Neuroangiography is a safe and effective technique for evaluating various intracranial and extracranial disorders. The diagnostic information obtained by neuroangiography, combined with other clinical and noninvasive imaging findings, can be used to plan or evaluate results of treatment.
Participation by the angiographer in preprocedural selection, intraprocedural monitoring, and postprocedural follow-up and management of the patient is an integral part of neuroangiography and will increase the success rate of the procedure.
These guidelines have been written for use in institution-wide quality improvement programs to assess the practice of neuroangiography. The most important processes of care are 1) patient selection, preparation, and education; 2) procedural performance; and 3) patient monitoring. The outcome measures or indicators for these processes are indications, success rates, and complication rates. Outcome measures are assigned threshold levels.
Definition and Procedural Overview (1–3)
Neuroangiography is a process by which the intracranial and extracranial head and neck circulation is evaluated. (Spinal and selective intracranial angiography will be addressed in a separate document.) It consists of selective catheter placement into extracranial cervical vessels by using imaging guidance, followed by contrast material injection, to delineate anatomy. The catheter is usually inserted via a common femoral arterial access site, but other access sites (eg, axillary, brachial) may be used in selected cases. Aortic arch flush injections may be performed to delineate the origins and/or tortuosity of the extracranial cervical vessels prior to selective catheterization. However, unless severe occlusive disease prohibits safe selective catheterization, a selective study should be performed. Selective catheter placement optimally shows the extracranial and intracranial circulation and better defines occlusive morphology, tandem occlusive lesions, and coincident or contributory pathology. Evaluation of the intracranial circulation is an essential component of the angiographic study of occlusive extracranial cerebrovascular disease. The injection of contrast media must be at a rate and volume that safely and adequately opacifies the vascular territory of interest. Optimal positioning, magnification, and filming rates are necessary to provide sufficient information regarding the disease and vascular territory being studied. Several projections may be necessary to show the targeted area, but a minimum of two orthogonal projections is essential. Findings are acquired and stored either on conventional film or digitally on computerized storage media. Imaging and image recording must be consistent with the As Low As Reasonably Achievable (ALARA) radiation safety guidelines (2). Image-based diagnosis and treatment planning require integrating the angiographic findings within the context of the patient's history, physical findings, and prior imaging studies. Therefore, the neuroangiographer must be clinically informed and understand the specific questions to be answered by neuroangiography prior to the procedure in order to plan and perform the procedure safely and effectively.
The physician performing the neuroangiogram must fully appreciate the benefits, alternatives, and risks of the procedure. S/he must have a thorough understanding of extracranial and intracranial vascular anatomy (including congenital and developmental variants and common collateral pathways), the angiographic equipment, radiation safety considerations, physiologic monitoring equipment, and have access to an adequate supply of catheters, guidewires, and personnel to perform the procedure safely. The physician must understand the principles of prevention of thromboembolic phenomena with anticoagulation and catheter flushing, the need for adequate hydration, puncture-site hemostasis, and management of neuroangiographic complications. Furthermore, the performing physician must be able to detect and understand the clinical significance of unsuspected findings.
Although practicing physicians should strive to achieve perfect outcomes (eg, 100% success, 0% complications), in practice, all physicians will fall short of this ideal to a variable extent. Thus, indicator thresholds may be used to assess the efficacy of ongoing quality improvement programs. For the purpose of these guidelines, a threshold is a specific level of an indicator that should prompt a review. “Procedure thresholds” or “overall thresholds” reference a group of indicators for a procedure, such as major complications for selective neuroangiography. Individual complications may also be associated with complication-specific thresholds. When measures such as indications or success rates fall below a (minimum) threshold, or when complication rates exceed a (maximum) threshold, a review should be performed to determine causes and to implement changes, if necessary.
Thresholds may vary from those listed herein; for example, patient referral patterns and selection factors may dictate a different threshold value for a particular indicator at a particular institution. Thus, setting universal thresholds is very difficult and each institution is urged to alter the thresholds as needed to higher or lower values, to meet its own quality improvement program needs. The threshold for the following indications is 99%. When fewer than 99% of procedures are performed for these indications, the institution will review the process of patient selection.
