a specific imaging finding.
Results
Kappa Analysis of Interobserver Variability
The highest kappa values for Group A (neonates for whom sonography findings were compared with CT results) were observed for the detection of GMH/IVH (κ = 0.712) and cortical HII (κ = 0.548) by CT (see Table 3). Both kappa values were significantly greater than were the corresponding values for sonography (P < .001). CT revealed 15 instances of cortical HII compared with seven revealed by sonography. CT also showed three instances of GMH/IVH compared with four seen by sonography.
TABLE 3:
Kappa analysis of interobserver variability
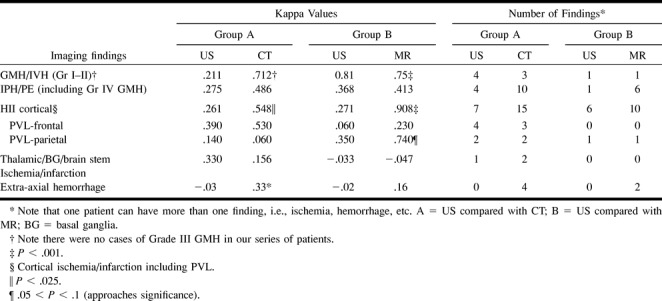
CT revealed 10 instances of IPH/PE, whereas sonography revealed four. CT, however, did not have a significantly higher degree of interobserver agreement for IPH/PE compared with sonography (κ = 0.486 versus κ = 0.275, respectively). In addition, all four examples of IPH/PE revealed by CT (Group A), which measured 1 cm or greater in diameter, were also revealed by sonography.
Both CT and sonography had poor interobserver agreement for the diagnosis of thalamic/basal ganglia/brain stem ischemia/infarction without a significant difference between kappa values. Sonography revealed only one instance of thalamic/basal ganglia/brain stem ischemia/infarction, compared with two found by CT. For the diagnosis of PVL, there were no significant differences in either the number of findings observed or the interobserver agreement for sonography compared with CT. CT revealed four cases of extra-axial hemorrhage (less than 1 cm maximal thickness) that were not revealed by sonography.
The highest kappa values for Group B (neonates for whom sonography findings were compared with MR imaging results) were found for the detection of cortical HII (κ = 0.908) and GMH/IVH (κ = 0.75) by MR imaging; both kappa values were significantly higher than the corresponding values for sonography (P < .001). MR imaging also showed four more instances of cortical HII than did sonography (10 versus six, respectively). MR imaging depicted five more findings of IPH/PE than did sonography, all 1 cm or greater in diameter, although the interobserver agreement was similar for both techniques. There was only one case of PVL in Group B revealed by both sonography and MR imaging, without a significant technique-related difference between kappa values. MR imaging revealed two cases of extra-axial hemorrhage (less than 1 cm maximal thickness) not revealed by sonography, although without a significant interobserver agreement difference.
Neurodevelopmental Outcome and Neuroimaging Findings
An analysis of specific neuroimaging findings with respect to short-term (2 months) and long-term (2 years) neurologic outcome was performed. Eighty-three and three-tenths percent (10 of 12 cases) of neonates with findings of ischemic injury (with and without associated hemorrhage) had abnormal neurologic examinations at age 2 months, whereas 100% (five of five cases) had abnormal neurodevelopment at age 2 years. Significant isolated hemorrhagic abnormalities (ie, parenchymal hemorrhages 1 cm or greater in diameter) had worse correlation with neurologic status at 2-month and 2-year follow-up examinations, with values of 60% (three of five cases) and 66% (two of three cases), respectively. Of note is that the neurologic examinations of neonates with normal imaging studies had low negative predictive values of 53.3% (ie, 16 normal neurodevelopmental outcomes in 30 normal pairs of sonography and CT or MR imaging studies) and 58.8% (10 of 17) at 2-month and 2-year follow-up examinations, respectively.