Indications (3–10)
1) Define presence/extent of vascular occlusive disease and thromboembolic phenomena.
2) Define etiology of hemorrhage (subarachnoid, intraventricular, parenchymal, and craniofacial).
3) Define presence, location, and anatomy of intracranial aneurysms and vascular malformations.
4) Evaluate vasospasm related to subarachnoid hemorrhage.
5) Define presence/extent of trauma to cervicocerebral vessels (eg, dissection and pseudoaneurysm).
6) Define vascular supply to tumors.
7) Define presence/extent of vasculitis (infectious, inflammatory, and drug-induced).
8) Diagnose and/or define congenital or anatomic anomaly (eg, vein of Galen fistula).
9) Define presence of venous occlusive disease (eg, dural sinus, cortical, and deep).
10) Outline vascular anatomy for planning and determining the effect of therapeutic measures.
11) Perform physiologic testing of brain function (eg, Wada's test).
There are no absolute contraindications to adult diagnostic neuroangiography. Relative contraindications include iodinated contrast media allergy, hypotension, severe hypertension, coagulopathy, renal insufficiency, and congestive heart failure. Patient management should address these relative contraindications prior to the procedure. Patients with diabetes who are taking metformin (Glucophage) should discontinue its use at the time of angiography and for the following 48 hours until renal function has been assessed as adequate.
Success Rate (11–13)
A successful examination is defined as sufficient selective neuroangiographic technical evaluation and image interpretation to establish or exclude pathology of the extracranial and intracranial circulation. Successful neuroangiography for the evaluation of atherosclerotic disease is performed in one sitting but, rarely, repeated neuroangiography may be necessary owing to limitation of vascular access, contrast media dose limit, patient intolerance, inadequate anesthesia, or comorbid illness such as congestive heart failure, which obviates prolonged supine positioning. Evaluation of certain conditions, such as intracranial hemorrhage, may require multiple studies to define or exclude pathology. Success rate for neuroangiography is as follows:
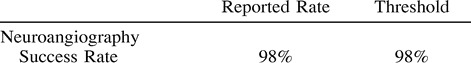 |
The rate of success is related to the patient's age, severity of atherosclerosis, and the presence of hypertensive disease.
Complications (14–71)
The risks of neuroangiography are generally higher among patients of advanced age with severe atherosclerosis, pre-existing symptomatic cerebrovascular disease, acute subarachnoid hemorrhage, certain vascular dysplasias such as Ehlers-Danlos Syndrome, and possibly a history of migraine headache. The risks are related to the length of the procedure, number of catheter exchanges, catheter size, extent of catheter manipulation, and amount of contrast media used. Femoral introduction of the diagnostic catheter is considered safer than retrograde axillobrachial catheterization and direct carotid/vertebral puncture. Nonionic low-osmolarity contrast media are generally safer than ionic, high-osmolarity contrast media among patients with a previous history of contrast media hypersensitivity or nephropathy. The risk of contrast media–induced nephropathy is greater for patients with pre-existing acute or chronic azotemia, particularly in association with diabetes.
Neurologic
Neurologic complications that occur within 24 hours of the angiogram are, by definition, attributed to the angiogram and are defined by the duration and severity of the neurologic deficit (Table 1). A deficit lasting fewer than 24 hours is a transient ischemic attack (TIA). Deficits lasting longer than 24 hours are considered strokes. Strokes may be divided, based on reversibility, into reversible and permanent strokes. A deficit that resolves within 7 days is defined as a reversible stroke, and one lasting longer than 7 days is defined as a permanent stroke. Permanent strokes range in severity from trivial to life-threatening. In order to evaluate the outcome of patients after cerebral angiography, an objective measure of stroke severity should be made. The Modified Rankin Disability Score (Table 2) is easily performed and allows stratification of stroke severity that can be compared with patient status prior to angiography.
TABLE 1:
Neurologic complications

TABLE 2:
Modified Rankin Disability Scores

Non-neurologic
Non-neurologic complications can be stratified on the basis of outcome. Major complications result in admission to a hospital for therapy (for outpatient procedures), an unplanned increase in the level of care, resulting in prolonged hospitalization, permanent adverse sequelae, or death (Table 3). Minor complications result in no sequelae; they may require nominal therapy or a short hospital stay for observation (generally overnight) (Appendix A). The complication rates and thresholds below refer to major complications. Any death for which the onset of cause is within 24 hours of the procedure or a puncture-site infection should be reviewed as part of the institution-wide quality improvement program.
TABLE 3:
Major complications (non-neurologic)
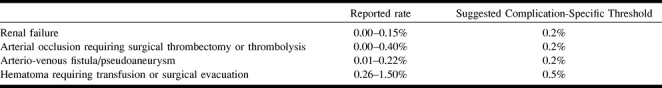
Published rates for individual types of complications are highly dependent on patient selection and are based on series comprising several hundred patients, which is a volume larger than most individual practitioners are likely to treat. It is also recognized that a single complication can cause a rate to cross above a complication-specific threshold when, for example, the complication occurs in a small volume of patients early in a quality improvement program. In this situation, the overall procedure threshold is more appropriate for use in a quality improvement program.
Overall Procedure Threshold
All major complications resulting from Adult Diagnostic Neuroangiography … 2%.
This threshold refers to any complication that requires additional therapy, prolonged hospitalization, or causes permanent adverse sequelae, as defined in Appendix A.
Disclaimer
The clinical practice guidelines of the Society of Cardiovascular and Interventional Radiology, the American Society of Interventional and Therapeutic Neuroradiology, and the American Society of Neuroradiology attempt to define practice principles that generally should assist in producing high-quality medical care. These guidelines are voluntary and not intended to be rules. A physician may deviate from these guidelines, as necessitated by the individual patient and available resources. These practice guidelines should not be deemed inclusive of all proper methods of care or exclusive of other methods of care that are reasonably directed toward the same result. Other sources of information may be used in conjunction with these principles to produce a process leading to high-quality medical care. The ultimate judgment regarding the conduct of any specific procedure or course of management must be made by the physician, who should consider all circumstances relevant to the individual clinical situation. Adherence to the Quality Improvement Program will not assure a successful outcome in every situation. It is prudent to document the rationale for any deviation from the suggested practice guidelines in the departmental policies and procedural manual or in the patient's medical record.
Society of Cardiovascular & Interventional Radiology Standards of Practice Committee Classification of Complications by Outcome
Minor Complications
A. No therapy, no consequence.
B. Nominal therapy, no consequence; includes overnight admission for observation only.
Major Complications
C. Require therapy, minor hospitalization (<48 hours).
D. Require major therapy, unplanned increase in level of care, prolonged hospitalization (>48 hours).
E. Permanent adverse sequelae.
F. Death.
Methodology
Reported complication-specific rates in some cases reflect the aggregate of major and minor complications. thresholds are derived from critical evaluation of the literature, evaluation of empirical data from standards of practice committee member practices, and, when available, the HI-IQ™ system national database.
Consensus on statements in this document was obtained utilizing a modified Delphi technique. (72, 73)
Footnotes
Technical documents specifying the exact consensus and literature review methodologies are available upon request from the Society of Cardiovascular & Interventional Radiology, 10201 Lee Highway Suite 500, Fairfax, VA 22030.
* Robert C. Wallace, MD, Co-Chair, ASITN and ASNR; Steven J. Citron, MD, Co-chair, SCVIR.
Curtis A. Lewis, MD; Robert C. Dawson, MD; Jacques E. Dion, MD; Allan J. Fox, MD; James V. Manzione, MD; Cynthia S. Payne, MD; Frank J. Rivera, MD; Eric J. Russell, MD; David Sacks, MD; Wayne F. Yakes, MD; Curtis W. Bakal, MD, MPH, participated on behalf of the Joint Standards of Practice Task Force of the Society of Cardiovascular and Interventional Radiology, the American Society of Interventional and Therapeutic Neuroradiology, and the American Society of Neuroradiology.