Discussion
A previous retrospective study of 72 neonates showed that CT and MR imaging had significant advantages over sonography for the detection of neonatal intracranial ischemia and hemorrhage (17). The weaknesses of this work were its retrospective nature and the time that had elapsed between sonography and CT or MR imaging (33.8 ± 24.0 hours). In the current study, we wanted to improve the study design by ensuring that most, if not all, sonography examinations (n = 44) were performed within 24 hours of CT or MR imaging (average, 14.4 ± 9.6 hours; range, 17 hours before to 30 hours after MR imaging or CT). Because patients were necessarily included in the study only when CT or MR imaging was ordered by the NICU because of clinical suspicion of HII, there was a strong selection bias toward term infants who, although ill, could be safely moved for CT or MR imaging, as opposed to preterm neonates who tended to be less stable and to have multiple medical difficulties.
For the diagnosis of cortical HII, both MR imaging and CT had significantly better interobserver agreement, with kappa values of 0.908 and 0.548 versus 0. 271 and 0.261 for MR imaging and CT, respectively, as compared with sonography. MR imaging and CT also revealed more cases of cortical HII than did sonography (presence of finding was a rating of 3 or greater by two or more independent observers), with the total number of cases of cortical HII revealed by MR imaging and CT of 25 versus 13 revealed by sonography. The differences between sonography, CT, and MR imaging for the detection of cortical HII could not be explained by the progressive evolution of ischemic injuries because there was a narrow window of time between the performance of each examination.
The finding of ischemic injury had a major adverse impact on neurodevelopmental outcome. Nonetheless, because most (74%) of our study population were term infants, there was a low incidence of PVL (watershed ischemia of the immature cerebrovascular system), making definitive conclusions about the relative values of sonography, CT, and MR imaging for the neuroimaging of preterm infants difficult.
Surprisingly, CT and MR imaging had better interobserver agreements for the finding of GMH/IVH (ie, Grade I and II bleeds only) compared with sonography, although sonography revealed one more case than did CT. This result was also noted previously by Blankenberg et al (17), although the previous study had possibly significant time lapses between MR imaging, CT, and sonography. The kappa values for the detection of GMH/IVH were relatively low in our series compared with those of previous investigations (12). The reasons for our low kappa values were a low prevalence of a particular finding, such as GMH/IVH, in our study group of predominantly term infants and multiple (three versus two) independent observers (17, 27, 28). These, in addition to other factors, may have affected our results. The presence of GMH/IVH (Grades I and II only; there were no Grade III hemorrhages in our study, and Grade IV hemorrhages were grouped with IPH/PE) were not associated with a worse neurodevelopmental outcome in our series.
CT and MR imaging depicted 16 instances of IPH (including Grade IV GMH) compared with sonography, which showed a total of five, despite similar interobserver agreements for all three techniques (κ = 0.275−0.486; ie, poor to fair interobserver agreement). Although the detection of parenchymal hemorrhage (at least 1 cm in diameter) had less of an association with abnormal neurodevelopmental outcome than did the detection of ischemic injury in our series, it still represented a major adverse prognostic finding.
All techniques performed poorly in the depiction of thalamic/basal ganglia/brain stem ischemia, a result found by previous investigators (17, 18). MR imaging and CT revealed six examples of extra-axial hemorrhage, whereas sonography showed none. The presence of isolated extra-axial hemorrhage (less than 1 cm in maximal thickness in the current series), however, did not adversely impact neurologic outcome (data not shown).
The prognostic significance of neuroimaging findings in our population for the exclusion of future abnormal neurodevelopment was, regardless of technique used, with negative predictive values of 53.3% and 58.8% at 2 months and 2 years of follow-up, respectively. This is partly because of the low previous probability (prevalence) of normal infants in our selected population in which all neonates underwent CT or MR imaging for suspected HII. In a less selected population, such as all premature infants with less than 1250-g birthweight and a 32-week estimated gestational age (a group usually screened for PVL and GMH/IVH by sonography), one would expect the negative predictive values (ie, the accuracy in the exclusion of future neurologic disease) to improve markedly. Because our study group was small in size and because 44% of the patients were lost to 2-year follow-up, we did not attempt to calculate absolute sensitivities and specificities of each technique with respect to neurodevelopmental outcome.