Address reprint requests to the American Journal of Neuroradiology, 2210 Midwest Rd, Oak Brook, IL 60521.
References
- 1.Cardella JF, Casarella WJ, DeWeese JA, et al. Optimal resources for the examination and endovascular treatment of the peripheral and visceral vascular systems: AHA intercouncil report on peripheral and visceral angiographic and interventional laboratories. Circulation 1994;89:1481-1493 [DOI] [PubMed] [Google Scholar]
- 2. National Council on Radiation Protection and Measurements Implementation of the principle ofAsLow as Reasonably Achievable (ALARA) for medical and dental personnel.. Bethesda, Md: 1990. NCRP Report No. 107
- 3.Ullrich CG, Moore AV, Parsons RG. The arteriographic diagnosis of extracranial cerebrovascular disease. In: Robicsek F, ed. Extracranial Cerebrovascular Disease: Diagnosis and Management. New York: Macmillan Inc; 1986:108-140 [Google Scholar]
- 4.Aletich VA, Debrun GM, Monsein LH, Nauta HJW, Spetzler RF. Giant serpentine aneurysms: a review and presentation of 5 cases. AJNR Am J Neuroradiol 1995;16:1061-1072 [PMC free article] [PubMed] [Google Scholar]
- 5.Batson RC, Sottiurai VS. Management of asymptomatic carotid stenosis. Int Surg 1984;69:239-246 [PubMed] [Google Scholar]
- 6.Biller J, Hingtgen WL, Adams HP, Smoker WRK, Godersky JC, Toffol GJ. Cervicocephalic arterial dissections: a ten year experience. Arch Neurol 1956;43:1234-1238 [DOI] [PubMed] [Google Scholar]
- 7.Connolly JE, Brownell DA, Levine EF, McCart PM. Accuracy and indications of diagnostic studies for extracranial carotid disease. Arch Surg 1985;120:1229-1232 [DOI] [PubMed] [Google Scholar]
- 8.Douglas DJ, Schuler JJ, Buchbinder D, Dillon BC, Flanigan DP, (The Association of Central Retinal Art) Occlusion and intracranial carotid artery disease. Arch Surg 1988;208:85-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dukes HT, Veith RG. Cerebral angiography during migraine prodromal and headache. Neurology 1964;14:636-639 [DOI] [PubMed] [Google Scholar]
- 10.Meyer JP, Walsh J, Barrett J, et al. Analysis of 18 recent cases of penetrating injuries to common and int. carotid arteries. Am J Surg 1988;156:96-99 [DOI] [PubMed] [Google Scholar]
- 11.Dion JE, Gates PC, Fox AJ, Barnett HJM, Blom RJ, Moulin D. Clinical events following neuroangiography: a prospective study. Stroke 1987;18:997-1004 [DOI] [PubMed] [Google Scholar]
- 12.Uchino A. Selective catheterization of the brachiocephalic arteries via the right brachial artery. Neuroradiology 1988;30:524-527 [DOI] [PubMed] [Google Scholar]
- 13.Vitek JJ. Femoro-cerebral angiography: analysis of 2000 consecutive exams, special emphasis on carotid artery catheterization in older patients. AJR Am J Roetgenol 1973;118:633-646 [DOI] [PubMed] [Google Scholar]
- 14.Allen JH, Parera C, Potts DG. The relation of arterial trauma to complications of cerebral angiography. AJR Am J Roentgenol 1965;95:845-857 [DOI] [PubMed] [Google Scholar]
- 15.Amagasa M, Yoshimoto T, Mizoi K, Suzuki J. Early cerebral angiography after aneurysm rupture: analysis of 197 cases. J Neurosurg 1986;65:776-778 [DOI] [PubMed] [Google Scholar]
- 16.Byrd L, Sherman RL. Radiologic contrast-induced acute renal failure: a clinical and patho-physiological review. Medicine 1979;58:270-279 [DOI] [PubMed] [Google Scholar]
- 17.Cali RL, Berg R, Rama K. Bilateral ICA agenesis: a case study and review of the literature. Surgery 1993;113:227-233 [PubMed] [Google Scholar]
- 18.Canhao P, Ferro JH, Pinto AN, Melo TP, Campos J. Perimesencephalic and non-perimesencephalic subarachnoid hemorrhage with negative angiograms. Acta Neuro Chir 1995;132:4-19 [DOI] [PubMed] [Google Scholar]
- 19.Crnic DM, Seifert FC, Ranniger K. Arterial injury in dogs after multiple percutaneous catheterizations at the same site of entry. Radiology 1973;108:295-299 [DOI] [PubMed] [Google Scholar]
- 20.Davies KN, Humphrey PR. Complications of cerebral angiography in patients with symptoms of carotid territory ischaemia screened by carotid ultrasound. J Neurol Neurosurg Psychiatry 1993;56:967-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Buxo JA, Wagoner RD, Hattery RR, Palumbo PJ. Acute renal failure after excretory urography in diabetic patients. Ann Int Med 1975;83:155-158 [DOI] [PubMed] [Google Scholar]
- 22.Dion JE, Gates PC, Fox AJ, Barnett HJM, Blom RJ. Clinical events following neuroangiography: a prospective study. Acta Radiol 1986;369S:29-33 [PubMed] [Google Scholar]
- 23.Fisher M, Ahmadi J, Zee CS, Terry R, Weiner JM. Arteriography of carotid bifrucation: oblique projections. Neurology 1985;35:1201-1204 [DOI] [PubMed] [Google Scholar]
- 24.Fox AJ. Carotid endarterectomy trials. Neuroimag Clin North Am 1996;6:931-938 [PubMed] [Google Scholar]
- 25.Ginsberg LE, Stump DA, King JC, Deal DD, Moody DM. Air embolus risk with glass versus plastic syringes: in vitro study and implications for neuroangiography. Radiology 1994;191:813-816 [DOI] [PubMed] [Google Scholar]
- 26.Grzyska U, Freitag J, Zeumer H. Selective cerebral intraarterial DSA. Neuroradiology 1990;32:296-299 [DOI] [PubMed] [Google Scholar]
- 27.Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke 1990;21:209-222 [DOI] [PubMed] [Google Scholar]
- 28.Hankey GJ, Warlow CP. Molyneux AJ. Complications of cerebral angiography for patients with mild carotid artery territory ischaemia being considered for carotid endarterectomy. J Neurol Neurosurg Psychiatry 1990;53:542-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hass WK, Fields WS, North RR, Kricheff II, Chase NE, Bauer RB. Joint Study of extracranial arterial occlusions. II. Arteriography, techniques, sites and complications. JAMA 1968;203:961-968 [PubMed] [Google Scholar]
- 30.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401-1407 [PMC free article] [PubMed] [Google Scholar]
- 31.Hellmann DB, Roubenoff R, Healy RA, Wang H. CNS angiography: safety and predictors of a positive result in 125 consecutive patients evaluated for possible vasculitis. J Rheum 1992;19:568-572 [PubMed] [Google Scholar]
- 32.Henry PY, Larre P, Aupy M, Lafforgue JL, Orgogozo JM. Reversible cerebral arteriopathy associated with the administration of ergot derivatives. Cephalalgia 1984;4:171-178 [DOI] [PubMed] [Google Scholar]
- 33.Hessel HJ, Adams DF, Abrams HL. Complications of angiography. Radiology 1981;138:273-281 [DOI] [PubMed] [Google Scholar]
- 34.Hughes DE, Patel U, Forbes WStC, Jones AP. Comparison of hand injection with mechanical injection for digital subtraction cerebral angiography. Br J Addict 1994;67:786-789 [DOI] [PubMed] [Google Scholar]