The presence of ischemic injury and hemorrhage (irrespective of the imaging technique used for diagnosis) seems to be a strong indicator of infants' being currently with, or at risk for, neurodevelopmental difficulties. Other techniques, such as a positron-emission tomography, single-photon-emission CT, MR spectroscopy, functional and diffusion-weighted MR imaging, and sonographic power Doppler may also prove to be more useful in the acute antenatal situation in the detection of regional cerebral blood flow/perfusion or metabolic disturbances than conventional sonography, CT, and MR imaging to future neurodevelopmental sequelae (29−38). These issues are particularly important for screening because sonography, CT, and MR imaging are all relatively poor at excluding future neurodevelopmental problems.
Cost containment issues unfortunately will impact on the clinical use of sonography, CT, and MR imaging and on the emerging imaging technologies listed above for the screening of neonates with suspected HII. Clearly, techniques that have high interobserver agreement and that are sensitive in the detection of abnormalities, particularly intracranial ischemia, despite cost concerns, may have ultimately better cost-benefit ratios in the management of neonates suffering form HII despite lower-cost techniques, such as sonography. Further prospective work needs to be conducted comparing both conventional and future neuroimaging techniques with respect to cost-benefit issues (17).
Conclusion
MR imaging and CT have significant advantages over sonography for the diagnosis of intracranial ischemia and hemorrhage in neonates with suspected HII. Nevertheless, early screening for infants at risk for future neurodevelopmental delays for more effective intervention remains problematic. New neuroimaging technologies that examine both physiologic and biochemical abnormalities may provide more insight into the complexity of the pathophysiology of HII and may allow for earlier diagnosis and therapeutic intervention in neonates suffering from HII.
Footnotes
Address reprint requests to Francis G. Blankenberg, MD, Department of Radiology, Neonatal Medicine, Stanford University School of Medicine, 300 Pasteur Drive, Stanford, CA 94305.
References
- 1.Quisling RG, Reeder JD, Setzer ES, Kaude JV. Temporal comparative analysis of computed tomography with ultrasound for intracranial hemorrhage in premature infants. Neuroradiology 1983;24:205-211 [DOI] [PubMed] [Google Scholar]
- 2.Enzmann D, Murphy-Irwin K, Stevenson D, Ariagno R, Barton J, Sunshine P. The natural history of subependymal germinal matrix hemorrhage. Am J Perinatol 1985;2:123-133 [DOI] [PubMed] [Google Scholar]
- 3.Hay CT, Rumack CM, Horgan JG. Cranial sonography: intracranial hemorrhage, periventricular leukomalacia, and asphyxia. Clin Diagn Ultrasound 1989;24:25-42 [PubMed] [Google Scholar]
- 4.Cohen HL, Haller JO. Advances in perinatal neurosonography. AJR Am J Radiol 1994;163:801-810 [DOI] [PubMed] [Google Scholar]
- 5.Matamoros A, Anderson JC, Mc Connell J, Bolam DL. Neurosonographic findings in infants treated with extracorporeal membrane oxygenation (ECMO). J Child Neurol 1989;4(suppl):52-61 [DOI] [PubMed] [Google Scholar]
- 6.Babcock DS, Matsumoto JS. Update on cranial sonography of the infant. In: Rifkin MD, Charboneau JW, Laing FC, eds. Syllabus: A Special Course in Ultrasound 1991. Oak Brook: Radiological Society of North America; 1991:337-345 [Google Scholar]