- 35.Jackson A, Stewart G, Wood A, Gillespie J. Transient global amnesia and cortical blindness after vertebral angiography: further evidence for the role of arterial spasm. AJNR Am J Neuroradiol 1995;16:955-959 [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson BS, Paulin S, Schlossman D. Thromboembolism of leg following percutaneous catheterization of femoral artery for angiography: signs & sx. Acta Rad Diagn 1969;8:97-108 [DOI] [PubMed] [Google Scholar]
- 37.Jungreis CA, Lunsford LD, Barker D. Angiographic complications during stereotactic radiosurgery for cerebral AVMs. AJNR Am J Neuroradiol 1992;13:946-948 [PMC free article] [PubMed] [Google Scholar]
- 38.Katzenschlager R, Ugurluoglu A, Ahmadi A, et al. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology 1995;195:463-466 [DOI] [PubMed] [Google Scholar]
- 39.Kothbauer K, Schroth G, Seiler RW, Do DD. Severe symptomatic vasospasm after rupture of AVM. AJNR Am J Neuroradiol 1995;16:1073-1075 [PMC free article] [PubMed] [Google Scholar]
- 40.Kurokawa Y, Abiko S, Okamura T, et al. Pulmonary embolism after cerebral angiography: 3 case reports. Neurol Med Chir 1995;35:305-309 [DOI] [PubMed] [Google Scholar]
- 41.Lang EK. Prevention and tyreatment of complications of arteriography. Radiology 1967;88:950-956 [DOI] [PubMed] [Google Scholar]
- 42.Lang EK. A survey of complications of percutaneous retrograde arteriography. Seldinger technique. Radiology 1963;81:257-263 [DOI] [PubMed] [Google Scholar]
- 43.Latchaw RE. The use of nonionic contrast agents in neuroangiography: a review of the literature and recommendations for clinical use. Invest Radiol 1993;28:S55-S59 [DOI] [PubMed] [Google Scholar]
- 44.Leow K, Murie JA. Cerebral angiography for cerebrovascular disease: the risks. Br J Surg 1988;75:428-430 [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein DA, Klapholz L, Vardy DA, et al. Chronic radio-dermatitis following cardiac catheterization. Arch Dermatology 1996;132:663-667 [PubMed] [Google Scholar]
- 46.Mani RL, Eisenberg RL, McDonald EJ, Pollock JA, Mani JR. Complications of catheter cerebral angiography: analysis of 5000 procedures. 1. Criteria and incidence. AJR Am J Roentgenol 1978;131:861-865 [DOI] [PubMed] [Google Scholar]
- 47.Markus H, Loh A, Israel D, Buckenham T, Clifton A, Brown MM. Microscopic air embolism during cerebral angiography and strategies for its avoidance. Lancet 1993;341:784-787 [DOI] [PubMed] [Google Scholar]
- 48.Marshall NW, Noble J, Faulkner K. Patient and staff dosimetry in neuroradiologic procedures. Br J Rad 1995;68:495-501 [DOI] [PubMed] [Google Scholar]
- 49.Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of ICA experience in 500 cases. AJNR Am J Neuroradiol 1995;16:749-754 [PMC free article] [PubMed] [Google Scholar]
- 50.Mattos MA, Hodgson KJ, Faught WE, et al. Carotid endarterectomy without angiography: is color-flow duplex scanning sufficient? Surgery 1994;116:776-783 [PubMed] [Google Scholar]
- 51.McIver J, Steiner TJ, Perkin GD, Greehalgh RM, Chir M, Rose FC. Neurological morbidity of arch and carotid arteriography in cerebrovascular disease: the influence of contrast media and radiologist. Br J Radiol 1987;60:117-122 [DOI] [PubMed] [Google Scholar]
- 52.Miller JDR, Grace MG, Russell DB, Zacks DJ. Complications of cerebral angiography and pneumography. Radiology 1977;124:741-744 [DOI] [PubMed] [Google Scholar]
- 53.Nakstad P, Bakke SJ, Kjartansson O, Nyhus S. Intra-arterial DSA of the carotid arteries. Neuroradiology 1986;28:195-198 [DOI] [PubMed] [Google Scholar]
- 54.Norbash AM, Busick D, Marks MP. Techniques for reducing interventional neuroradiologic skin dose: tube position rotation and supplemental beam filtration. AJNR Am J Neuroradiol 1996;17:41-49 [PMC free article] [PubMed] [Google Scholar]
- 55.Numaguchi Y, Fleming MS, Hasuo K, Puyau FA, Nice CM. Blood-brain barrier disruption due to cerebral angio: CT findings. JCAT 1984;8:936-939 [DOI] [PubMed] [Google Scholar]
- 56.Olivecrona H. Complications of cerebral angiography. Neuroradiology 1977;14:175-181 [DOI] [PubMed] [Google Scholar]
- 57.Patterson RH, Goodell H, Dunning HS. Complications of carotid arteriography. Arch Neurol 1964;10:513-520 [DOI] [PubMed] [Google Scholar]
- 58.Saitoh H, Hayakawa K, Nishimura K, et al. Rerupture of cerebral aneurysms during angiography. AJR Am J Roentgenol 1995;16:539-542 [PMC free article] [PubMed] [Google Scholar]
- 59.Shope TB. Radiation-induced skin injuries from flouroscopy. RadioGraphics 1996;16:1195-1199 [DOI] [PubMed] [Google Scholar]
- 60.Shuaib A, Hachinski VC. Migraine and the risks of angiography. Arch Neurol 1988;45:911-912 [DOI] [PubMed] [Google Scholar]
- 61.Skalpe IO. Complications in cerebral angiography with iohexal (Omnpaque) and meglumine metrizoate (Isopaque cerebral). Neuroradiology 1988;30:69-72 [DOI] [PubMed] [Google Scholar]
- 62.Slingenberg EJ. Complications during intravascular diagnostic manipulations in the Ehlers-Danlos syndrome. Neth J Surg 1980;32:56-58 [PubMed] [Google Scholar]
- 63.Spies JB, Berlin L. Complications of femoral artery puncture. AJR Am J Roentgenol 1998;170:9-11 [DOI] [PubMed] [Google Scholar]
- 64.Theodotou BC, Whaley R, Mahaley MS. Complications following transfemoral cerebral angiography for cerebral ischemia. Surg Neurol 1987;28:90-92 [DOI] [PubMed] [Google Scholar]
- 65.Thomson KR, Thomson SMcA. Complications of cerebral angiography in a teaching hospital. Australasian Radiol 1986;30:206-208 [DOI] [PubMed] [Google Scholar]
- 66.Warnock NG, Gandhi MR, Bergvall U, Powell T. Complications of intraarterial DSA in patients investigated for cerebral vascular disease. Br J Radiol 1993;66:855-858 [DOI] [PubMed] [Google Scholar]
- 67.Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology 1992;182:243-246 [DOI] [PubMed] [Google Scholar]
- 68.Earnest F IV, Forbes G, Sandok BA, et al. Complications of cerebral angiography: prospective assessment of risk. AJNR Am J Neuroradiol 1983;4:1191-1197 [DOI] [PubMed] [Google Scholar]
- 69.Eisenberg RL, Bank WD, Hedgcock MW. Neurologic complications of angiography in patients with critical stenosis of the carotid artery. Neurology 1980;30:892-895 [DOI] [PubMed] [Google Scholar]
- 70.Feild JR, Robertson JT, DeSaussure Jr RL. Complications of cerebral case angiography in 2000 consecutive cases. J Neurosurg 1962;19:775-781 [DOI] [PubMed] [Google Scholar]
- 71.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Inter-observer agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-607 [DOI] [PubMed] [Google Scholar]
- 72.Fink A, Kosefcoff J, Chassin M, Brook RH, Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979-983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leape LL, Hilborne LH, Park RE, et al. The appropriateness of use of coronary artery bypass graft surgery in New York State. JAMA 1993;269:753-760 [PubMed] [Google Scholar]