- 7.von Allmen D, Babcock D, Matsumoto J, et al. The predictive value of head ultrasound in the ECMO candidate. J Pediatr Surg 1992;27:36-39 [DOI] [PubMed] [Google Scholar]
- 8.Keeney SE, Adcock EW, McArdle CB. Prospective observation of 100 high-risk neonates by high-field (1.5 Tesla) magnetic resonance imaging of the central nervous system: I: intraventricular and extracerebral lesions. Pediatrics 1991;87:421-430 [PubMed] [Google Scholar]
- 9.Keeney SE, Adcock EW, McArdle CB. Prospective observation of 100 high-risk neonates by high-field (1.5 Tesla) magnetic resonance imaging of the central nervous system: II: lesions associated with hypoxic-ischemic encephalopathy. Pediatrics 1991;87:431-438 [PubMed] [Google Scholar]
- 10.Christophe C, Clercx A, Blum D, Hasaerts D, Segebarth C, Perlmutter N. Early MR detection of cortical and subcortical hypoxic-ischemic encephalopathy in full-term infants. Pediatr Radiol 1994;24:581-584 [DOI] [PubMed] [Google Scholar]
- 11.Byrne P, Welch R, Johnson MA, Darrah J, Piper M. Serial magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. J Pediatr 1990;117:694-700 [DOI] [PubMed] [Google Scholar]
- 12.Pinto-Martin J, Paneth N, Witomski T, et al. The Central New Jersey Neonatal Brain Haemorrhage Study: design of the study and reliability of ultrasound diagnosis. Paediatr Perinat Epidemiol 1992;6:273-284 [DOI] [PubMed] [Google Scholar]
- 13.Pinto-Martin JA, Riolo S, Cnaan A, Holzman C, Susser MW, Paneth N. Cranial ultrasound prediction of disabling and nondisabling cerebral palsy at age two in a low birth weight population. Pediatrics 1995;95:249-254 [PubMed] [Google Scholar]
- 14.Bulas DL, Glass P, O'Donnell RM, Taylor GA, Short BL, Verzina GL. Neonates treated with ECMO: Predictive value of CT and US neuroimaging findings on short-term neurodevelopmental outcome. Radiology 1995;195:407-412 [DOI] [PubMed] [Google Scholar]
- 15.Aziz K, Vickar DB, Sauve RS, Etches PC, Pain KS, Robertson CMT. Province-based study of neurologic disability of children weighing 500 through 1249 grams at birth in relation to neonatal cerebral ultrasound findings. Pediatrics 1995;95:837-844 [PubMed] [Google Scholar]
- 16.Boal DKB, Watterberg KL, Miles S, Gifford KL. Optimal cost-effective timing of cranial ultrasound screening in low-birth-weight infants. Pediatri Radiol 1995;25:425-428 [DOI] [PubMed] [Google Scholar]
- 17.Blankenberg FG, Norbash AM, Barton L, Stevenson DK, Bracci PM, Enzmann DR. Neonatal intracranial ischemia and hemorrhage: diagnosis with US, CT and MR imaging. Radiology 1996;199:253-259 [DOI] [PubMed] [Google Scholar]
- 18.Barkovich JA, Westmark K, Partridge C, Sola A, Ferriero DM. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J of Neuroradiol 1995;16:427-438 [PMC free article] [PubMed] [Google Scholar]
- 19.Bulas DL, Taylor GA, O'Donnell RM, Short BL, Fitz CR, Vezina G. Intracranial abnormalities in infants treated with extracorporeal membrane oxygenation: update on sonographic and CT findings. AJNR Am J Neuroradiol 1996;17:287-294 [PMC free article] [PubMed] [Google Scholar]
- 20.van de Bor M, den Ouden L, Guit GL. Value of cranial ultrasound and magnetic resonance imaging in predicting neurodevelopmental outcome in preterm infants. Pediatrics 1992;90:196-199 [PubMed] [Google Scholar]
- 21.Skranes JS, Vik T, Nilsen G, et al. Cerebral magnetic resonance imaging (MRI) and mental and motor function of very low birth weight infants at one year of corrected age. Neuropediatrics 1993;24:256-262 [DOI] [PubMed] [Google Scholar]
- 22.Scher MS, Belfar H, Martin J, Painter MJ. Destructive brain lesions of presumed fetal onset: antepartum causes of cerebral palsy. Pediatrics 1991;88:898-906 [PubMed] [Google Scholar]
- 23.Allan WC, Riviello JJ. Perinatal cerebrovascular disease in the neonate: parenchymal ischemic lesions in term and preterm infants. Pediatr Clin North Am 1992;39:621-650 [DOI] [PubMed] [Google Scholar]
- 24.Menkes JH, Curran J. Clinical and MR correlates in children with extra pyramidal cerebral palsy. AJNR Am J Neuroradiol 1994;15:451-457 [PMC free article] [PubMed] [Google Scholar]
- 25.Ford LM, Steichen J, Steichen Asch PA, Babcock D, Fogelson MH. Neurologic status and intracranial hemorrhage in very-low-birth-weight preterm infants. AJDC 1989;143:1186-1190 [DOI] [PubMed] [Google Scholar]
- 26.Papile LA, Burnstein J, Burnstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants less than 1500 gm. J Pediatr 1978;92:529-534 [DOI] [PubMed] [Google Scholar]
- 27.Fleiss JL. Statistical Method for Rates and Proportions. 2nd ed. New York: Wiley; 1981;
- 28.Donker DK, Hasman A, Van Geijn HP. Interpretation of low kappa values. Int J Biomed Comput 1993;33:55-64 [DOI] [PubMed] [Google Scholar]
- 29.Blankenberg FG, Loh N-N, Norbash AM, et al. Impaired cerebrovascular autoregulation after hypoxic-ischemic injury in extremely low-birth-weight neonates: detection with power and pulsed wave Doppler US. Radiology 1997;205:563-568 [DOI] [PubMed] [Google Scholar]
- 30.Azzarelli B, Caldemeyer KS, Phillips JP, DeMyer WE. Hypoxic-ischemic encephalopathy in areas of primary myelination: a neuroimaging and PET study. Pediatr Neurol 1996;14:108-116 [DOI] [PubMed] [Google Scholar]
- 31.Volpe JS, Herscovitch P, Perlman JM, Raichle ME. Position emission tomography in the newborn: Extensive impairment of regional blood flow with intraventricular hemorrhage and hemorrhagic intracerebral involvement. Pediatrics 1983;72:589-601 [PubMed] [Google Scholar]
- 32.Mountz JM, Deutsch G, Khan SH. Regional cerebral blood flow changes in stroke imaged by Tc-99m HMPAO SPECT with corresponding anatomic image comparison. Clin Nucl Med 1993;18:1067-1082 [DOI] [PubMed] [Google Scholar]
- 33.Martin E, Buchli R, Ritter S, et al. Diagnostic and prognostic value of cerebral 31P magnetic resonance spectroscopy in neonates with perinatal asphyxia. Pediatr Res 1996;40:749-758 [DOI] [PubMed] [Google Scholar]
- 34.Toft PB, Leth H, Lou HC, Pyrds O, Peitersen B, Henriksen O. Local vascular CO2 reactivity in the infant brain assessed by functional MRI. Pediatr Radiol 1995;25:420-424 [DOI] [PubMed] [Google Scholar]
- 35.Cowan FM, Pennock JM, Hanrahan JD, Manji KP, Edwards AD. Early detection of cerebral infarction and hypoxic ischemic encephalopathy in neonates using diffusion-weighted magnetic resonance imaging. Neuropediatrics 1994;25:172-175 [DOI] [PubMed] [Google Scholar]
- 36.Taylor GA, Short BL, Walker LK, Traystman RJ. Intracranial blood flow: quantification with duplex Doppler color doppler flow US. Radiology 1990;176:231-236 [DOI] [PubMed] [Google Scholar]
- 37.Taylor GA, Trescher WA, Traystman RJ, Johnston MV. Acute experimental neuronal injury in the newborn lamb: US characterization and demonstration of hemodynamic effects. Pediatr Radiol 1993;23:268-275 [DOI] [PubMed] [Google Scholar]
- 38.Taylor GA. Regional cerebral blood flow estimates in newborn lamb using amplitude-mode color doppler ultrasound. Pediatr Radiol 1996;26:282-286 [DOI] [PubMed] [Google Scholar]


